化工学报 ›› 2024, Vol. 75 ›› Issue (9): 3163-3175.DOI: 10.11949/0438-1157.20240178
收稿日期:2024-02-18
修回日期:2024-03-13
出版日期:2024-09-25
发布日期:2024-10-10
通讯作者:
王雅君
作者简介:张佳颖(1998—),女,硕士研究生,zhangjyjia@163.com
基金资助:
Jiaying ZHANG1,2( ), Cong WANG1, Yajun WANG1(
), Cong WANG1, Yajun WANG1( )
)
Received:2024-02-18
Revised:2024-03-13
Online:2024-09-25
Published:2024-10-10
Contact:
Yajun WANG
摘要:
在过硫酸盐高级氧化技术中,Co2+的再生效率低是Co高效活化过一硫酸盐(PMS)的主要问题。成功制备了富含氧缺陷氧化铋负载碳纳米管(x%CNT-Co/Bi2O3)光催化剂,用于光催化协同过硫酸盐活化降解污染物。在紫外线照射下,催化剂添加量为20 mg/L、PMS浓度为0.5 mmol/L和初始pH为4.68时,70%CNT-Co/Bi2O3对四环素(TC)的降解率高达91.3%,具有优异的可重复利用性和稳定性。其降解活性的提高归因于CNT-Co具有极大的比表面积,有利于TC吸附,光生电子加速Co2+→Co3+→Co2+的循环速率,既促进光生电荷的分离与迁移,又促进PMS的活化,实现TC的快速降解。此外,Bi2O3均匀分散在CNT-Co外管壁上,避免了Bi2O3纳米颗粒的团聚,CNT-Co具有较大的比表面积,有利于污染物吸附于催化剂表面,增加吸附效果。该体系产生多种活性物种,其贡献度为1O2>·SO
中图分类号:
张佳颖, 王聪, 王雅君. CNT-Co/Bi2O3催化剂光催化协同过硫酸盐活化高效降解四环素[J]. 化工学报, 2024, 75(9): 3163-3175.
Jiaying ZHANG, Cong WANG, Yajun WANG. CNT-Co/Bi2O3 catalyst photocatalytic synergistic activation of persulfate for efficient degradation of tetracycline[J]. CIESC Journal, 2024, 75(9): 3163-3175.
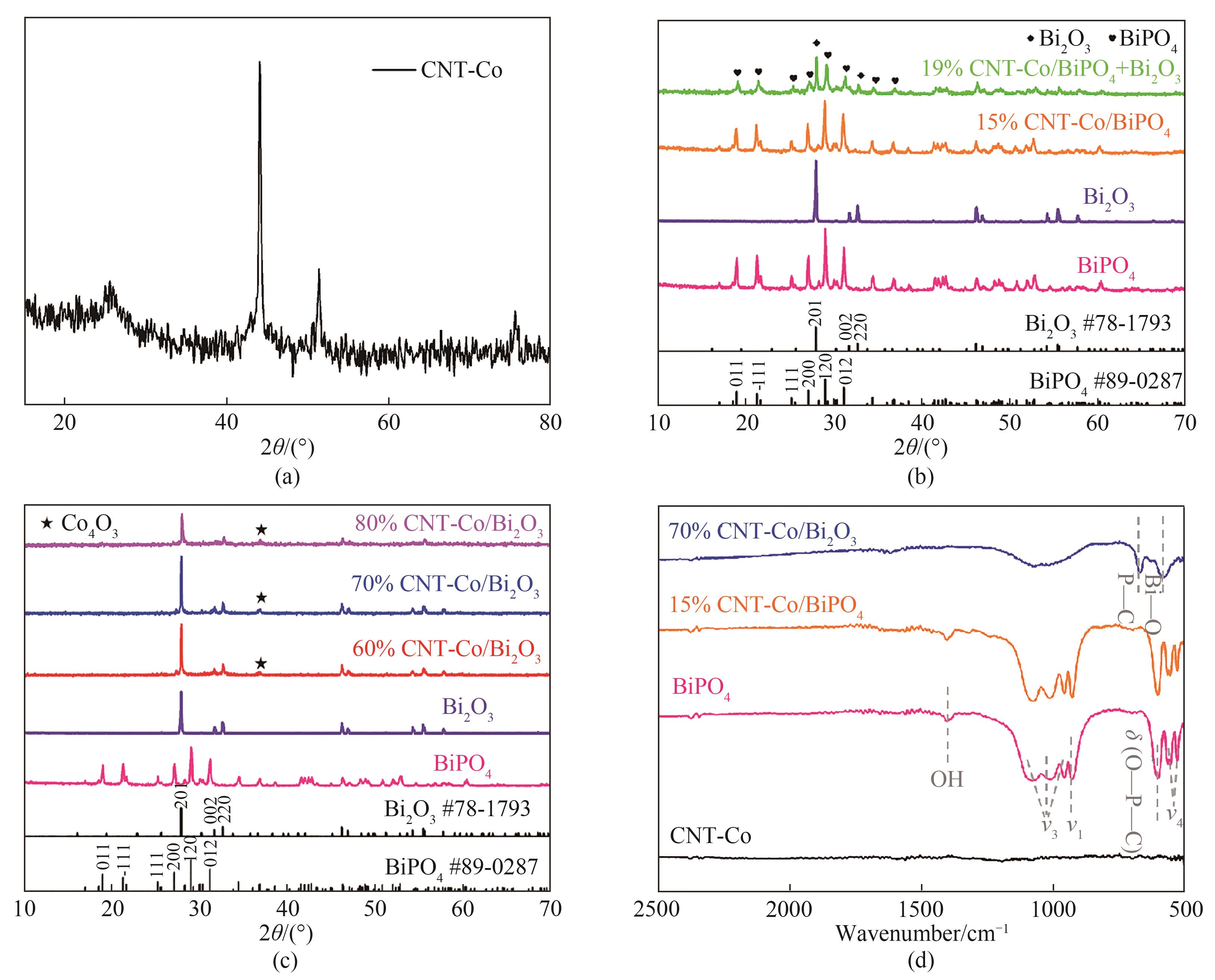
图1 CNT-Co(a),BiPO4、Bi2O3、15%CNT-Co/BiPO4和19%CNT-Co/BiPO4+Bi2O3(b),BiPO4、Bi2O3、60%CNT-Co/Bi2O3、70%CNT-Co/Bi2O3和80%CNT-Co/Bi2O3(c)的XRD谱图;CNT-Co、BiPO4、15%CNT-Co/BiPO4和70%CNT-Co/Bi2O3的红外光谱(d)
Fig.1 XRD patterns of CNT-Co (a), BiPO4, Bi2O3, 15%CNT-Co/BiPO4 and 19%CNT-Co/BiPO4+Bi2O3 (b), BiPO4, Bi2O3, 60%CNT-Co/Bi2O3, 70%CNT-Co/Bi2O3 and 80%CNT-Co/Bi2O3 (c); FTIR spectra of CNT-Co, BiPO4, 15%CNT-Co/BiPO4 and 70%CNT-Co/Bi2O3 (d)
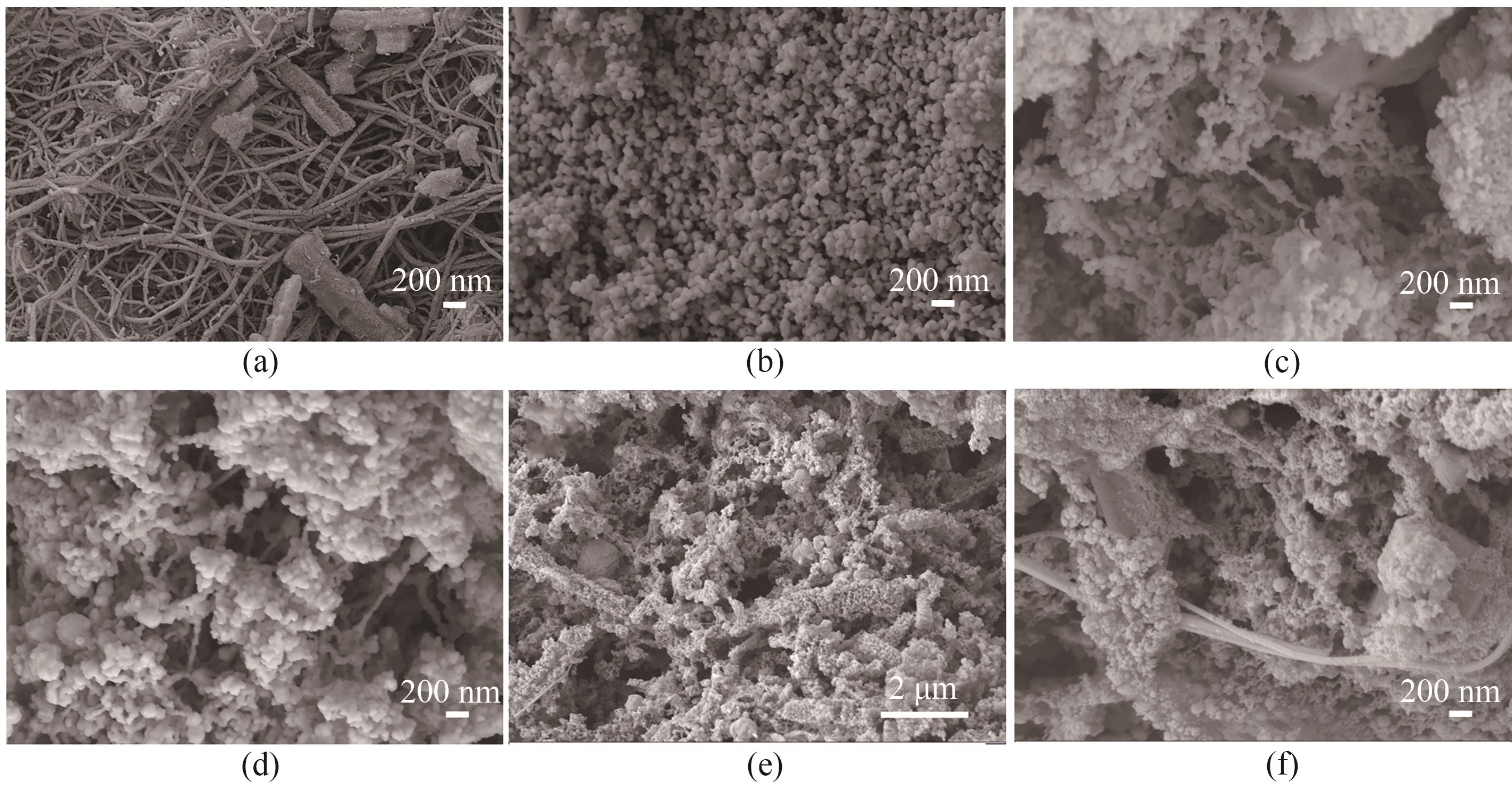
图2 CNT-Co(a)、BiPO4(b)、15%CNT-Co/BiPO4(c)、19%CNT-Co/BiPO4+Bi2O3(d)和70%CNT-Co/Bi2O3[(e)~(f)]的SEM图
Fig.2 SEM images of CNT-Co (a), BiPO4 (b), 15%CNT-Co/BiPO4 (c), 19%CNT-Co/BiPO4+Bi2O3 (d) and 70%CNT-Co/Bi2O3 [(e)—(f)]
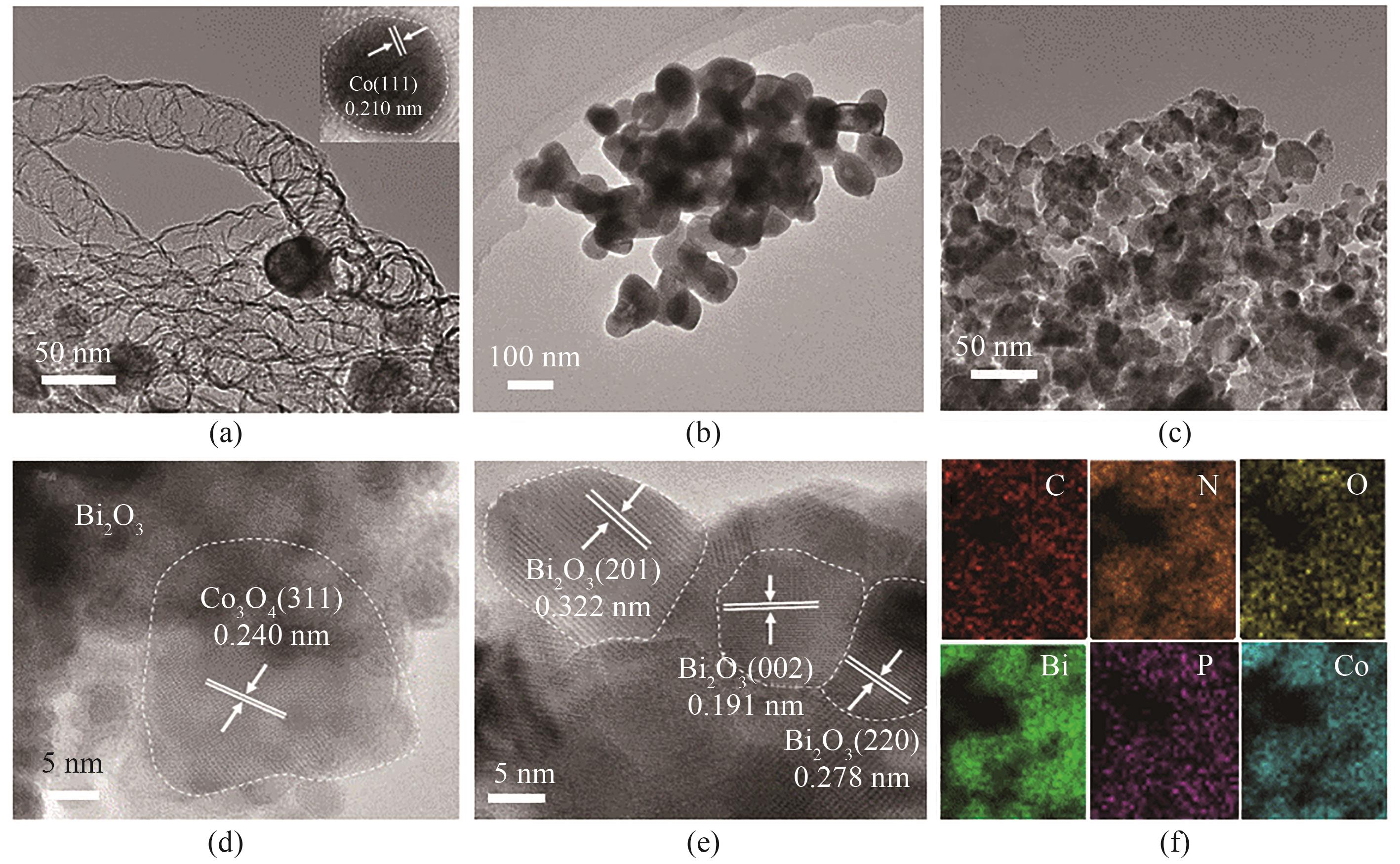
图3 CNT-Co(a)、BiPO4(b)和70%CNT-Co/BiPO4[(c)~(e)]的HRTEM图像;70%CNT-Co/Bi2O3的元素扫描图像(f)
Fig.3 HRTEM images of CNT-Co (a), BiPO4 (b), and 70%CNT-Co/BiPO4 [(c)—(e)]; EDX mapping of the 70%CNT-Co/Bi2O3 (f)
