化工学报 ›› 2020, Vol. 71 ›› Issue (3): 1326-1334.DOI: 10.11949/0438-1157.20191104
收稿日期:2019-10-07
修回日期:2019-12-07
出版日期:2020-03-05
发布日期:2020-03-05
通讯作者:
吴玮
作者简介:王柯晴(1996—),女,硕士研究生,基金资助:
Keqing WANG1( ),Jie XU1,Zhixuan SHEN1,Jiabin CHEN2,Wei WU1(
),Jie XU1,Zhixuan SHEN1,Jiabin CHEN2,Wei WU1( )
)
Received:2019-10-07
Revised:2019-12-07
Online:2020-03-05
Published:2020-03-05
Contact:
Wei WU
摘要:
在催化活化过一硫酸盐(PMS)降解水中污染物的反应中,通过添加钴基钙钛矿提高反应效率。利用溶胶凝胶法制备了LaCoO3钙钛矿,通过实验评估LaCoO3/PMS体系对非甾体抗炎药萘普生(NAP)的降解效果。分析了LaCoO3投加量、PMS投加量、反应初始pH、Cl-浓度和腐殖酸(HA)对NAP去除率的影响以及该体系的矿化能力。结果表明NAP降解的反应速率随LaCoO3和PMS投加量增加而增大;反应初始pH在5.0时NAP降解效果最好;溶液中存在Cl-对降解有促进效果,且Cl-浓度越大促进效果越明显;腐殖酸(HA)对反应有一定程度的抑制效果;LaCoO3在重复利用5次时仍有较好的稳定性。此外,自由基淬灭实验结果表明在LaCoO3/PMS体系中SO4·-为主要活性物质。
中图分类号:
王柯晴, 徐劼, 沈芷璇, 陈家斌, 吴玮. LaCoO3钙钛矿活化过一硫酸盐降解萘普生[J]. 化工学报, 2020, 71(3): 1326-1334.
Keqing WANG, Jie XU, Zhixuan SHEN, Jiabin CHEN, Wei WU. Degradation of naproxen by peroxymonosulfate activated with LaCoO3[J]. CIESC Journal, 2020, 71(3): 1326-1334.
| LaCoO3投加量/(mg·L-1) | Kabs/min-1 |
|---|---|
| 20 | 0.074 |
| 50 | 0.1315 |
| 100 | 0.2241 |
| 200 | 0.2858 |
表1 不同LaCoO3投加量的一级反应动力学常数
Table 1 First order kinetic reaction rate constants of different LaCoO3 dosage
| LaCoO3投加量/(mg·L-1) | Kabs/min-1 |
|---|---|
| 20 | 0.074 |
| 50 | 0.1315 |
| 100 | 0.2241 |
| 200 | 0.2858 |
| n(NAP)∶n(PMS) | 反应前/ (mmol·L-1) | 反应后/ (mmol·L-1) | PMS分解量/ (mmol·L-1) |
|---|---|---|---|
| 1∶5 | 0.25 | 0 | 0.25 |
| 1∶10 | 0.5 | 0.03 | 0.47 |
| 1∶20 | 1 | 0.32 | 0.68 |
| 1∶40 | 2 | 0.92 | 1.08 |
表2 PMS的分解量
Table 2 Decomposition of PMS
| n(NAP)∶n(PMS) | 反应前/ (mmol·L-1) | 反应后/ (mmol·L-1) | PMS分解量/ (mmol·L-1) |
|---|---|---|---|
| 1∶5 | 0.25 | 0 | 0.25 |
| 1∶10 | 0.5 | 0.03 | 0.47 |
| 1∶20 | 1 | 0.32 | 0.68 |
| 1∶40 | 2 | 0.92 | 1.08 |
| n(NAP)∶n(PMS) | Kabs/min-1 |
|---|---|
| 1∶5 | 0.0262 |
| 1∶10 | 0.0994 |
| 1∶20 | 0.2254 |
| 1∶40 | 0.4558 |
表3 不同PMS投加量的一级反应动力学常数
Table 3 First order kinetic reaction rate constants with different PMS dosage
| n(NAP)∶n(PMS) | Kabs/min-1 |
|---|---|
| 1∶5 | 0.0262 |
| 1∶10 | 0.0994 |
| 1∶20 | 0.2254 |
| 1∶40 | 0.4558 |
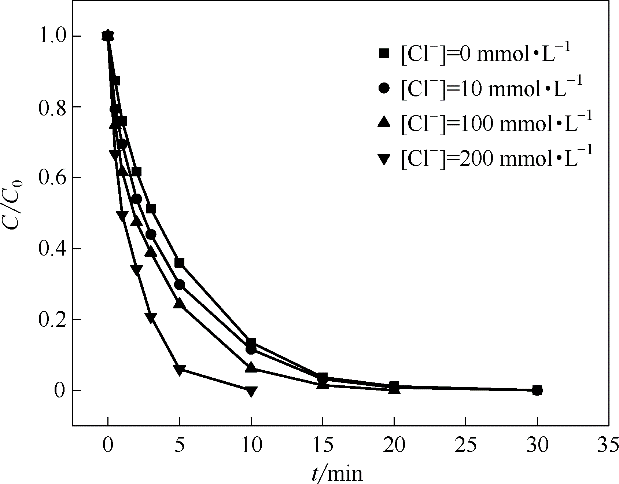
图10 反应中不同浓度的Cl-对NAP降解效果的影响
Fig.10 Effects of different concentration of Cl- on degradation of NAP(pH=7.0, NAP = 50 μmol·L-1, PMS = 1.0 mmol·L-1, LaCoO3 = 100 mg·L-1)

图11 反应中不同浓度的HA对NAP降解效果的影响
Fig.11 Effect of different concentration of HA on NAP degradation(pH=7.0, NAP = 50 μmol·L-1, PMS = 1.0 mmol·L-1, LaCoO3 = 100 mg·L-1)
| 1 | Dearmond B, Francisco C A, Lin J S, et al. Safety profile of over the counter naproxen sodium[J]. Clinical Therapeutics, 1995, 17(4): 587. |
| 2 | Ekman E. The non-selective anti-inflammatory, Naproxen at an over the counter dose during arthroscopic surgery[J]. Journal of Pain, 2012, 13(4): S85. |
| 3 | Ternes T A, Hirsch R. Occurrence and behavior of X-ray contrast media in sewage facilities and the aquatic environment[J]. Environmental Science & Technology, 2000, 34(13): 2741-2748. |
