化工学报 ›› 2023, Vol. 74 ›› Issue (2): 642-652.DOI: 10.11949/0438-1157.20221368
收稿日期:2022-10-17
修回日期:2022-12-08
出版日期:2023-02-05
发布日期:2023-03-21
通讯作者:
程文婷
作者简介:程文婷(1983—),女,博士,副教授,wenting_cheng@outlook.com
基金资助:
Wenting CHENG1( ), Jie LI1, Li XU2, Fangqin CHENG1, Guoji LIU2
), Jie LI1, Li XU2, Fangqin CHENG1, Guoji LIU2
Received:2022-10-17
Revised:2022-12-08
Online:2023-02-05
Published:2023-03-21
Contact:
Wenting CHENG
摘要:
如何将AlCl3·6H2O从众多组分中选择性地结晶分离是从煤矸石中提Al的关键,而AlCl3·6H2O在煤矸石酸浸体系中的热力学平衡数据对于结晶过程的控制至关重要。在25~85℃的温度范围内,测定了不同温度和溶液浓度下AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl-FeCl3溶液中的溶解度。实验发现温度对AlCl3·6H2O在所有溶液体系中溶解度的影响均不明显,溶解度只随温度的升高略有增加;溶液浓度是影响溶解度的主要因素,AlCl3·6H2O在所有溶液体系中的溶解度均随溶液浓度的升高而明显下降,分析其原因是由于溶液浓度的增大使得Cl–同离子效应增强。为了提高OLI软件预测结果的准确性,对其嵌入Bromley-Zemaitis模型中“Al3+–Cl-”离子对的交互参数进行了修正。随后验证了修正模型的实用性,结果表明它可应用于AlCl3·6H2O在FeCl3、CaCl2及KCl的任意单、混溶液中溶解度的预测。
中图分类号:
程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652.
Wenting CHENG, Jie LI, Li XU, Fangqin CHENG, Guoji LIU. Experiment and prediction for the solubility of AlCl3·6H2O in FeCl3, CaCl2, KCl and KCl-FeCl3 solutions[J]. CIESC Journal, 2023, 74(2): 642-652.
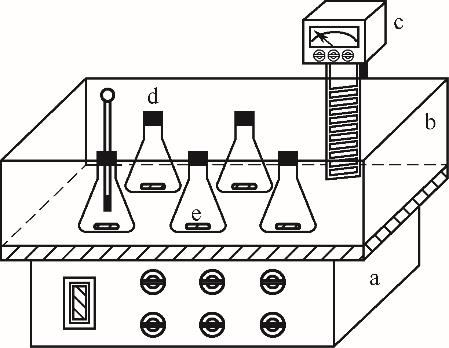
图1 溶解度测定装置a—磁力搅拌器;b—水槽;c—投入式恒温器;d—碘瓶;e—磁力转子
Fig.1 Experimental set-up used in the solubility measurementa—magnetic stirrer; b—water tank; c—input-thermostat; d—iodine flask; e—magnetic rotor
| 组分 | (cm3·mol-1) | (J·K-1·mol-1) | (J·K-1·mol-1) | (kJ·mol-1) | (kJ·mol-1) |
|---|---|---|---|---|---|
| H+ | 0 | 0 | 0 | 0 | 0 |
| OH- | -4.18 | -137.19 | -10.711 | -229.99 | -157.3 |
| H2O | 18.1 | 75.3 | 69.95 | -285.83 | -237.19 |
| Al3+ | 44.4 | -135.98 | -325.1 | -530.67 | -483.7 |
| Fe3+ | 42.8 | -142.737 | -277.522 | -49.6023 | -17.2457 |
| Ca2+ | 18.06 | -31.50 | -56.48 | -543.083 | -552.79 |
| K+ | 9.06 | 8.28432 | 101.044 | -252.17 | -282.462 |
| Cl– | 17.8 | -123.18 | 56.735 | -167.08 | -131.29 |
表1 在用OLI-HKF计算热力学平衡常数时所涉及到主要组分的热力学数据
Table 1 Thermochemical data for the main species used by OLI-HKF to calculate equilibrium constants
| 组分 | (cm3·mol-1) | (J·K-1·mol-1) | (J·K-1·mol-1) | (kJ·mol-1) | (kJ·mol-1) |
|---|---|---|---|---|---|
| H+ | 0 | 0 | 0 | 0 | 0 |
| OH- | -4.18 | -137.19 | -10.711 | -229.99 | -157.3 |
| H2O | 18.1 | 75.3 | 69.95 | -285.83 | -237.19 |
| Al3+ | 44.4 | -135.98 | -325.1 | -530.67 | -483.7 |
| Fe3+ | 42.8 | -142.737 | -277.522 | -49.6023 | -17.2457 |
| Ca2+ | 18.06 | -31.50 | -56.48 | -543.083 | -552.79 |
| K+ | 9.06 | 8.28432 | 101.044 | -252.17 | -282.462 |
| Cl– | 17.8 | -123.18 | 56.735 | -167.08 | -131.29 |