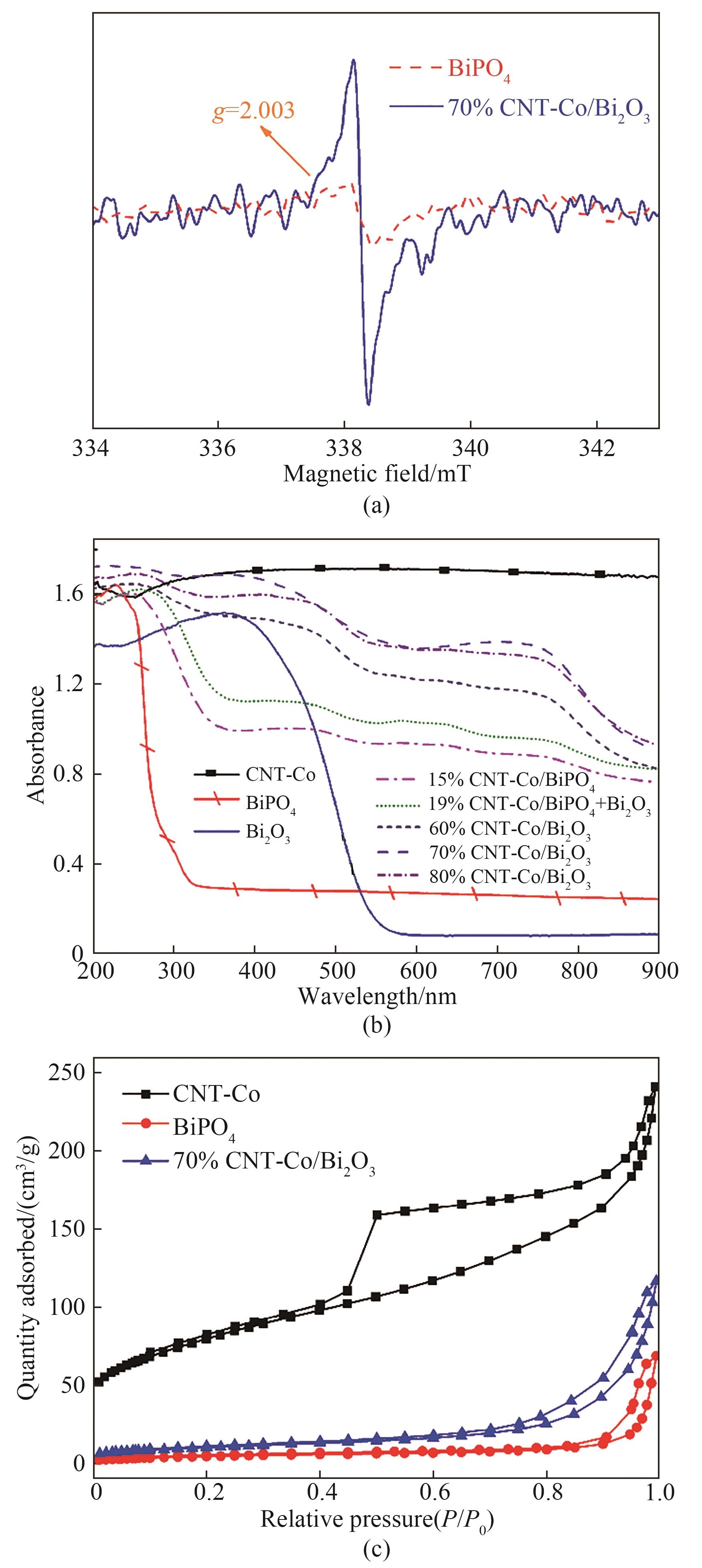
图4 BiPO4和70%CNT-Co/Bi2O3的EPR光谱(a);CNT-Co、BiPO4、Bi2O3、15%CNT-Co/BiPO4、19%CNT-Co/BiPO4+Bi2O3、60%CNT-Co/Bi2O3、70%CNT-Co/Bi2O3和80%CNT-Co/Bi2O3的UV-Vis DRS光谱(b);CNT-Co、BiPO4和70%CNT-Co/Bi2O3的N2吸附-脱附等温线(c)
Fig.4 EPR spectra of BiPO4 and 70%CNT-Co/Bi2O3(a); UV-Vis DRS spectra of CNT-Co, BiPO4, Bi2O3, 15%CNT-Co/BiPO4, 19%CNT-Co/BiPO4+Bi2O3, 60%CNT-Co/Bi2O3, 70%CNT-Co/Bi2O3 and 80%CNT-Co/Bi2O3(b); N2 adsorption-desorption isotherms of CNT-Co, BiPO4 and 70%CNT-Co/Bi2O3(c)
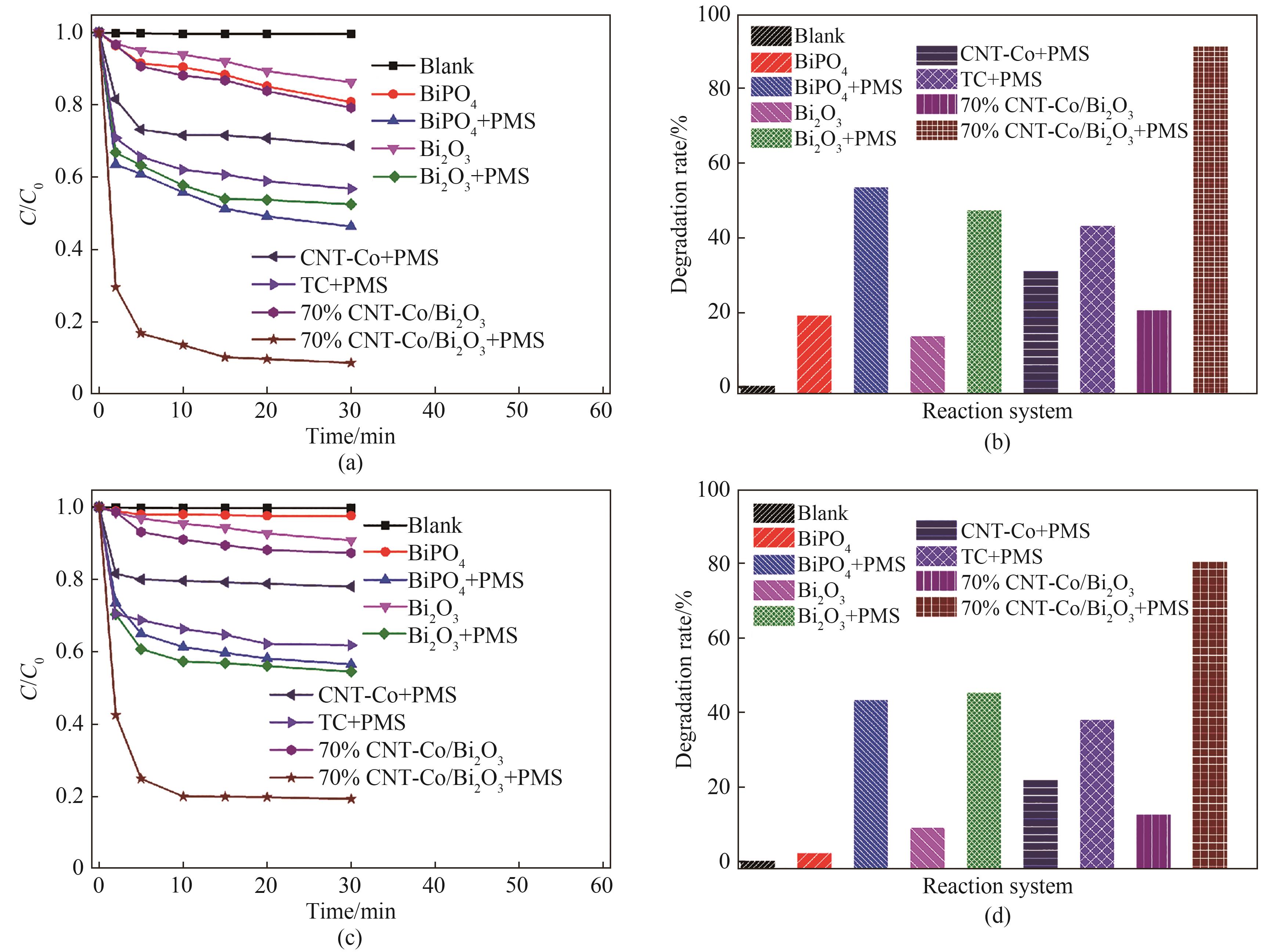
图6 紫外线(λ=254 nm)照射下TC在不同反应体系中的降解曲线(a)和降解率(b);可见光(λ≥420 nm)照射下TC在不同反应体系中的降解曲线(c)和降解率(d)
Fig.6 The degradation curve (a) and degradation rate (b) of TC in different reaction systems under ultraviolet light irradiation (λ=254 nm); The degradation curve (c) and degradation rate (d) of TC in different reaction systems under visible light irradiation (λ≥420 nm)
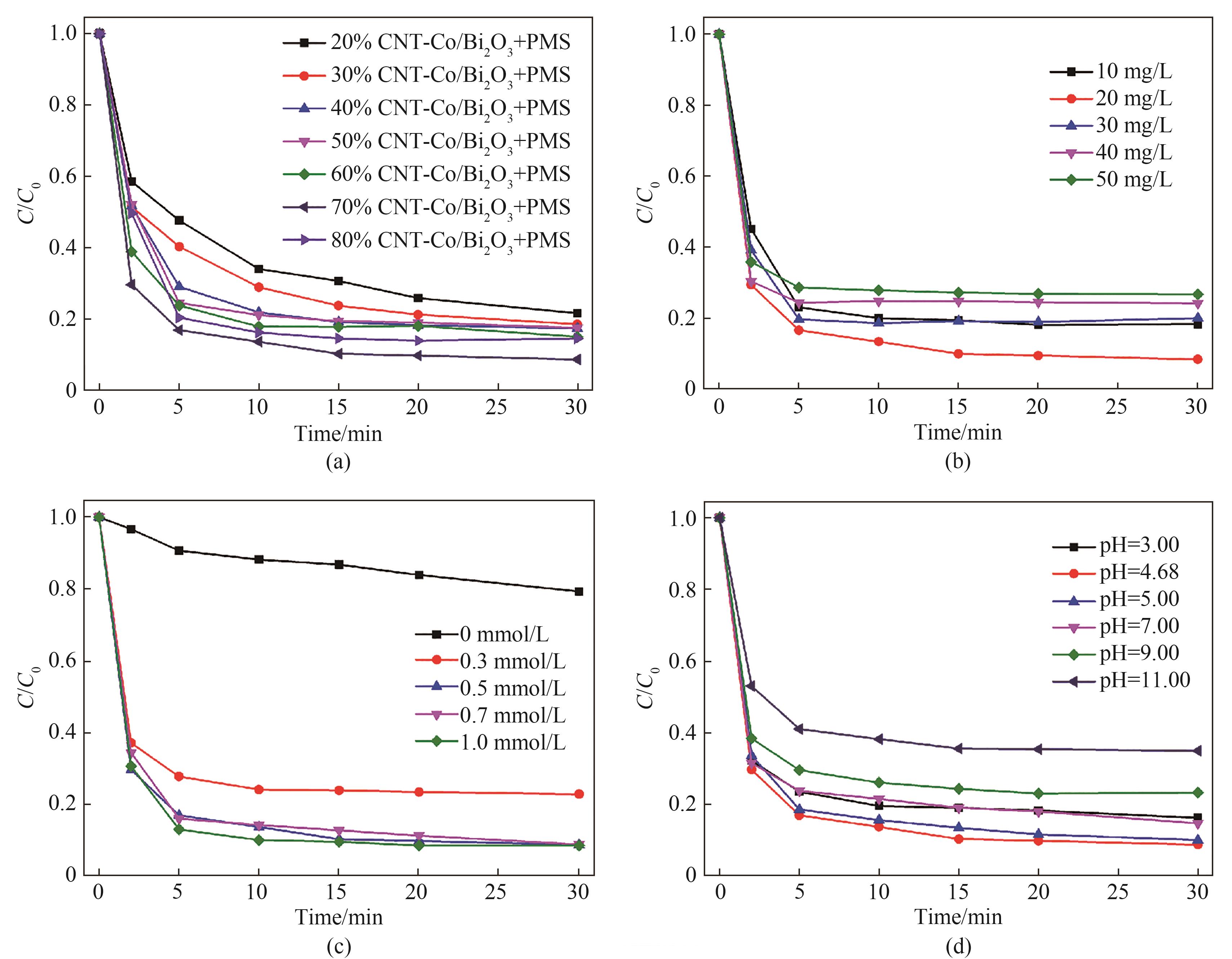
图8 CNT-Co/Bi2O3质量分数(a)、催化剂投加量(b)、PMS浓度(c)和pH(d)对TC降解效能的影响
Fig.8 Eeffect of mass fraction of CNT-Co/Bi2O3 (a), catalyst dosages (b), PMS concentration(c) and pH (d) on degradation of TC
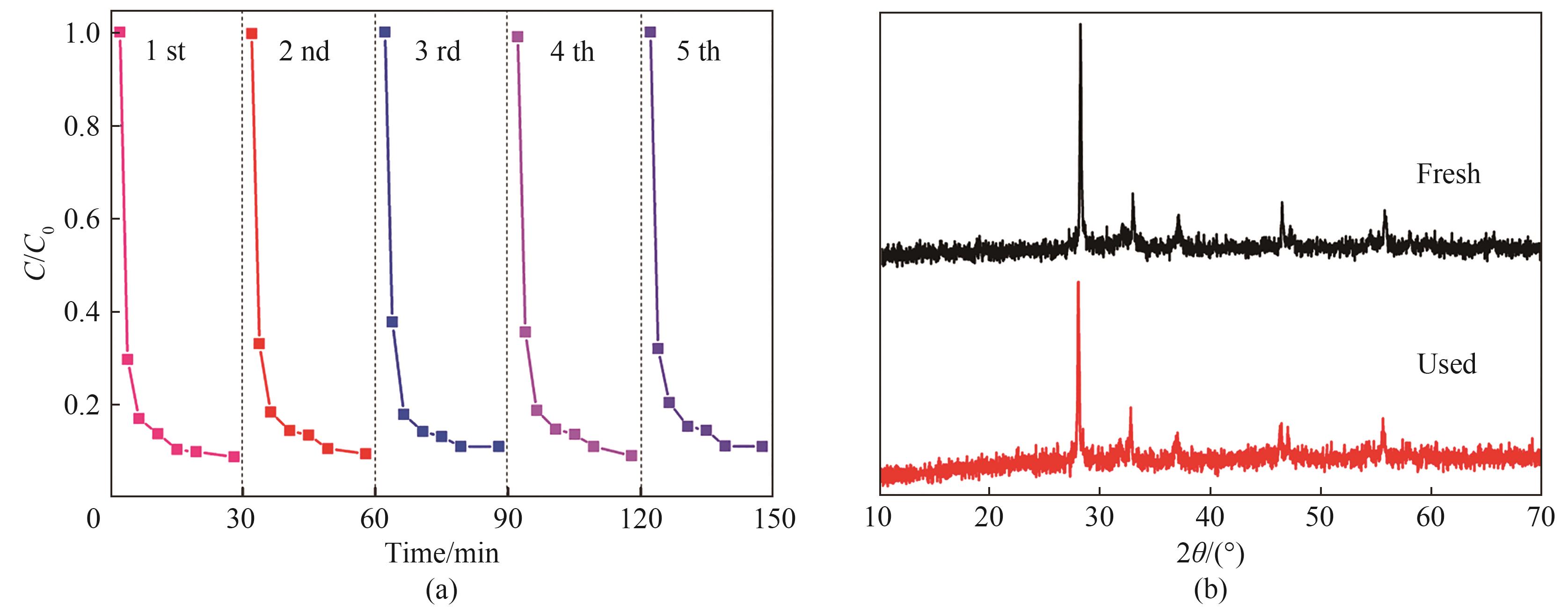
图9 70%CNT-Co/Bi2O3样品循环活性(a);70%CNT-Co/Bi2O3反应前后XRD谱图(b)
Fig.9 Cycle activity of 70%CNT-Co/Bi2O3 sample(a); XRD patterns of 70%CNT-Co/Bi2O3 before and after reaction(b)
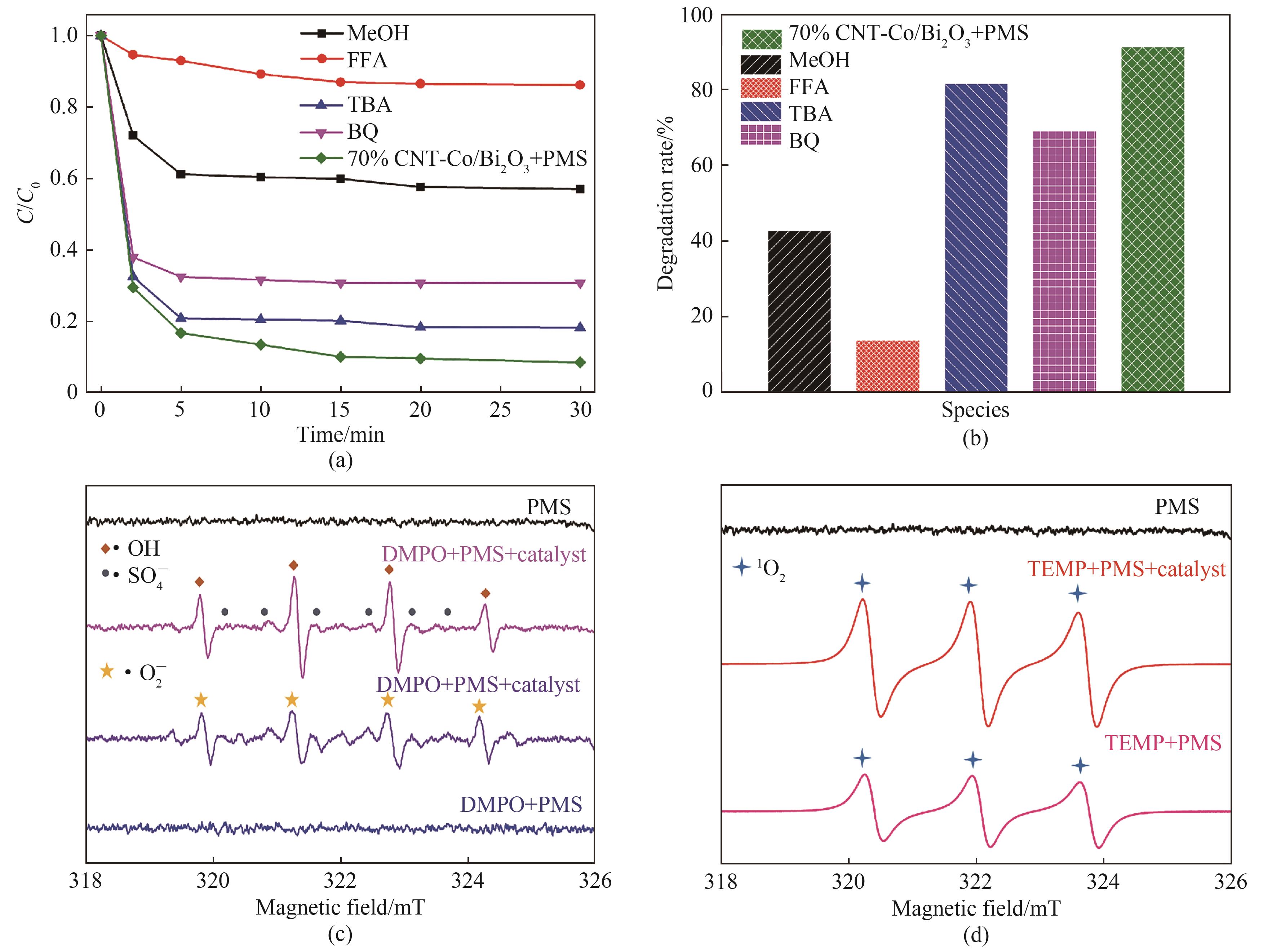
图10 70%CNT-Co/Bi2O3的活性物种捕获实验(a)和降解率(b);70%CNT-Co/Bi2O3体系中DMPO-·OH,·SO4-和·O2-(c), TEMP-1O2(d)的EPR谱
Fig.10 Active species capture experiment of 70%CNT-Co/Bi2O3(a) and degradation rate(b); EPR spectra of DMPO-·OH, ·SO4- and ·O2- (c), TEMP-1O2 adducts (d) of 70%CNT-Co/Bi2O3 system
| 1 | Li J C, Zhao L, Zhang R C, et al. Transformation of tetracycline antibiotics with goethite: mechanism, kinetic modeling and toxicity evaluation[J]. Water Research, 2021, 199: 117196. |
| 2 | Kumar Ray S, Dhakal D, Gyawali G, et al. Transformation of tetracycline in water during degradation by visible light driven Ag nanoparticles decorated α-NiMoO4 nanorods: mechanism and pathways[J]. Chemical Engineering Journal, 2019, 373: 259-274. |
