化工学报 ›› 2020, Vol. 71 ›› Issue (3): 923-935.DOI: 10.11949/0438-1157.20190852
詹世平1,2( ),丁仕强1,2,王卫京2,李鸣明2,赵启成2
),丁仕强1,2,王卫京2,李鸣明2,赵启成2
收稿日期:2019-07-24
修回日期:2019-10-06
出版日期:2020-03-05
发布日期:2020-03-05
通讯作者:
詹世平
作者简介:詹世平(1959—),女,博士,教授,基金资助:
Shiping ZHAN1,2( ),Shiqiang DING1,2,Weijing WANG2,Mingming LI2,Qicheng ZHAO2
),Shiqiang DING1,2,Weijing WANG2,Mingming LI2,Qicheng ZHAO2
Received:2019-07-24
Revised:2019-10-06
Online:2020-03-05
Published:2020-03-05
Contact:
Shiping ZHAN
摘要:
生物可降解聚合物/药物纳米微粒在药物靶向递送、有效成分封装和医疗诊断等领域具有突出的优势。超临界流体超细微粒制备技术具有绿色环保、制备方法种类多、粒径易调节和后续分离纯化容易等特点,得到了广泛的研究。为了得到满足使用要求的聚合物/药物纳米微粒,超临界流体制粒技术是有效的手段之一。论述了生物可降解聚合物纳米材料的特点和应用情况,简要介绍了超临界流体及特性,重点介绍了超临界溶液快速膨胀(RESS)、超临界抗溶剂沉淀(SAS)、超临界CO2辅助雾化(SAA)和超临界流体乳液萃取(SFEE)的工艺特点、制备方法、基本原理和研究进展,并对超临界流体技术制备聚合物/药物纳米微粒的发展方向进行了展望。
中图分类号:
詹世平, 丁仕强, 王卫京, 李鸣明, 赵启成. 超临界流体技术制备生物可降解聚合物/药物纳米微粒研究进展[J]. 化工学报, 2020, 71(3): 923-935.
Shiping ZHAN, Shiqiang DING, Weijing WANG, Mingming LI, Qicheng ZHAO. Research progress of biodegradable polymers/drug nanoparticles prepared by supercritical fluid technology[J]. CIESC Journal, 2020, 71(3): 923-935.
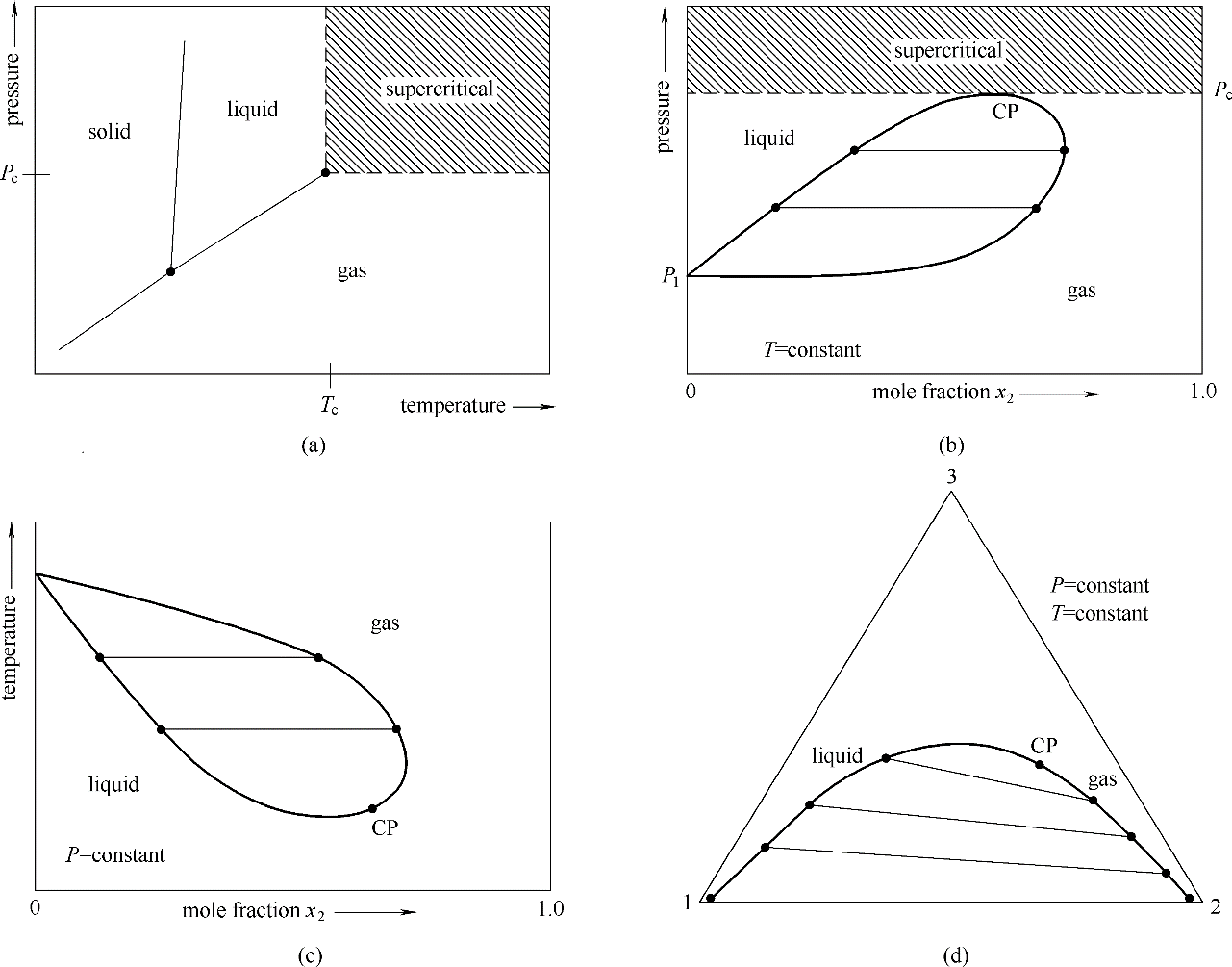
图1 超临界状态和临界点纯物质(a),恒温二元混合物(b),恒压二元混合物(c),恒温恒压三元混合物(d)
Fig.1 Supercritical states and critical points pure substance (a), binary mixture at constant temperature (b), binary mixture at constant pressure (c) and ternary mixture at constant temperature and pressure (d)
| 1 | Wang G, Zhou F, Li X, et al. Controlled synthesis of L-cysteine coated cobalt ferrite nanoparticles for drug delivery[J]. Ceramics International, 2018, 44(12): 13588-13594. |
| 2 | Al-Kassas R, Bansal M, Shaw J. Nanosizing techniques for improving bioavailability of drugs[J]. Journal of Controlled Release, 2017, 260(8): 202-212. |
| 3 | Kamaly N, Yameen B, Wu J, et al. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release[J]. Chemical Reviews, 2016, 116(4): 2602-2663. |
| 4 | 施萍,曾贤伍,何文涛, 等. 肿瘤靶向pH响应药物递送⁃成像体系的合成与控制释放研究[J]. 化学研究, 2019, 30(2): 182-188. |
| Shi P, Zeng X W, He W T, et al. Synthesis and controlled release of tumor-targeted pH-responsive drug delivery-imaging system[J]. Chemical Research, 2019, 30(2): 182-188. | |
| 5 | Barım Ş B, Bayrakçeken A, Bozbağ S E, et al. Control of average particle size of carbon aerogel supported platinum nanoparticles by supercritical deposition[J]. Microporous and Mesoporous Materials, 2017, 245(6): 94-103. |