| 组分 | α1×10 | α2×10-2 | α3 | α4×10-4 | γ1 | γ2×10-4 | ω×10-5 |
|---|---|---|---|---|---|---|---|
| H+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OH- | 1.2527 | 0.0738 | 1.8423 | -2.782 | 4.15 | -10.35 | 1.724 |
| Al3+ | -3.3404 | -17.1108 | 14.9917 | -2.0716 | 10.7 | -8.06 | 2.8711 |
| Fe3+ | -3.1784 | -15.542 | 11.859 | -2.1365 | 11.08 | -9.9808 | 2.7025 |
| Ca2+ | -0.1974 | 7.252 | 5.2966 | -2.4792 | 9 | -2.522 | 1.2366 |
| K+ | 3.559 | -1.473 | 5.435 | -2.712 | 7.4 | -1.791 | 0.1927 |
| Cl- | 4.032 | 4.801 | 5.563 | -2.847 | -4.40 | -5.714 | 1.456 |
表2 主要组分的七个HKF参数值
Table 2 Values of seven HKF parameters for the main species
| 组分 | α1×10 | α2×10-2 | α3 | α4×10-4 | γ1 | γ2×10-4 | ω×10-5 |
|---|---|---|---|---|---|---|---|
| H+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OH- | 1.2527 | 0.0738 | 1.8423 | -2.782 | 4.15 | -10.35 | 1.724 |
| Al3+ | -3.3404 | -17.1108 | 14.9917 | -2.0716 | 10.7 | -8.06 | 2.8711 |
| Fe3+ | -3.1784 | -15.542 | 11.859 | -2.1365 | 11.08 | -9.9808 | 2.7025 |
| Ca2+ | -0.1974 | 7.252 | 5.2966 | -2.4792 | 9 | -2.522 | 1.2366 |
| K+ | 3.559 | -1.473 | 5.435 | -2.712 | 7.4 | -1.791 | 0.1927 |
| Cl- | 4.032 | 4.801 | 5.563 | -2.847 | -4.40 | -5.714 | 1.456 |
| A | B | C | D |
|---|---|---|---|
| -93.1444 | -7955.7916 | 16.7452 | -0.0419 |
表3 AlCl3·6H2O热力学平衡方程的经验参数
Table 3 Coefficients for thermodynamic equilibrium constants of AlCl3·6H2O
| A | B | C | D |
|---|---|---|---|
| -93.1444 | -7955.7916 | 16.7452 | -0.0419 |
| 温度/℃ | 用不同单位形式表示的溶解度(以AlCl3计) | ||
|---|---|---|---|
| 25 | 386.124 | 2.8958 | 3.3622 |
| 35 | 385.658 | 2.8923 | 3.3754 |
| 45 | 385.598 | 2.8918 | 3.3896 |
| 50 | 386.728 | 2.9003 | 3.4100 |
| 55 | 386.437 | 2.8981 | 3.4112 |
| 65 | 387.280 | 2.9045 | 3.4287 |
| 70 | 388.439 | 2.9131 | 3.4453 |
| 75 | 388.881 | 2.9165 | 3.4514 |
| 85 | 390.593 | 2.9293 | 3.4682 |
表4 AlCl3·6H2O在水中的溶解度(平衡时间:6 h)
Table 4 Solubility of AlCl3·6H2O (1) in H2O (2) (Equilibration time: 6 h)
| 温度/℃ | 用不同单位形式表示的溶解度(以AlCl3计) | ||
|---|---|---|---|
| 25 | 386.124 | 2.8958 | 3.3622 |
| 35 | 385.658 | 2.8923 | 3.3754 |
| 45 | 385.598 | 2.8918 | 3.3896 |
| 50 | 386.728 | 2.9003 | 3.4100 |
| 55 | 386.437 | 2.8981 | 3.4112 |
| 65 | 387.280 | 2.9045 | 3.4287 |
| 70 | 388.439 | 2.9131 | 3.4453 |
| 75 | 388.881 | 2.9165 | 3.4514 |
| 85 | 390.593 | 2.9293 | 3.4682 |
| 溶液的浓度和密度 | 用不同单位形式表示的溶解度 (以AlCl3计) | ||||||
|---|---|---|---|---|---|---|---|
| M2/(mol·L-1) | M3/(mol·L-1) | m2/(mol·(kg H2O)-1) | m3/(mol·(kg H2O)-1) | ρs/(g·ml-1) | C1/(g·L-1) | M1/(mol·L-1) | m1/(mol·(kg H2O)-1) |