| 3 | Zuo J X, Wang B Y, Kang J, et al. Activation of peroxymonosulfate by nanoscaled NiFe2O4 magnetic particles for the degradation of 2,4-dichlorophenoxyacetic acid in water: efficiency, mechanism and degradation pathways[J]. Separation and Purification Technology, 2022, 297: 121459. |
| 4 | Peng X M, Wu J Q, Zhao Z L, et al. Activation of peroxymonosulfate by single atom Co-N-C catalysts for high-efficient removal of chloroquine phosphate via non-radical pathways: electron-transfer mechanism[J]. Chemical Engineering Journal, 2022, 429: 132245. |
| 5 | Wang X Y, Ma Y H, Jiang J J, et al. Cl-based functional group modification MIL-53(Fe) as efficient photocatalysts for degradation of tetracycline hydrochloride[J]. Journal of Hazardous Materials, 2022, 434: 128864. |
| 6 | Tang S F, Wang Z T, Deling, et al. Enhanced photocatalytic performance of BiVO4 for degradation of methylene blue under LED visible light irradiation assisted by peroxymonosulfate[J]. International Journal of Electrochemical Science, 2020, 15(3): 2470-2480. |
| 7 | Zhang H X, Xie C H, Chen L, et al. Different reaction mechanisms of SO4•- and •OH with organic compound interpreted at molecular orbital level in Co(Ⅱ)/peroxymonosulfate catalytic activation system[J]. Water Research, 2023, 229: 119392. |
| 8 | Jiang J, Luan X, Chen M, et al. Facile synthesis and photocatalytic activity of Bi2O3/BiVO4 nanocomposites[J]. Optoelectronics and Advanced Materials-Rapid Communications, 2015, 9: 464-467. |
| 9 | Kim D, Jung D. Enhancement of photocatalytic activity over Bi2O3/black-BiOCl heterojunction[J]. Chemical Physics Letters, 2017, 674: 130-135. |
| 10 | Dai J F, Chen X F, Yang H. Visible light photocatalytic degradation of dyes by a new polyaniline/β-Bi2O3 composite[J]. Inorganic and Nano-Metal Chemistry, 2017, 47(9): 1364-1368. |
