化工学报 ›› 2019, Vol. 70 ›› Issue (7): 2786-2794.DOI: 10.11949/0438-1157.20190060
朱啸林1( ),徐存英1,2(
),徐存英1,2( ),唐杰1,华一新1,2,张启波1,2,刘海1,王祥1,黄梦婷1
),唐杰1,华一新1,2,张启波1,2,刘海1,王祥1,黄梦婷1
收稿日期:2019-01-21
修回日期:2019-04-28
出版日期:2019-07-05
发布日期:2019-07-05
通讯作者:
徐存英
作者简介:朱啸林(1990—),男,硕士研究生,<email>zhuxiaolinzxl@163.com</email>
基金资助:
Xiaolin ZHU1( ),Cunying XU1,2(
),Cunying XU1,2( ),Jie TANG1,Yixin HUA1,2,Qibo ZHANG1,2,Hai LIU1,Xiang WANG1,Mengting HUANG1
),Jie TANG1,Yixin HUA1,2,Qibo ZHANG1,2,Hai LIU1,Xiang WANG1,Mengting HUANG1
Received:2019-01-21
Revised:2019-04-28
Online:2019-07-05
Published:2019-07-05
Contact:
Cunying XU
摘要:
研究了氯化铬(CrCl3·6H2O)和氯化镍(NiCl2·6H2O)浓度和存在形式对氯化胆碱-乙二醇(ChCl-EG)低共熔溶剂的黏度和电导率的影响。电喷雾质谱(ESI-MS)分析结果表明,在溶解有CrCl3?6H2O和NiCl2?6H2O 的ChCl-EG(ChCl-EG-NiCl2·6H2O-CrCl3·6H2O)溶液中出现了配阴离子[Cr(H2O)2Cl4]–和[Ni(H2O)2Cl4]2-。由此可以推断,Cr3+(或Ni2+)的两个d轨道、4s 和4p轨道发生d2sp3杂化,形成6个等同的杂化轨道,接受6个配体(Cl-和H2O)形成阴离子配合物。该溶液的电导率随温度的升高而增大,随总金属离子浓度的增大而减小。此外,溶液黏度随温度和总金属离子浓度的变化趋势与电导率相反。这主要是由于镍和铬配离子的形成改变了溶液中的离子组成。
中图分类号:
朱啸林, 徐存英, 唐杰, 华一新, 张启波, 刘海, 王祥, 黄梦婷. 氯化铬和氯化镍浓度及其存在形式对ChCl-EG低共熔溶剂电导率的影响[J]. 化工学报, 2019, 70(7): 2786-2794.
Xiaolin ZHU, Cunying XU, Jie TANG, Yixin HUA, Qibo ZHANG, Hai LIU, Xiang WANG, Mengting HUANG. Effects of existence form and concentrations of NiCl2•6H2O and CrCl3•6H2O on conductivity of ChCl-EG deep eutectic solvent[J]. CIESC Journal, 2019, 70(7): 2786-2794.
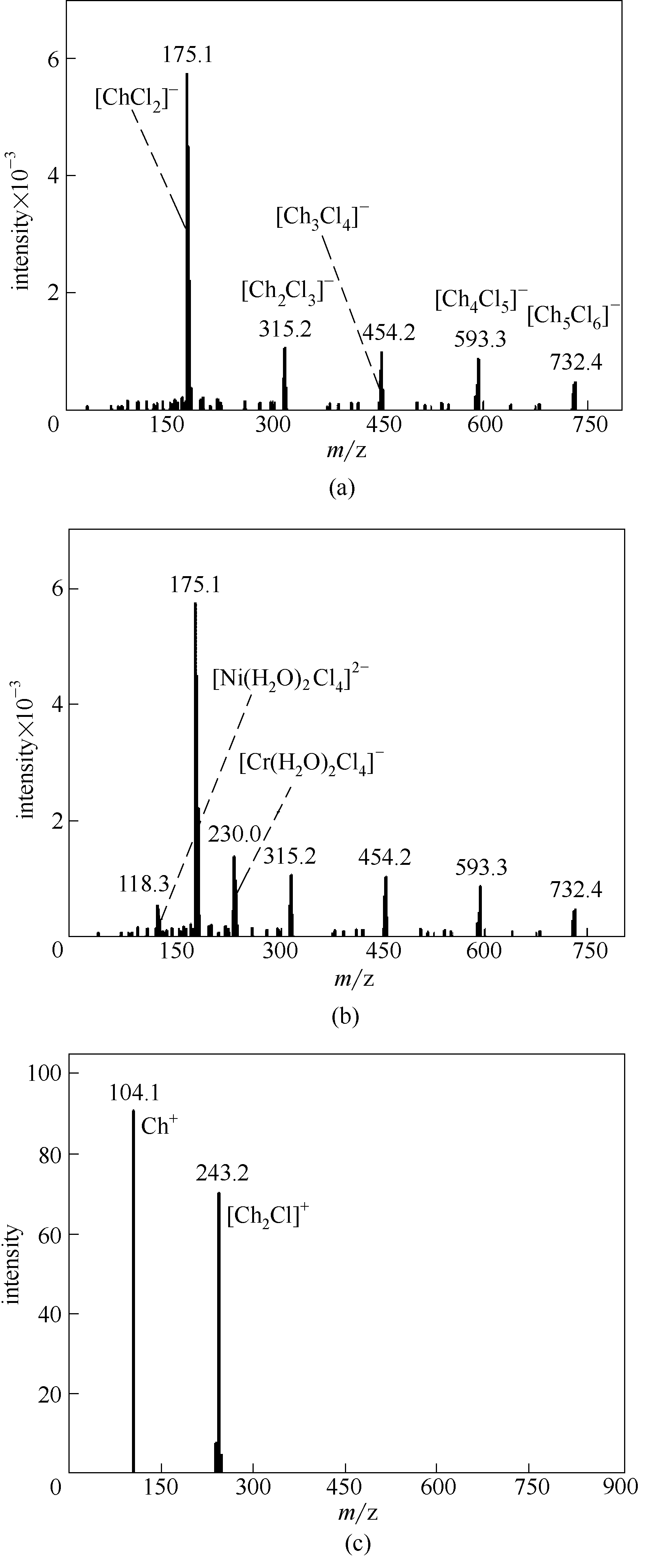
图1 ChCl-EG低共熔溶剂(a)和ChCl-EG-NiCl2·6H2O-CrCl3·6H2O DES(b)的负离子电喷雾质谱图,以及ChCl-EG-NiCl2·6H2O-CrCl3·6H2O DES的正离子电喷雾质谱图(c)