| T=25℃ | |||||||
| 0.25 | 0 | 0.2923 | 0 | 1.2523 | 377.606 | 2.8319 | 3.3091 |
| 0.25 | 0.1 | 0.2921 | 0.1002 | 1.2626 | 374.724 | 2.8103 | 3.2856 |
| 0.25 | 0.2 | 0.2920 | 0.1940 | 1.2718 | 370.003 | 2.7749 | 3.2409 |
| 0.25 | 0.4 | 0.2916 | 0.3150 | 1.2837 | 363.840 | 2.7287 | 3.1823 |
| 0.25 | 0.5 | 0.2907 | 0.4265 | 1.2947 | 356.597 | 2.6743 | 3.1098 |
| 0.25 | 0.7 | 0.2906 | 0.5452 | 1.3062 | 349.680 | 2.6225 | 3.0435 |
| 0.25 | 0.8 | 0.2905 | 0.6707 | 1.3180 | 345.142 | 2.5884 | 3.0076 |
| 0.25 | 0.9 | 0.2899 | 0.7579 | 1.3263 | 338.541 | 2.5389 | 2.9418 |
| 0.25 | 1.0 | 0.2898 | 0.8715 | 1.3371 | 333.771 | 2.5032 | 2.9013 |
| 0.25 | 1.1 | 0.2897 | 0.9957 | 1.3486 | 328.333 | 2.4624 | 2.8553 |
| 0.25 | 1.2 | 0.2888 | 1.0793 | 1.3561 | 320.330 | 2.4024 | 2.7753 |
| T=50℃ | |||||||
| 0.25 | 0 | 0.2968 | 0 | 1.2420 | 379.939 | 2.8494 | 3.3795 |
| 0.25 | 0.1 | 0.2965 | 0.1098 | 1.2521 | 375.231 | 2.8141 | 3.3375 |
| 0.25 | 0.2 | 0.2962 | 0.2080 | 1.2613 | 370.293 | 2.7771 | 3.2906 |
| 0.25 | 0.4 | 0.2956 | 0.3138 | 1.2712 | 363.734 | 2.7279 | 3.2252 |
| 0.25 | 0.5 | 0.2954 | 0.4281 | 1.2813 | 357.475 | 2.6809 | 3.1674 |
| 0.25 | 0.7 | 0.2950 | 0.5561 | 1.2930 | 348.889 | 2.6165 | 3.0824 |
| 0.25 | 0.8 | 0.2945 | 0.6793 | 1.3040 | 344.391 | 2.5828 | 3.0473 |
| 0.25 | 0.9 | 0.2944 | 0.7662 | 1.3115 | 336.976 | 2.5272 | 2.9723 |
| 0.25 | 1.0 | 0.2943 | 0.8997 | 1.3232 | 331.210 | 2.4840 | 2.9245 |
| 0.25 | 1.1 | 0.2942 | 1.0065 | 1.3324 | 325.814 | 2.4435 | 2.8771 |
| 0.25 | 1.2 | 0.2940 | 1.0873 | 1.3394 | 320.644 | 2.4047 | 2.8284 |
表8 AlCl3·6H2O在KCl-FeCl3-H2O中的溶解度 (平衡时间:6 h)
Table 8 Solubility of AlCl3·6H2O (1) in KCl (2)-FeCl3 (3)-H2O (4) (Equilibration time: 6 h)
| 溶液的浓度和密度 | 用不同单位形式表示的溶解度 (以AlCl3计) | ||||||
|---|---|---|---|---|---|---|---|
| M2/(mol·L-1) | M3/(mol·L-1) | m2/(mol·(kg H2O)-1) | m3/(mol·(kg H2O)-1) | ρs/(g·ml-1) | C1/(g·L-1) | M1/(mol·L-1) | m1/(mol·(kg H2O)-1) |
| T=25℃ | |||||||
| 0.25 | 0 | 0.2923 | 0 | 1.2523 | 377.606 | 2.8319 | 3.3091 |
| 0.25 | 0.1 | 0.2921 | 0.1002 | 1.2626 | 374.724 | 2.8103 | 3.2856 |
| 0.25 | 0.2 | 0.2920 | 0.1940 | 1.2718 | 370.003 | 2.7749 | 3.2409 |
| 0.25 | 0.4 | 0.2916 | 0.3150 | 1.2837 | 363.840 | 2.7287 | 3.1823 |
| 0.25 | 0.5 | 0.2907 | 0.4265 | 1.2947 | 356.597 | 2.6743 | 3.1098 |
| 0.25 | 0.7 | 0.2906 | 0.5452 | 1.3062 | 349.680 | 2.6225 | 3.0435 |
| 0.25 | 0.8 | 0.2905 | 0.6707 | 1.3180 | 345.142 | 2.5884 | 3.0076 |
| 0.25 | 0.9 | 0.2899 | 0.7579 | 1.3263 | 338.541 | 2.5389 | 2.9418 |
| 0.25 | 1.0 | 0.2898 | 0.8715 | 1.3371 | 333.771 | 2.5032 | 2.9013 |