| 4 | Möhle E, Kempter C, Kern A, et al. Untersuchungen zum abbau von pharmaka in kommunalen kläranlagen mit HPLC electrospray massenspektrometrie[J]. CLEAN - Soil, Air, Water, 2010, 27(6): 430-436. |
| 5 | Soufan M, Deborde M, Delmont A, et al. Aqueous chlorination of carbamazepine: kinetic study and transformation product identification[J]. Water Research, 2013, 47(14): 5076-5087. |
| 6 | Olmez-Hanci T, Arslan-Alaton I. Comparison of sulfate and hydroxyl radical based advanced oxidation of phenol[J]. Chemical Engineering Journal, 2013, 224(1): 10-16. |
| 7 | Li H, Ma Y, Wan J, et al. Degradation of refractory dibutyl phthalate by peroxymonosulfate activated with novel catalysts cobalt metal organic frameworks: mechanism, performance, and stability[J]. Journal of hazardous materials, 2016, 318(15): 154-163. |
| 8 | Pang X, Guo Y, Zhang Y, et al. LaCoO3 perovskite oxide activation of peroxymonosulfate for aqueous 2-phenyl-5-sulfobenzimidazole degradation: effect of synthetic method and the reaction mechanism[J]. Chemical Engineering Journal, 2016, 304: 897-907. |
| 9 | Ribeiro A R, Nunes O C, Pereira M F R, et al. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched directive[J]. Environment International, 2015, 75: 33-51. |
| 10 | Oh W D, Lua S K, Dong Z, et al. A novel three-dimensional spherical CuBi2O4 nanocolumn arrays with persulfate and peroxymonosulfate activation functionalities for 1H-benzotriazole removal[J]. Nanoscale, 2015, 7(17): 8149-8158. |
| 11 | Ji Y, Dong C, Kong D, et al. Heat-activated persulfate oxidation of atrazine: implications for remediation of groundwater contaminated by herbicides[J]. Chemical Engineering Journal, 2015, 263: 45-54. |
| 12 | Cai C, Zhang H, Zhong X, et al. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a bimetallic Fe-Co/SBA-15 catalyst for the degradation of orange Ⅱ in water[J]. Journal of Hazardous Materials, 2015, 283(283): 70-79. |
| 13 | Guan Y H, Ma J, Li X C, et al. Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system[J]. Environmental Science & Technology, 2011, 45(21): 9308. |
| 14 | Wei C, Zhang J, Zhang Y, et al. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a Co-NiOx catalyst[J]. Water Science & Technology, 2017, 76(6): t2017316. |
| 15 | Chen X Y, Chen J W, Qiao X L, et al. Performance of nano-Co3O4/peroxymonosulfate system: kinetics and mechanism study using acid orange 7 as a model compound[J] Applied Catalysis B Environmental, 2008, 80(1/2): 116-121. |
| 16 | Yang S, Xiao T, Zhang J, et al. Activated carbon fiber as heterogeneous catalyst of peroxymonosulfate activation for efficient degradation of acid orange 7 in aqueous solution[J]. Separation & Purification Technology, 2015, 143: 19-26. |