| 11 | Sun Q, Zhao Y J, Zhang J, et al. Efficient degradation of antibiotics over Co(Ⅱ)-doped Bi2MoO6 nanohybrid via the synergy of peroxymonosulfate activation and photocatalytic reaction under visible irradiation[J]. Chemosphere, 2022, 302: 134807. |
| 12 | Dai H W, Zhou W J, Wang W, et al. Unveiling the role of cobalt species in the Co/N-C catalysts-induced peroxymonosulfate activation process[J]. Journal of Hazardous Materials, 2022, 426: 127784. |
| 13 | Wang Y, Zhao S, Fan W C, et al. The synthesis of novel Co-Al2O3 nanofibrous membranes with efficient activation of peroxymonosulfate for bisphenol A degradation[J]. Environmental Science: Nano, 2018, 5(8): 1933-1942. |
| 14 | Zhao Y J, Dang P, Gao Y Q, et al. Double Z-scheme Co3O4/Bi4O7/Bi2O3 composite activated peroxymonosulfate to efficiently degrade tetracycline under visible light[J]. Environmental Science and Pollution Research International, 2022, 29(52): 79184-79198. |
| 15 | Lin X, Nie Z Z, Zhang L Y, et al. Nitrogen-doped carbon nanotubes encapsulate cobalt nanoparticles as efficient catalysts for aerobic and solvent-free selective oxidation of hydrocarbons[J]. Green Chemistry, 2017, 19(9): 2164-2173. |
| 16 | Wang Y J, Qiang Z S, Zhu W, et al. BiPO4 nanorod/graphene composite heterojunctions for photocatalytic degradation of tetracycline hydrochloride[J]. ACS Applied Nano Materials, 2021, 4(9): 8680-8689. |
| 17 | Li B, Xu H Y, Liu Y L, et al. Fabricating an oxygen-vacancy-rich urchin-like Co3O4 nanocatalyst to boost peroxymonosulfate activation to degrade high-concentration crystal violet[J]. Ceramics International, 2022, 48(18): 26553-26564. |
| 18 | Zhu Y Y, Ling Q, Liu Y F, et al. Photocatalytic performance of BiPO4 nanorods adjusted via defects[J]. Applied Catalysis B: Environmental, 2016, 187: 204-211. |
| 19 | Dong S Y, Cui L F, Liu C Y, et al. Fabrication of 3D ultra-light graphene aerogel/Bi2WO6 composite with excellent photocatalytic performance: a promising photocatalysts for water purification[J]. Journal of the Taiwan Institute of Chemical Engineers, 2019, 97: 288-296. |