| 0.25 | 1.1 | 0.2897 | 0.9957 | 1.3486 | 328.333 | 2.4624 | 2.8553 |
| 0.25 | 1.2 | 0.2888 | 1.0793 | 1.3561 | 320.330 | 2.4024 | 2.7753 |
| T=50℃ | |||||||
| 0.25 | 0 | 0.2968 | 0 | 1.2420 | 379.939 | 2.8494 | 3.3795 |
| 0.25 | 0.1 | 0.2965 | 0.1098 | 1.2521 | 375.231 | 2.8141 | 3.3375 |
| 0.25 | 0.2 | 0.2962 | 0.2080 | 1.2613 | 370.293 | 2.7771 | 3.2906 |
| 0.25 | 0.4 | 0.2956 | 0.3138 | 1.2712 | 363.734 | 2.7279 | 3.2252 |
| 0.25 | 0.5 | 0.2954 | 0.4281 | 1.2813 | 357.475 | 2.6809 | 3.1674 |
| 0.25 | 0.7 | 0.2950 | 0.5561 | 1.2930 | 348.889 | 2.6165 | 3.0824 |
| 0.25 | 0.8 | 0.2945 | 0.6793 | 1.3040 | 344.391 | 2.5828 | 3.0473 |
| 0.25 | 0.9 | 0.2944 | 0.7662 | 1.3115 | 336.976 | 2.5272 | 2.9723 |
| 0.25 | 1.0 | 0.2943 | 0.8997 | 1.3232 | 331.210 | 2.4840 | 2.9245 |
| 0.25 | 1.1 | 0.2942 | 1.0065 | 1.3324 | 325.814 | 2.4435 | 2.8771 |
| 0.25 | 1.2 | 0.2940 | 1.0873 | 1.3394 | 320.644 | 2.4047 | 2.8284 |
| 离子对 | B1 | B2 | B3 | C1 | C2 | C3 | D1 | D2 | D3 |
|---|---|---|---|---|---|---|---|---|---|
| Al3+-Cl- | 0.09215 | 6.4151×10-5 | 1.2147×10-6 | -3.415×10-4 | -7.513×10-6 | -6.851×10-8 | -1.084×10-5 | 1.1475×10-7 | 1.1082×10-9 |
表9 Al3+-Cl-离子对的Bromley-Zemaitis活度系数模型修正参数
Table 9 Modified Bromley-Zemaitis model parameters for Al3+-Cl- interactions
| 离子对 | B1 | B2 | B3 | C1 | C2 | C3 | D1 | D2 | D3 |
|---|---|---|---|---|---|---|---|---|---|
| Al3+-Cl- | 0.09215 | 6.4151×10-5 | 1.2147×10-6 | -3.415×10-4 | -7.513×10-6 | -6.851×10-8 | -1.084×10-5 | 1.1475×10-7 | 1.1082×10-9 |
| 1 | Li C, Wan J H, Sun H H, et al. Investigation on the activation of coal gangue by a new compound method[J]. Journal of Hazardous Materials, 2010, 179(1/2/3): 515-520. |
| 2 | Qureshi A A, Kazi T G, Baig J A, et al. Exposure of heavy metals in coal gangue soil, in and outside the mining area using BCR conventional and vortex assisted and single step extraction methods. Impact on orchard grass[J]. Chemosphere, 2020, 255: 1-11. |
| 3 | 常纪文, 杜根杰, 杜建磊, 等. 我国煤矸石综合利用的现状、问题与建议[J]. 中国环保产业, 2022(8): 13-17. |
| Chang J W, Du G J, Du J L, et al. Current situation of the comprehensive utilization of coal gangue in China and the related problems and recommendations[J]. China Environmental Protection Industry, 2022(8): 13-17. | |
| 4 | Stracher G B, Taylor T P. Coal fires burning out of control around the world: thermodynamic recipe for environmental catastrophe[J]. International Journal of Coal Geology, 2004, 59(1/2): 7-17. |