Fig.1 ESI-MS spectra of ChCl-EG DES (a) and ChCl-EG-NiCl2·6H2O-CrCl3·6H2O DES in negative ion mode(b),and ESI-MS spectra of ChCl-EG-NiCl2·6H2O-CrCl3·6H2O DES (c) in positive ion mode

图2 总金属离子浓度和温度对ChCl-EG-NiCl2·6H2O-CrCl3·6H2O DES黏度的影响
Fig.2 Effect of temperature and total metal ion concentration (ctotal) on viscosity of ChCl-EG-NiCl2·6H2O-CrCl3·6H2O DES
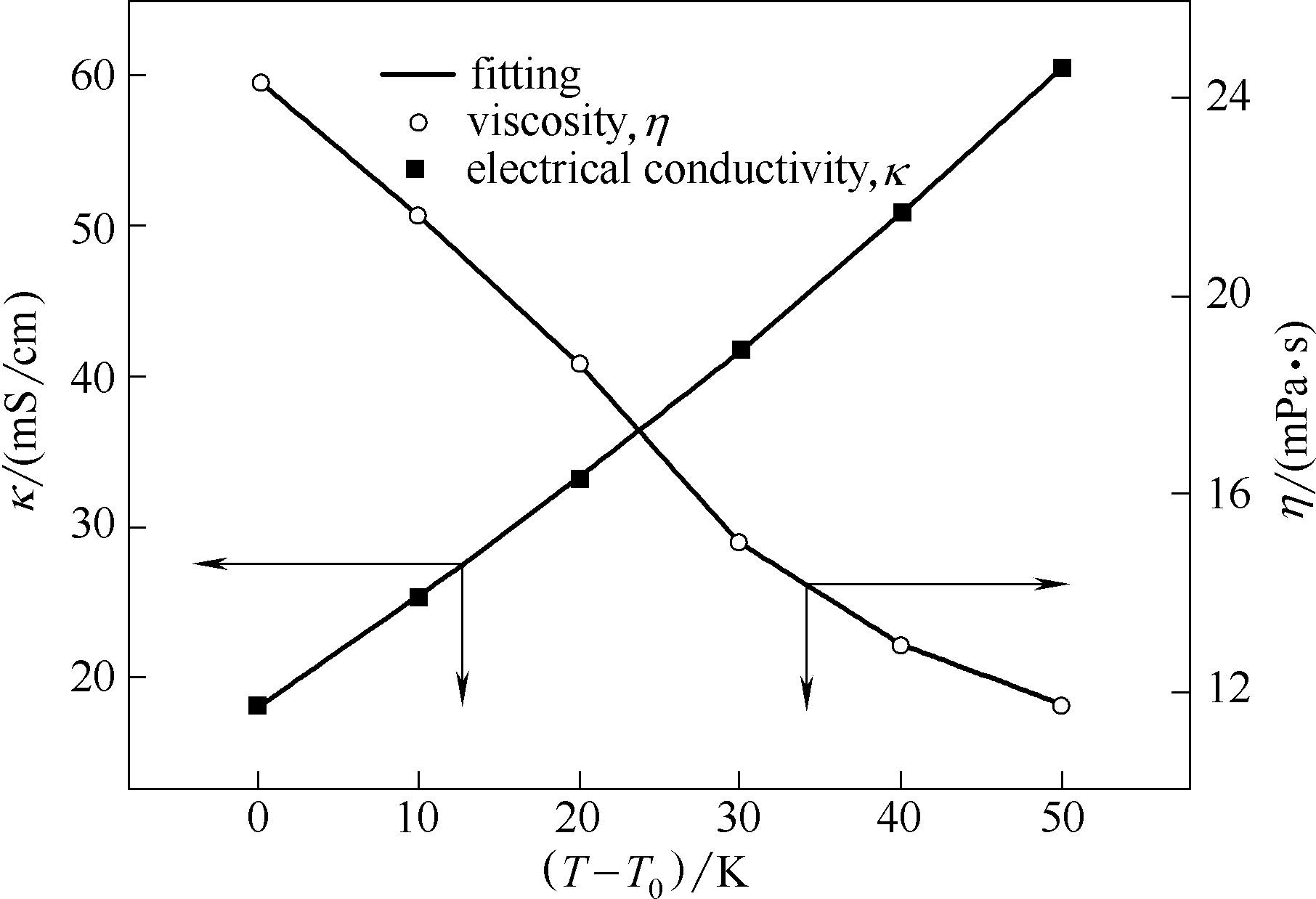
图3 总金属离子浓度为400 mmol/L的ChCl-EG-NiCl2·6H2O-CrCl3·6H2O DES的黏度和电导率与温度的关系
Fig.3 Viscosity and electrical conductivity of ChCl-EG-NiCl2·6H2O-CrCl3·6H2O solution as function of temperature (ctotal=400 mmol/L)
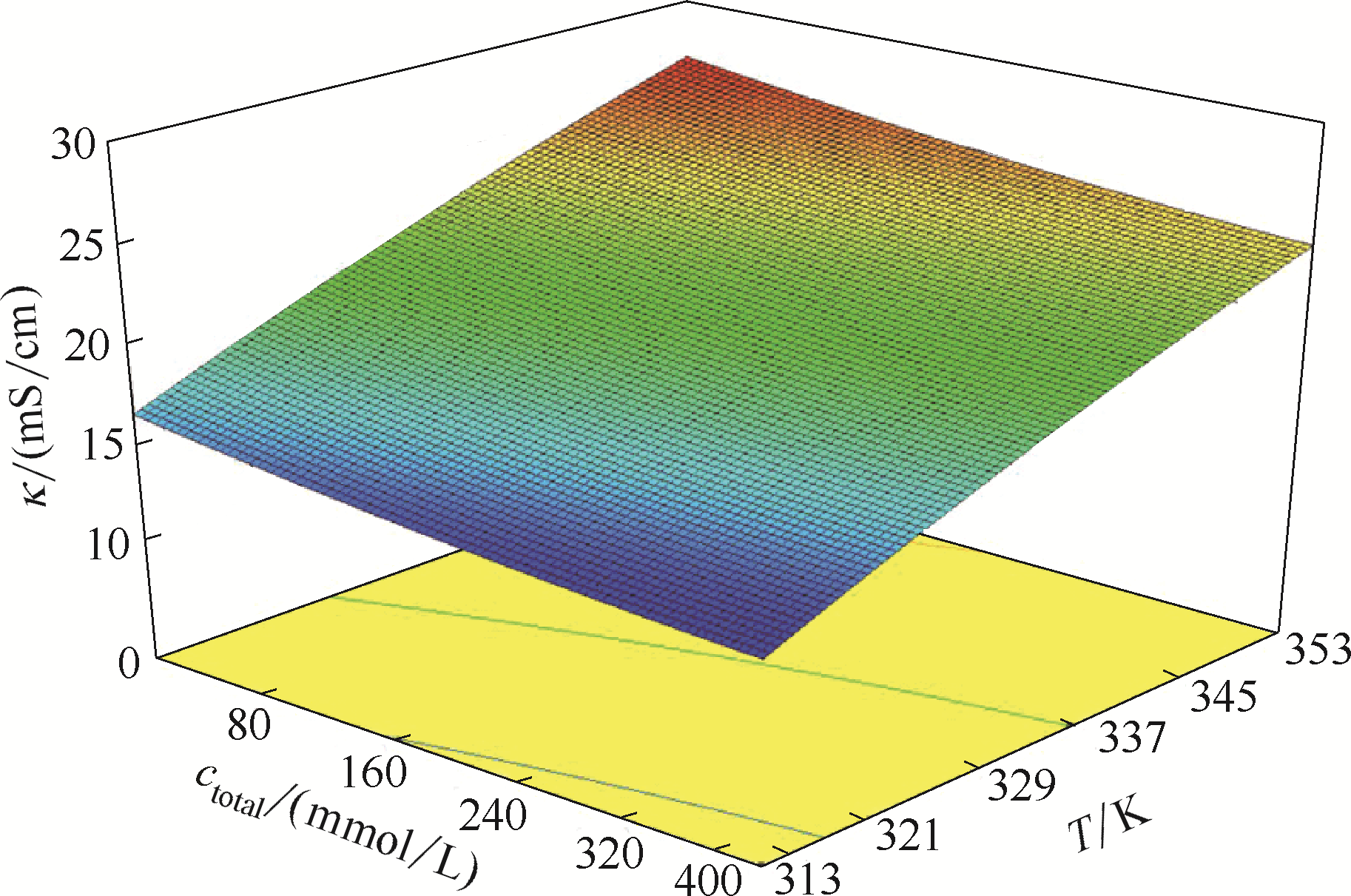
图5 温度和总金属离子浓度对ChCl-EG-NiCl2·6H2O-CrCl3·6H2O DES电导率的影响
Fig.5 Effects of temperature and total metal ion concentration on conductivity of ChCl-EG-NiCl2·6H2O-CrCl3·6H2O DES
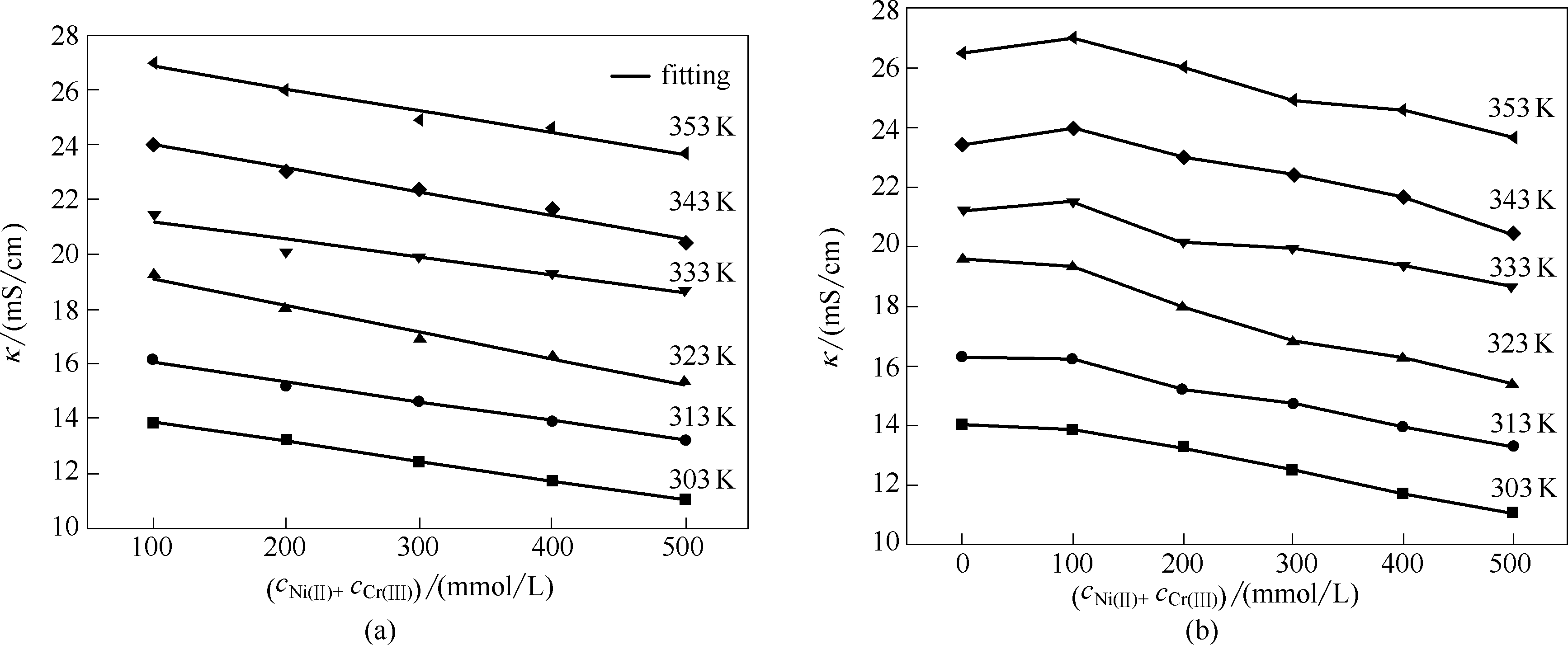
图6 不同温度下ChCl-EG-NiCl2·6H2O-CrCl3·6H2O DES的总金属离子浓度与其电导率的关系(a),以及对(a)中数据的线性拟合(b)
Fig.6 Electrical conductivity of ChCl-EG-NiCl2·6H2O-CrCl3·6H2O DES as function of total metal ion concentration at different temperature(a), (b) is linear fitting of data shown in (a)
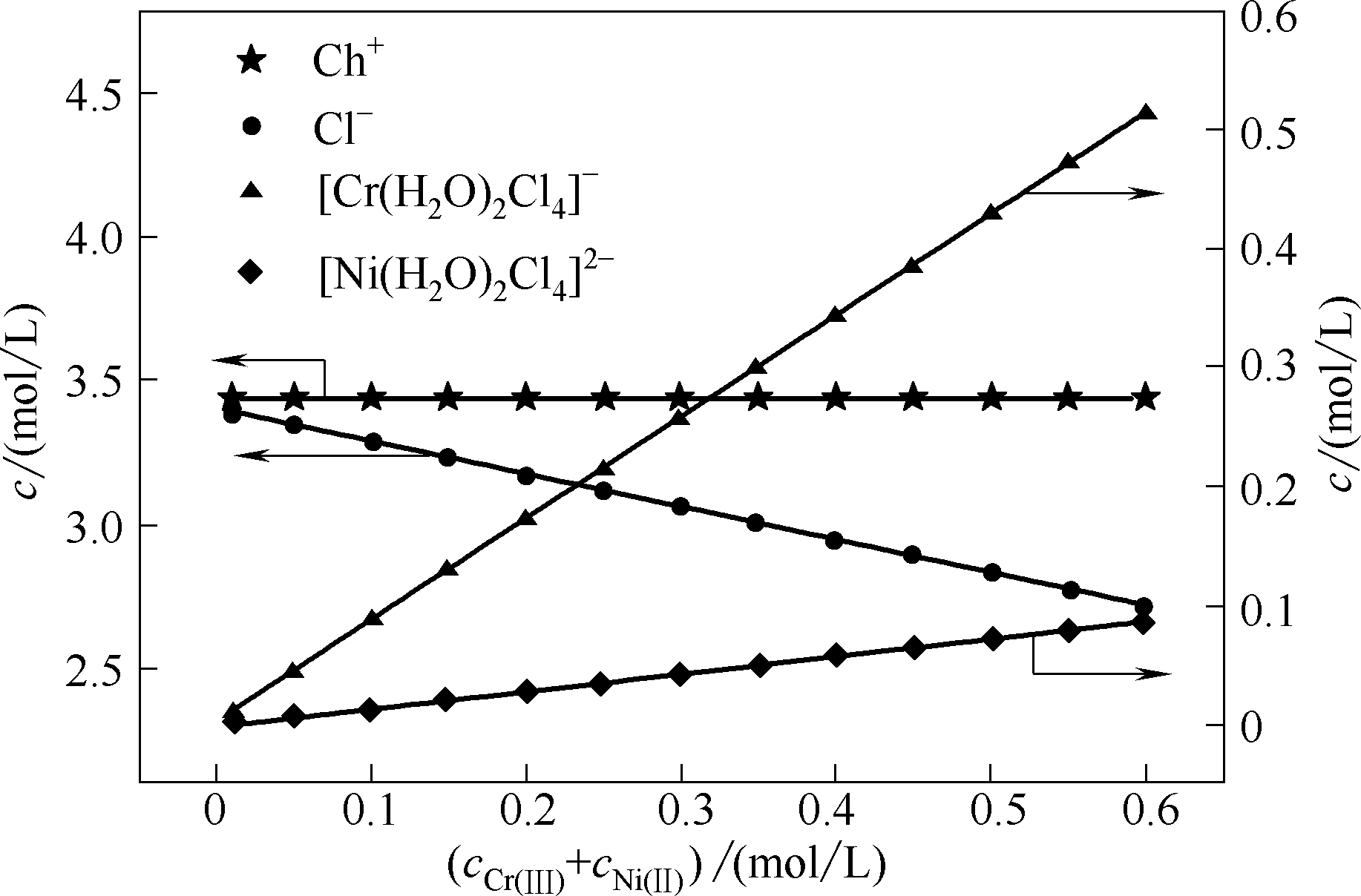
图7 ChCl-EG DES中(Ch+)、Cl-、[Cr(H2O)2Cl4]-和[Ni(H2O)2Cl4]2-的浓度与总金属离子浓度的关系
Fig.7 Concentration of Ch+ Cl-, [Cr(H2O)2Cl4]- and [Ni(H2O)2Cl4]2- as function of total metal ions concentration in ChCl-EG DES
| ctotal/(mmol/L) | Eκ/(kJ/mol) |
|---|---|
| 0 | 10.91 |
| 100 | 11.26 |
| 200 | 12.16 |
| 300 | 12.38 |
| 400 | 12.74 |
| 500 | 13.60 |
表1 ChCl-EG-NiCl2·6H2O-CrCl3·6H2O DES电导活化能与总金属离子浓度的关系