| 17 | Zhang J, Shao X, Shi C, et al. Decolorization of acid orange 7 with peroxymonosulfate oxidation catalyzed by granular activated carbon[J]. Chemical Engineering Journal, 2013, 232(10): 259-265. |
| 18 | Zhu J, Li H, Zhong L, et al. Perovskite oxides: preparation, characterizations, and applications in heterogeneous catalysis[J]. ACS Catalysis, 2014, 4(9): 2917-2940. |
| 19 | Rao Y F, Zhang Y F, Han F M, et al. Heterogeneous activation of peroxymonosulfate by LaFeO3 for diclofenac degradation: DFT-assisted mechanistic study and degradation pathways[J]. Chemical Engineering Journal, 2018, 352: 601-611. |
| 20 | Miao J, Duan X G, Li J, et al. Boosting performance of lanthanide magnetism perovskite for advanced oxidation through lattice doping with catalytically inert element[J]. Chemical Engineering Journal, 2019, 355: 721-730. |
| 21 | Liang C, Huang C F, Mohanty N, et al. A rapid spectrophotometric determination of persulfate anion in ISCO[J]. Chemosphere, 2008, 73(9): 1540-1543. |
| 22 | Merino N A, Barbero B P, Eloy P, et al. La1-xCaxCoO3 perovskite-type oxides: identification of the surface oxygen species by XPS[J]. Applied Surface Science, 2006, 253(3): 1489-1493. |
| 23 | Chen X, Qiao X, Wang D, et al. Kinetics of oxidative decolorization and mineralization of acid orange 7 by dark and photoassisted Co2+-catalyzed peroxymonosulfate system[J]. Chemosphere, 2007, 67(4): 802-808. |
| 24 | Jie L, Zhao Z, Shao P, et al. Activation of peroxymonosulfate with magnetic Fe3O4–MnO2 core–shell nanocomposites for 4-chlorophenol degradation[J]. Chemical Engineering Journal, 2015, 262(9): 854-861. |
| 25 | Duan X, Donnell K O, Sun H, et al. Sulfur and nitrogen co-doped graphene for metal-free catalytic oxidation reactions[J]. Small, 2015, 11(25): 3036-3044. |
| 26 | Liu J, Zhou J, Ding Z, et al. Ultrasound irritation enhanced heterogeneous activation of peroxymonosulfate with Fe3O4 for degradation of azo dye[J]. Ultrasonics Sonochemistry, 2017, 34: 953-959. |
| 27 | 徐蕾, 袁瑞霞, 郭耀广, 等. 氯离子对钴/单过氧硫酸盐体系降解2, 4, 6-三氯苯酚的影响[J]. 武汉大学学报(理学版), 2013, 59(1): 51-56. |
| Xu L, Yuan R X, Guo Y G, et al. Effects of chloride ions on degradation of 2, 4, 6-trichlorophenol by Co(Ⅱ)/peroxymonosulfate (Co/PMS) system[J]. Journal of Wuhan University (Science Edition), 2013, 59(1): 51-56. | |
| 28 | Fei G, Wang L, Li D, et al. An effective heterogeneous iron-based catalyst to activate peroxymonosulfate for organic contaminants removal[J]. Chemical Engineering Journal, 2015, 267: 102-110. |
| 29 | Yuan R, Ramjaun S N, Wang Z, et al. Effects of chloride ion on degradation of acid orange 7 by sulfate radical-based advanced oxidation process: implications for formation of chlorinated aromatic compounds[J]. Journal of Hazardous Materials, 2011, 196(1): 173-179. |
| 30 | Du E, Li J, Zhou S, et al. Transformation of naproxen during the chlorination process: products identification and quantum chemistry validation[J]. Chemosphere, 2018, 211: 1007-1017. |