| 5 | Pone J D N, Hein K A A, Stracher G B, et al. The spontaneous combustion of coal and its by-products in the Witbank and Sasolburg coalfields of South Africa[J]. International Journal of Coal Geology, 2007, 72(2): 124-140. |
| 6 | Leroy C, Ferro M C, Monteiro R C C, et al. Production of glass-ceramics from coal ashes[J]. Journal of the European Ceramic Society, 2001, 21(2): 195-202. |
| 7 | Haugsten K E, Gustavson B. Environmental properties of vitrified fly ash from hazardous and municipal waste incineration[J]. Waste Management, 2000, 20(2/3): 167-176. |
| 8 | 马志斌, 张森, 单雪媛, 等. 煤、煤泥和煤矸石燃烧过程锂镓稀土元素的迁移规律[J]. 化工学报, 2021, 72(6): 3349-3358. |
| Ma Z B, Zhang S, Shan X Y, et al. Migration of lithium, gallium and rare earth elements in coal, coal slime, and coal gangue during combustion[J]. CIESC Journal, 2021, 72(6): 3349-3358. | |
| 9 | 郭志强, 燕可洲, 张吉元, 等. 煤矸石/粉煤灰对赤泥钠化还原焙烧反应的影响机制[J]. 化工学报, 2022, 73(5): 2194-2205. |
| Guo Z Q, Yan K Z, Zhang J Y, et al. Influence mechanism of coal gangue/coal fly ash on the sodium reduction roasting reaction of red mud[J]. CIESC Journal, 2022, 73(5): 2194-2205. | |
| 10 | Zha J F, Guo G L, Wang Q, et al. Study of in-situ sieving experiment and gradation optimization of gangue[J]. Procedia Earth and Planetary Science, 2009, 1(1): 754-759. |
| 11 | Wang H J, Wang X L, Wang L X. Adsorption performance of methylene blue on modified coal gangue[J]. Advanced Materials Research, 2013, 807/808/809: 521-525. |
| 12 | Agirre I, Griessacher T, Roesler G, et al. Production of charcoal as an alternative reducing agent from agricultural residues using a semi-continuous semi-pilot scale pyrolysis screw reactor[J]. Fuel Processing Technology, 2013, 106: 114-121. |
| 13 | Shemi A, Mpana R N, Ndlovu S, et al. Alternative techniques for extracting alumina from coal fly ash[J]. Minerals Engineering, 2012, 34: 30-37. |
| 14 | Brown R R, Daut G E, Mrazek R V, et al. Solubility and activity of aluminum chloride in aqueous hydrochloric acid solutions[R]. Report of Investigations 8379, United States Department of the Interior, Bureau of Mines, 1979. |
| 15 | Richter U, Brand P, Bohmhammel K, et al. Thermodynamic investigations of aqueous solutions of aluminum chloride[J]. The Journal of Chemical Thermodynamics, 2000, 32(2): 145-154. |
| 16 | Farelo F, Fernandes C, Avelino A. Solubilities for six ternary systems: NaCl+NH4Cl+H2O, KCl+NH4Cl+H2O, NaCl+LiCl+H2O, KCl+LiCl+H2O, NaCl+AlCl3+H2O, and KCl+AlCl3+H2O at T = (298 to 333) K[J]. Journal of Chemical and Engineering Data, 2005, 50(4): 1470-1477. |