Table 1 Relationship between conductance activation energy and total metal ion concentration of ChCl-EG-NiCl2·6H2O-CrCl3·6H2O DES
| ctotal/(mmol/L) | Eκ/(kJ/mol) |
|---|---|
| 0 | 10.91 |
| 100 | 11.26 |
| 200 | 12.16 |
| 300 | 12.38 |
| 400 | 12.74 |
| 500 | 13.60 |
| 1 | 曹玉鹏, 戴志强, 刘建涛,等. Ni-Cr合金层研究现状及展望[J]. 热加工工艺, 2017, 22(7): 29-32. |
| CaoY P, DaiZ Q, LiuJ T, et al. Research status and prospect of Ni-Cr alloy layer[J]. Hot Working Technology, 2017, 22(7): 29-32. | |
| 2 | 张志. 氯化胆碱-乙二醇-三氯化铬低共熔体系中电沉积铬的研究[D]. 昆明: 昆明理工大学, 2014. |
| ZhangZ. Study on electrodeposition of chromium in ChCl-EG-CrCl3 eutectic system[D]. Kunming: Kunming University of Technology, 2014. | |
| 3 | AghdamA S, AllahkaramS R, MahdaviS. Corrosion and tribological behavior of Ni-Cr alloy coatings electrodeposited on low carbon steel in Cr(Ⅲ)-Ni(Ⅱ) bath[J]. Surf. Coat. Technol., 2015, 281: 144-149. |
| 4 | SurvilienėS, ČešūnienėA, JasulaitienėV, et al. The use of XPS for study of the surface layers of CrNi alloys electrodeposited from the Cr(Ⅲ)+Ni(Ⅱ) bath[J]. Appl. Surf. Sci., 2012, 258: 9902-9906. |
| 5 | 王茂全. 化学方法处理电镀废水[J]. 中国资源综合利用, 2018, 375(2): 55-57. |
| WangM Q. Chemical treatment of electroplating wastewater[J]. China Resources Comprehensive Utilization, 2018, 375(2): 55-57. | |
| 6 | 李冰, 严琪, 宋皓. 喷射电沉积制备替代六价铬的三价铬镀层[J]. 机械工程师, 2018, 327(9): 50-52. |
| LiB, YanQ, SongH. Trivalent chromium coatings prepared by jet electrodeposition to replace the hexavalent chromium[J]. Mechanical Engineer, 2018, 327(9): 50-52. | |