| 31 | Manzak A, Kurşun C, Yıldız Y. Characterization of humic acid extracted from aqueous solutions with polymer inclusion membranes[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 81: 14-20. |
| 32 | Whitby H, Berg C M G V. Evidence for copper-binding humic substances in seawater[J]. Marine Chemistry, 2015, 173: 282-290. |
| 33 | Zhang N, Li J M, Liu G G, et al. Photodegradation of diclofenac in aqueous solution by simulated sunlight irradiation: kinetics, thermodynamics and pathways[J]. Water Science & Technology A Journal of the International Association on Water Pollution Research, 2017, 75(9/10): 2163. |
| 34 | Nie M, Yi Y, Zhang Z, et al. Degradation of chloramphenicol by thermally activated persulfate in aqueous solution[J]. Chemical Engineering Journal, 2014, 246(246): 373-382. |
| 35 | Furman O S, Teel A L, Ahmad M, et al. Effect of basicity on persulfate reactivity[J]. Journal of Environmental Engineering, 2011, 137(4): 241-247. |
| 36 | Yan J, Lei M, Zhu L, et al. Degradation of sulfamonomethoxine with Fe3O4 magnetic nanoparticles as heterogeneous activator of persulfate[J]. Journal of Hazardous Materials, 2011, 186(2/3): 1398-1404. |
| 37 | Wang Y, Sun H, Ang M, et al. 3D-hierarchically structured MnO2 for catalytic oxidation of phenol solutions by activation of peroxymonosulfate: structure dependence and mechanism[J]. Applied Catalysis B Environmental, 2015, 164: 159-167. |
| [1] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [2] | 李瑞康, 何盈盈, 卢维鹏, 王园园, 丁皓东, 骆勇名. 电化学强化钴基阴极活化过一硫酸盐的研究[J]. 化工学报, 2023, 74(5): 2207-2216. |
| [3] | 吴学红, 栾林林, 陈亚南, 赵敏, 吕财, 刘勇. 可降解柔性相变薄膜的制备及其热性能[J]. 化工学报, 2023, 74(4): 1818-1826. |
| [4] | 杨庆云, 李青松, 陈泽铭, 邓靖, 李玉瑛, 杨帆, 陈国元, 李国新. UV/PMS、UV/PDS、UV/SPC工艺降解尼泊金甲酯[J]. 化工学报, 2023, 74(3): 1322-1331. |
| [5] | 谢煜, 张民, 胡卫国, 王玉军, 骆广生. 利用膜分散微反应器高效溶解D-7-ACA的研究[J]. 化工学报, 2023, 74(2): 748-755. |
| [6] | 李彩风, 王晓, 李岗建, 林军章, 汪卫东, 束青林, 曹嫣镔, 肖盟. 嗜烃乳化菌SL-1与内源菌协同驱油的菌群作用关系研究[J]. 化工学报, 2022, 73(9): 4095-4102. |
| [7] | 靳文章, 张玉玲, 贾晓宇. 电化学高级氧化对HEDP的降解效能研究[J]. 化工学报, 2022, 73(9): 4062-4069. |
| [8] | 许贤伦, 钱旸, 张兴旺, 雷乐成. 高压脉冲介质阻挡放电降解土壤中芘的研究[J]. 化工学报, 2022, 73(9): 4025-4033. |
| [9] | 徐振和, 李泓江, 高雨, 礼峥, 张含烟, 徐宝彤, 丁茯, 孙亚光. In2O3/Ag:ZnIn2S4“Type Ⅱ”型异质结构材料的制备及可见光催化性能[J]. 化工学报, 2022, 73(8): 3625-3635. |
| [10] | 黄仕元, 邓简, 袁瀚钦, 王国华, 吴兴良. 钴强化铁磁体活化过一硫酸盐的实验研究[J]. 化工学报, 2022, 73(7): 3045-3056. |
| [11] | 贾艳萍, 丁雪, 刚健, 佟泽为, 张海丰, 张兰河. Mn强化Fe/C微电解工艺条件优化及降解油墨废水机理[J]. 化工学报, 2022, 73(5): 2183-2193. |
| [12] | 韩雪, 高生旺, 王国英, 夏训峰. 铈掺杂强化碳纳米管活化过一硫酸盐实验研究[J]. 化工学报, 2022, 73(4): 1743-1753. |
| [13] | 万丽, 梁德青. 一种可生物降解水合物动力学抑制剂的研究[J]. 化工学报, 2022, 73(2): 894-903. |
| [14] | 侯晓松, 刘晨星, 任爱玲, 郭斌, 郭渊明. 超声雾化/表面活性剂强化吸收耦合生物洗涤净化甲苯废气[J]. 化工学报, 2022, 73(10): 4692-4706. |
| [15] | 朱振林, 王松林, 姜冰雪, 李家旭, 邓维, 吴海强, 杨轩, 刘平伟, 王文俊. 聚酯生物降解及评价方法研究[J]. 化工学报, 2022, 73(1): 110-121. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号