| 20 | Qian Y, Jiang S, Li Y, et al. In situ revealing the electroactivity of P—O and P—C bonds in hard carbon for high-capacity and long-life Li/K-ion batteries[J]. Advanced Energy Materials, 2019, 9(34): 1901676. |
| 21 | Wang L L, Tang G G, Liu S, et al. Interfacial active-site-rich 0D Co3O4/1D TiO2 p-n heterojunction for enhanced photocatalytic hydrogen evolution[J]. Chemical Engineering Journal, 2022, 428: 131338. |
| 22 | Wang D B, Yu X, Feng Q G, et al. In-situ growth of β-Bi2O3 nanosheets on g-C3N4 to construct direct Z-scheme heterojunction with enhanced photocatalytic activities[J]. Journal of Alloys and Compounds, 2021, 859: 157795. |
| 23 | Lv Y H, Zhu Y Y, Zhu Y F. Enhanced photocatalytic performance for the BiPO4- x nanorod induced by surface oxygen vacancy[J]. The Journal of Physical Chemistry C, 2013, 117(36): 18520-18528. |
| 24 | Yin Z L, Zhang X X, Yuan X H, et al. Constructing TiO2@Bi2O3 multi-heterojunction hollow structure for enhanced visible-light photocatalytic performance[J]. Journal of Cleaner Production, 2022, 375: 134112. |
| 25 | Li D G, Zhang G Z, Li W J, et al. Magnetic nitrogen-doped carbon nanotubes as activators of peroxymonosulfate and their application in non-radical degradation of sulfonamide antibiotics[J]. Journal of Cleaner Production, 2022, 380: 135064. |
| 26 | Cheng X X, Zhang Y R, Fan Q S, et al. Preparation of Co3O4@carbon nanotubes modified ceramic membrane for simultaneous catalytic oxidation and filtration of secondary effluent[J]. Chemical Engineering Journal, 2023, 454: 140450. |
| 27 | Liu C H, Dai H L, Tan C Q, et al. Photo-Fenton degradation of tetracycline over Z-scheme Fe-g-C3N4/Bi2WO6 heterojunctions: mechanism insight, degradation pathways and DFT calculation[J]. Applied Catalysis B: Environmental, 2022, 310: 121326. |
| 28 | Ma R, Chen Z J, Xu W H, et al. Fe-MOFs/CuS nanocomposite-mediated peroxymonosulfate activation for tetracycline degradation: boosted dual redox cycles[J]. Journal of Cleaner Production, 2024, 442: 140885. |