| 7 | GenganS, SubramanianM. Electrodeposition of Fe-Ni-Cr alloy from deep eutectic system containing choline chloride and ethylene glycol[J]. Int. J. Electrochem. Sci., 2011, 6: 1468-1478. |
| 8 | SurvilieneS, Lisowska-OleksiakA, SelskisA, et al. Corrosion behaviour of Cr coatings deposited from Cr(Ⅲ) formate-urea electrolytes[J]. Trans. IMF, 2006, 84: 241-245. |
| 9 | SrimathiS N, MayannaS M. Electroplating of thin films of magnetic FeNi alloys[J]. Materials Chemistry and Physics, 1984, 11: 351-364. |
| 10 | 苏波, 张贤杰, 李坚,等. CH3CONH2-EG低共熔溶剂的物化性质[J]. 昆明理工大学学报(自然科学版), 2017, 3(2):16-21+52. |
| SuB, ZhangX J, LiJ, et al. Physicochemical properties of CH3CONH2-EG deep eutectic solvents[J]. Journal of Kunming University of Science and Technology (Natural Science Edition), 2017, 3(2): 16-21+52. | |
| 11 | 雷震, 徐存英, 卢东辉,等. 低共熔溶剂处理含锌烟尘的研究[J]. 有色金属(冶炼部分), 2017, 8(2): 5-8. |
| LeiZ, XuC Y, LuD H, et al. Study on treatment of zinc dust with deep eutectic solvent[J]. Nonferrous Metals (Extractive Metallurgy), 2017, 8(2): 5-8. | |
| 12 | 张盈盈, 陆小华, 冯新, 等. 胆碱类低共熔溶剂的物性及应用[J]. 化学进展, 2013, 25(6): 881-892. |
| ZhangY Y, LuX H, FengX, et al. Physical properties and application of choline eutectic solvents[J]. Progress in Chemical, 2013, 25(6): 881-892. | |
| 13 | HuoC, ChanT H. A novel liquid-phase strategy for organic synthesis using organic ions as soluble supports[J]. Chemical Society Reviews, 2010, 39(8): 2977-3006. |