| 17 | Wang J F, Petit C, Zhang X P, et al. Phase equilibrium study of the AlCl3-CaCl2-H2O system for the production of aluminum chloride hexahydrate from Ca-rich flue ash[J]. Journal of Chemical and Engineering Data, 2016, 61(1): 359-369. |
| 18 | 袁梦霞, 乔秀臣. 三元体系AlCl3+CaCl2+H2O, AlCl3+FeCl3+H2O和CaCl2+FeCl3+H2O在35℃时的相平衡[J]. 化工学报, 2017, 68(7): 2653-2659. |
| Yuan M X, Qiao X C. Phase equilibria of AlCl3+CaCl2+H2O, AlCl3+FeCl3 +H2O and CaCl2+FeCl3+H2O ternary systems at 35℃[J]. CIESC Journal, 2017, 68(7): 2653-2659. | |
| 19 | Pitzer K S. Thermodynamics of electrolytes(Ⅰ): Theoretical basis and general equations[J]. The Journal of Physical Chemistry, 1973, 77(2): 268-277. |
| 20 | Cheng W T, Li Z B, Cheng F Q. Solubility of Li2CO3 in Na-K-Li-Cl brines from 20 to 90oC[J]. The Journal of Chemical Thermodynamics, 2013, 67: 74-82. |
| 21 | Cheng W T, Li Z B. Precipitation of nesquehonite from homogeneous supersaturated solutions[J]. Crystal Research and Technology, 2009, 44(9): 937-947. |
| 22 | Cheng W T, Li Z B. Controlled supersaturation precipitation of hydromagnesite for the MgCl2-Na2CO3 system at elevated temperatures: chemical modeling and experiment[J]. Industrial & Engineering Chemistry Research, 2010, 49(4): 1964-1974. |
| 23 | Ma J Y, Li Z B. Chemical equilibrium modeling and experimental measurement of solubility for Friedel's salt in the Na-OH-Cl-NO3-H2O systems up to 200oC[J]. Industrial & Engineering Chemistry Research, 2010, 49(19): 8949-8958. |
| 24 | Thomsen K, Rasmussen P, Gani R. Simulation and optimization of fractional crystallization processes[J]. Chemical Engineering Science, 1998, 53(8): 1551-1564. |
| 25 | Sun S P, Wang J F, Li Z B. Solubility and self-consistent modeling of aniline hydrochloride in H-Mg-Na-Ca-Al-Cl-H2O system at the temperature range of 288—348 K[J]. Industrial & Engineering Chemistry Research, 2012, 51(9): 3783-3790. |
| 26 | Berthold J. An Overview of Data Analysis and Data Entry for OLI Programs[M]. New Jersey: OLI Systems Inc., 2006. |
| 27 | de Lucas A, Rodríguez L, Sánchez P, et al. Comparative study of the solubility of the crystalline layered silicates α-Na2Si2O5 and δ-Na2Si2O5 and the amorphous silicate Na2Si2O5 [J]. Industrial & Engineering Chemistry Research, 2004, 43(6): 1472-1477. |
| 28 | Power W H, Fabuss B M. Transient solubilities in the calcium sulfate-water system[J]. Journal of Chemical & Engineering Data, 1964, 9(3): 437-442. |
| 29 | Helgeson H C, Kirkham D H, Flowers G C. Theoretical prediction of the thermodynamic behavior of aqueous electrolytes by high pressures and temperatures ( Ⅳ ) : Calculation of activity coefficients, osmotic coefficients, and apparent molal and standard and relative partial molal properties to 600oC and 5kb[J]. American Journal of Science, 1981, 281(10): 1249-1516. |