| 29 | Su R, Wang Z Y, Xiao F, et al. In-situ fabrication of MOF-derived MnO-C modified graphite felt for electro-activation of peroxymonosulfate toward degradation of tetracycline: performance, mechanism and degradation pathway[J]. Separation and Purification Technology, 2024, 342: 126766. |
| 30 | Guo Y C, Zhao L D, Fang J, et al. Tetracycline degradation by activated persulfate with enhancement of ZIF-67 loaded wood-microreactor[J]. Journal of Environmental Chemical Engineering, 2024, 12(2): 111901. |
| 31 | An B Y, Liu J L, Zhu B J, et al. Returnable MoS2@carbon nitride nanotube composite hollow spheres drive photo-self-Fenton-PMS system for synergistic catalytic and photocatalytic tetracycline degradation[J]. Chemical Engineering Journal, 2023, 478: 147344. |
| 32 | Xu X S, Shao W F, Tai G Y, et al. Single-atomic Co-N site modulated exciton dissociation and charge transfer on covalent organic frameworks for efficient antibiotics degradation via peroxymonosulfate activation[J]. Separation and Purification Technology, 2024, 333: 125890. |
| 33 | Song J J, Yuan X Y, Sun M K, et al. Oxidation of tetracycline hydrochloride with a photoenhanced MIL-101(Fe)/g-C3N4/PMS system: synergetic effects and radical/nonradical pathways[J]. Ecotoxicology and Environmental Safety, 2023, 251: 114524. |
| 34 | Tang C S, Cheng M, Lai C, et al. Multiple path-dominated activation of peroxymonosulfate by MOF-derived Fe2O3/Mn3O4 for catalytic degradation of tetracycline[J]. Journal of Environmental Chemical Engineering, 2023, 11(5): 110395. |
| 35 | Wang Q R, Xiao P F. Self-synthesized heterogeneous CuFe2O4-MoS2@BC composite as an activator of peroxymonosulfate for the oxidative degradation of tetracycline[J]. Separation and Purification Technology, 2023, 305: 122550. |
| 36 | Chen Q, Ning S Y, Yang J R, et al. In situ interfacial engineering of CeO2/Bi2WO6 heterojunction with improved photodegradation of tetracycline and organic dyes: mechanism insight and toxicity assessment[J]. Small, 2024, 20(18): e2307304. |