| 14 | YangL, LiuY E, Zu, et al. Optimize the process of ionic liquid-based ultrasonic-assisted extraction of aesculin and aesculetin from Correx fraxini by response surface methodology[J]. Chemical Engineering Journal, 2011, 175(1): 539-547. |
| 15 | LiZ, FriedrichA, TaubertA. Gold microcrystal synthesis via reduction of HAuCl4 by cellulose in the ionic liquid 1-butyl-3-methyl imidazolium chloride[J]. Journal of Materials Chemistry, 2008, 18(9): 1008-1014. |
| 16 | 方园, 魏琦峰, 任秀莲,等. 低共熔溶剂中电化学沉积的研究进展[J]. 电镀与精饰, 2015, 37(10): 12-17. |
| FangY, WeiQ F, RenX L, et al. Advances in electrochemical deposition in low eutectic solvents[J]. Plating & Fishing, 2015, 37(10): 12-17. | |
| 17 | AbbottA P, CapperG, DaviesD L, et al. Electrodeposition of chromium black from ionic liquids[J]. Transactions of the IMF, 2004, 82(1/2): 14-17. |
| 18 | AbbottA P, Al-BarzinjyA A, AbbottP D, et al. Speciation, physical and electrolytic properties of eutectic mixtures based on CrCl3·6H2O and urea[J]. Physical Chemistry Chemical Physics, 2014, 16(19): 9047-9055. |
| 19 | ZhangQ, VigierK D O, RoyerS, et al. Deep eutectic solvents: syntheses, properties and applications[J]. Chemical Society Reviews, 2012, 41(21): 7108-7146. |
| 20 | AbbottA P, CapperG, DaviesD L, et al. Selective extraction of metals from mixed oxide matrixes using choline-based ionic liquids[J]. Inorganic Chemistry, 2005, 44(19): 6497-6499. |
| 21 | ZouY, XuH, WuG, et al. Structural analysis of [ChCl]m[ZnCl2]n ionic liquid by X-ray absorption fine structure spectroscopy[J]. The Journal of Physical Chemistry B, 2009, 113(7): 2066-2070. |