| 30 | Shock E L, Helgeson H C. Erratum to geochim. Cosmochim.: E. L. Shock and H. C. Helgeson: cosmochimica acta 52, pp. 2009–2036[J]. Geochimica et Cosmochimica Acta, 1989, 53(1): 215. |
| 31 | Shock E L, Sassani D C, Willis M, et al. Inorganic species in geologic fluids: correlations among standard molal thermodynamic properties of aqueous ions and hydroxide complexes[J]. Geochimica et Cosmochimica Acta, 1997, 61(5): 907-950. |
| 32 | Bromley L A. Thermodynamic properties of strong electrolytes in aqueous solutions[J]. AIChE Journal, 1973, 19(2): 313-320. |
| 33 | Zemaitis J F Jr. Predicting vapor-liquid-solid equilibria in multicomponent aqueous solutions of electrolytes[M] //Thermodynamics of Aqueous Systems with Industrial Applications. Washington, D. C.: American Chemical Society, 1980: 227-246. |
| 34 | Meissner H P, Kusik C L. Aqueous solutions of two or more strong electrolytes. Vapor pressures and solubilities[J]. Industrial & Engineering Chemistry Process Design and Development, 1973, 12(2): 205-208. |
| [1] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [2] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [3] | 姚晓宇, 沈俊, 李健, 李振兴, 康慧芳, 唐博, 董学强, 公茂琼. 流体气液临界参数测量方法研究进展[J]. 化工学报, 2023, 74(5): 1847-1861. |
| [4] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [5] | 毛元敬, 杨智, 莫松平, 郭浩, 陈颖, 罗向龙, 陈健勇, 梁颖宗. C6~C10烷醇的SAFT-VR Mie状态方程参数回归及其热物性研究[J]. 化工学报, 2023, 74(3): 1033-1041. |
| [6] | 高靖博, 孙强, 李青, 王逸伟, 郭绪强. 考虑水合物结构转变的含氢气体水合物相平衡模型[J]. 化工学报, 2023, 74(2): 666-673. |
| [7] | 蔡进, 王晓辉, 汤涵, 陈光进, 孙长宇. TBAB水溶液体系中半笼型水合物的相平衡预测模型[J]. 化工学报, 2023, 74(1): 408-415. |
| [8] | 杨松涛, 李东洋, 牛玉清, 李鑫钢, 康绍辉, 李洪, 叶开凯, 周志全, 高鑫. 氟化物势能函数和热力学性质的分子模拟研究进展[J]. 化工学报, 2022, 73(9): 3828-3840. |
| [9] | 周桓, 张梦丽, 郝晴, 吴思, 李杰, 徐存兵. 硫酸镁型光卤石转化钾盐镁矾的过程机制与动态规律[J]. 化工学报, 2022, 73(9): 3841-3850. |
| [10] | 刘潜, 张香兰, 李志平, 李玉龙, 韩梦醒. 油酚分离过程低共熔溶剂的筛选及萃取性能研究[J]. 化工学报, 2022, 73(9): 3915-3928. |
| [11] | 孙哲, 金华强, 李康, 顾江萍, 黄跃进, 沈希. 基于知识数据化表达的制冷空调系统故障诊断方法[J]. 化工学报, 2022, 73(7): 3131-3144. |
| [12] | 任玉鑫, 徐润峰, 王婉颖, 陈鹏忠, 彭孝军. 彩色光刻胶用蒽醌染料的合成及稳定性研究[J]. 化工学报, 2022, 73(5): 2251-2261. |
| [13] | 张家仁, 刘海超. 大豆油与甲醇酯交换反应体系的相平衡研究[J]. 化工学报, 2022, 73(5): 1920-1929. |
| [14] | 任嘉辉, 刘豫, 刘朝, 刘浪, 李莹. 基于分子指纹和拓扑指数的工质临界温度理论预测[J]. 化工学报, 2022, 73(4): 1493-1500. |
| [15] | 门文欣, 彭庆收, 桂霞. 不同季铵盐作用下的CO2水合物相平衡[J]. 化工学报, 2022, 73(4): 1472-1482. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号