| 37 | Wang Z X, Han Y F, Fan W L, et al. Shell-core MnO2/carbon@carbon nanotubes synthesized by a facile one-pot method for peroxymonosulfate oxidation of tetracycline[J]. Separation and Purification Technology, 2021, 278: 119558. |
| 38 | Alnaggar G, Hezam A, Drmosh Q A, et al. Sunlight-driven activation of peroxymonosulfate by microwave synthesized ternary MoO3/Bi2O3/g-C3N4 heterostructures for boosting tetracycline hydrochloride degradation[J]. Chemosphere, 2021, 272: 129807. |
| 39 | Li D, Li H M, Long M Y, et al. Synergetic effect of photocatalysis and peroxymonosulfate activation by MIL-53Fe@TiO2 on efficient degradation of tetracycline hydrochloride under visible light irradiation[J]. CrystEngComm, 2022, 24(23): 4283-4293. |
| 40 | Li S, Yang Y L, Zheng H S, et al. Introduction of oxygen vacancy to manganese ferrite by Co substitution for enhanced peracetic acid activation and 1O2 dominated tetracycline hydrochloride degradation under microwave irradiation[J]. Water Research, 2022, 225: 119176. |
| 41 | Deng Y C, Li L, Zeng H, et al. Unveiling the origin of high-efficiency charge transport effect of C3N5/C3N4 homojunction for activating peroxymonosulfate to degrade atrazine under visible light[J]. Chemical Engineering Journal, 2023, 457: 141261. |
| 42 | Wei K X, Armutlulu A, Wang Y X, et al. Visible-light-driven removal of atrazine by durable hollow core-shell TiO2@LaFeO3 heterojunction coupling with peroxymonosulfate via enhanced electron-transfer[J]. Applied Catalysis B: Environmental, 2022, 303: 120889. |
| 43 | Ming H B, Wei D L, Yang Y, et al. Photocatalytic activation of peroxymonosulfate by carbon quantum dots functionalized carbon nitride for efficient degradation of bisphenol A under visible-light irradiation[J]. Chemical Engineering Journal, 2021, 424: 130296. |
| 44 | Mertah O, Gómez-Avilés A, Kherbeche A, et al. Peroxymonosulfate enhanced photodegradation of sulfamethoxazole with TiO2@CuCo2O4 catalysts under simulated solar light[J]. Journal of Environmental Chemical Engineering, 2022, 10(5): 108438. |