| 22 | LinI J B, VasamC S. Metal-containing ionic liquids and ionic liquid crystals based on imidazolium moiety[J]. Journal of Organometallic Chemistry, 2005, 690(15): 3498-3512. |
| 23 | AbbottA P, CapperG, DaviesD L, et al. Solubility of metal oxides in deep eutectic solvents based on choline chloride[J]. Journal of Chemical & Engineering Data, 2006, 51(4): 1280-1282. |
| 24 | VorobievaS N, BaidinaI A, BelyaevA V, et al. Crystal structure of [Rh(H2O)5Cl] (C7H7O3S)2 and Cs[Rh(H2O)5NO3] (C7H7O3S)3·H2O[J]. Journal of Structural Chemistry, 2015, 56(8): 1606-1612. |
| 25 | MccalmanD C, SunL, ZhangY, et al. Speciation, conductivities, diffusivities, and electrochemical reduction as a function of water content in mixtures of hydrated chromium chloride/ choline chloride[J]. Journal of Physical Chemistry B, 2015, 119(19): 6018-6023. |
| 26 | ShupackS I. The chemistry of chromium and some resulting analytical problems[J]. Environmental Health Perspectives, 1991, 92: 7-11. |
| 27 | AbbottA P, GlenC, DaviesD L, et al. Ionic liquid analogues formed from hydrated metal salts[J]. Chemistry - A European Journal, 2010, 10(15): 3769-3774. |
| 28 | ElvingP J, ZemelB. Absorption in the ultraviolet and visible regions of chloroaquochromium(Ⅲ) ions in acid media[J]. Journal of the American Chemical Society, 1957, 79(6): 1281-1285. |
| 29 | AbbottA P, CapperG, DaviesD L, et al. Novel ambient temperature ionic liquids for zinc and zinc alloy electrodeposition[J]. Transactions of the IMF, 2001, 79(6): 204-206. |