| 45 | Ruiz-Castillo A L, Hinojosa-Reyes M, Camposeco-Solis R, et al. Photocatalytic activity of Bi2O3/BiOCl heterojunctions under UV and visible light illumination for degradation of caffeine[J]. Topics in Catalysis, 2022, 65(9): 1071-1087. |
| 46 | Liu W, Zhou J B, Zhou Y, et al. Peroxymonosulfate-assisted g-C3N4@Bi2MoO6 photocatalytic system for degradation of nimesulide through phenyl ether bond cleavage under visible light irradiation[J]. Separation and Purification Technology, 2021, 264: 118288. |
| [1] | 王树振, 王玉婷, 马梦茜, 张巍, 向江南, 鲁海莹, 王琰, 范彬彬, 郑家军, 代卫炯, 李瑞丰. 两步晶化合成ZSM-22分子筛及其临氢异构反应性能[J]. 化工学报, 2024, 75(9): 3176-3187. |
| [2] | 王冉, 王焕, 熊晓云, 关慧敏, 郑云锋, 陈彩琳, 秦玉才, 宋丽娟. FCC催化剂传质强化活性位利用效率的可视化分析[J]. 化工学报, 2024, 75(9): 3198-3209. |
| [3] | 邢登雪, 张良, 李文强, 梁建华, 秦磊, 张根林, 李春. 酵母细胞催化合成18α-甘草酸[J]. 化工学报, 2024, 75(9): 3266-3276. |
| [4] | 刘亚超, 谭晓杰, 李旭东, 王瑞, 王慧, 韩璇, 赵青山. DES合成高活性CoCO3纳米片及析氧反应性能研究[J]. 化工学报, 2024, 75(9): 3320-3328. |
| [5] | 张梦婷, 王书林, 桑熙, 元兴昊, 徐刚. 人工Cu-TM1459金属酶催化不对称迈克尔加成反应[J]. 化工学报, 2024, 75(9): 3255-3265. |
| [6] | 豆少军, 郝亮. PEMFC催化层耦合气体电荷传输过程的介观模拟[J]. 化工学报, 2024, 75(8): 3002-3010. |
| [7] | 罗莉, 陈文尧, 张晶, 钱刚, 周兴贵, 段学志. 氧化铝结构与表面性质调控及其催化甲醇脱水制二甲醚性能研究[J]. 化工学报, 2024, 75(7): 2522-2532. |
| [8] | 王寅, 初鹏飞, 刘虎, 吕静, 黄守莹, 王胜平, 马新宾. 不同pH铝溶胶对二甲醚羰基化成型丝光沸石催化剂性能的影响[J]. 化工学报, 2024, 75(7): 2533-2543. |
| [9] | 杨露, 刘聪聪, 孟彤彤, 张博远, 杨腾飞, 邓文安, 王晓斌. 分散型催化剂在煤/重油共炼体系中的加氢抑焦作用[J]. 化工学报, 2024, 75(7): 2556-2564. |
| [10] | 刘旭升, 李泽洋, 杨宇森, 卫敏. 电催化二氧化碳还原制备气态产物的研究进展[J]. 化工学报, 2024, 75(7): 2385-2408. |
| [11] | 吴哲明, 张碧云, 郑仁朝. 腈水解酶立体选择性改造及其合成布瓦西坦[J]. 化工学报, 2024, 75(7): 2633-2643. |
| [12] | 王天闻, 闫肃, 赵梦园, 杨天让, 刘建国. 固体氧化物电池空气电极铬中毒机理及抗铬性能研究进展[J]. 化工学报, 2024, 75(6): 2091-2108. |
| [13] | 寇梦瑶, 郑芳菲, 胥雯, 郭娜, 廖兵. 碱催化过氧化氢体系降解四环素的作用规律与机制解析[J]. 化工学报, 2024, 75(6): 2362-2374. |
| [14] | 丁禹, 杨昌泽, 李军, 孙会东, 商辉. 原子尺度钼系加氢脱硫催化剂的研究进展与展望[J]. 化工学报, 2024, 75(5): 1735-1749. |
| [15] | 裴欣哲, 孙朱行, 林钰翔, 张朝阳, 钱勇, 吕兴才. 电催化分解液氨阳极材料的研究[J]. 化工学报, 2024, 75(5): 1843-1854. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号