| 30 | AbbottA P, DavidB, GlenC, et al. Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids[J]. Journal of the American Chemical Society, 2004, 126(29): 9142-9147. |
| 31 | ParkerJ F, CheekG T, RoeperD F, et al. Electronic absorption and voltammetric analysis of Ni(Ⅱ) coordination in the room temperature ionic liquid: 1-ethyl-3-Methylimidizolium chloride/aluminum chloride[J]. ECS Transactions, 2012, 41(36): 23-33. |
| 32 | 华一新. 冶金动力学导论[M]. 北京: 冶金工业出版社, 2004: 27-29. |
| HuaY X. Introduction to Metallurgical Dynamics[M]. Beijing: Metallurgical Industry Press, 2004: 27-29. | |
| 33 | 刘春萍, 刘刚, 胡玉才, 等. 1-苄基-4-甲基吡啶盐离子液体的合成及其电导性能的研究[J]. 石油化工, 2011, 40(7): 764-769. |
| LiuC P, LiuG, HuY C, et al. Synthesis and electric conductivity of 1-benzyl-4-methylpyridine ionic liquids[J]. Petrochemical Technology, 2011, 40(7): 764-769. | |
| 34 | 何志强, 鄢浩, 王骑虎,等. 温度对氯化胆碱/多元醇型低共熔溶剂物性的影响[J]. 上海大学学报(自然科学版), 2015, 21(3): 384-392. |
| HeZ Q, YanH, WangQ H, et al. Effect of temperature on physic-chemical properties of deep eutectic solvent based on choline chloride and polyols[J]. Journal of Shanghai University (Natural Science Edition), 2015, 21(3): 384-392. | |
| 35 | 王喜然, 华一新, 赵秋凝, 等. AlCl3-BMIC 离子液体率 [J]. 中国有色金属学 2006, 16 (12): 2138-2141. |
| WangX R, HuaY X, ZhaoQ N, et al. Electrical conductivity of AlCl3-BMIC room temperature ionic liquids[J]. The Chinese Journal of Nonferrous Metals, 2006, 16 (12): 2138-2141. | |
| 36 | 雷震, 徐存英, 华一新, 等. ChCl-urea-ZnO低共熔溶剂体系的电化学行为[J]. 化工学报, 2017, 68(8): 3301-3309. |
| LeiZ, XuC Y, HuaY X, et al. Eelectrochemical behaviors of ZnO in choline chloride-urea deep eutectic solvents[J]. CIESC Journal, 2017, 68(8): 3301-3309. | |
| 37 | 高颖, 邬冰. 电化学基础[M]. 北京: 化学工业出版社, 2004: 11-14. |
| GaoY, WuB. Foundations of Electrochemistry[M]. Beijing: Chemical Industry Press, 2004: 11-14. | |
| 38 | AbbottA P, CapperG, MckenzieK J, et al. Electrodeposition of zinc-tin alloys from deep eutectic solvents based on choline chloride[J]. Journal of Electroanalytical Chemistry, 2007, 599(2): 288-294. |
| [1] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [2] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [3] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [4] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [5] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [6] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [7] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [8] | 雷博雯, 吴建华, 吴启航. R290低压比热泵高补气过热度循环研究[J]. 化工学报, 2023, 74(5): 1875-1883. |
| [9] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [10] | 周必茂, 许世森, 王肖肖, 刘刚, 李小宇, 任永强, 谭厚章. 烧嘴偏转角度对气化炉渣层分布特性的影响[J]. 化工学报, 2023, 74(5): 1939-1949. |
| [11] | 张永泉, 玄伟伟. 碱金属/(FeO+CaO+MgO)对硅酸盐灰熔渣结构和黏度的影响机理[J]. 化工学报, 2023, 74(4): 1764-1771. |
| [12] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [13] | 靳志远, 单国荣, 潘鹏举. AM/AMPS/SSS三元共聚物的制备及耐温耐盐性能[J]. 化工学报, 2023, 74(2): 916-923. |
| [14] | 张家庆, 蒋榕培, 史伟康, 武博翔, 杨超, 刘朝晖. 煤基/石油基火箭煤油高参数黏温特性与组分特性研究[J]. 化工学报, 2023, 74(2): 653-665. |
| [15] | 谢煜, 张民, 胡卫国, 王玉军, 骆广生. 利用膜分散微反应器高效溶解D-7-ACA的研究[J]. 化工学报, 2023, 74(2): 748-755. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号