| 6 | Guamán-Balcázar M C, Montes A, Fernández-Ponce M T, et al. Generation of potent antioxidant nanoparticles from mango leaves by supercritical antisolvent extraction[J]. Journal of Supercritical Fluids, 2018, 138(4): 92-101. |
| 7 | Sodeifian G, Sajadian A S. Solubility measurement and preparation of nanoparticles of an anticancer drug (Letrozole) using rapid expansion of supercritical solutions with solid cosolvent (RESS-SC)[J]. Journal of Supercritical Fluids, 2018, 133(1): 239-252. |
| 8 | Nuchuchua O, Nejadnik M R, Goulooze S C, et al. Characterization of drug delivery particles produced by supercritical carbon dioxide technologies[J]. Journal of Supercritical Fluids, 2017, 128(10): 244-262. |
| 9 | 陈爱政, 康永强, 王士斌, 等. 超临界流体技术构建壳聚糖纳米粒/PLLA-PEG-PLLA 复合微粒及其表征[J]. 化工学报, 2015, 66(4): 1565-1576. |
| Chen A Z, Kang Y Q, Wang S B, et al. Preparation and characterization of chitosan nanoparticles/PLLA-PEG-PLLA composite microparticles by supercritical fluid technology [J]. CIESC Journal, 2015, 66(4): 1565-1576. | |
| 10 | Esfandiari N. Production of micro and nano particles of pharmaceutical by supercritical carbon dioxide[J]. Journal of Supercritical Fluids, 2015, 100(5): 129-141. |
| 11 | Lee L Y, Wang C H, Smith K A. Supercritical antisolvent production of biodegradable micro- and nanoparticles for controlled delivery of paclitaxel[J]. Journal of Controlled Release, 2008, 125(2): 96-106. |
| 12 | Lee L Y, Ranganath S H, Fu Y, et al. Paclitaxel release from micro-porous PLGA disks[J]. Chemical Engineering Science, 2009, 64(21): 4341-4349. |
| 13 | Badens E, Masmoudi Y, Mouahid A, et al. Current situation and perspectives in drug formulation by using supercritical fluid technology[J]. Journal of Supercritical Fluids, 2018, 134(4): 274–283. |
| 14 | Gooneh-Farahani S, Naimi-Jamal M R, Naghib S M. Stimuli-responsive graphene-incorporated multifunctional chitosan for drug delivery applications: a review[J]. Expert Opinion on Drug Delivery, 2019, 16(1): 79-99. |
| 15 | Verma D, Gulati N, Kaul S, et al. Protein based nanostructures for drug delivery[J]. Journal of Pharmaceutics, 2018, 2018: 9285854. |
| 16 | Choi S Y, Rhie M N, Kim H T, et al. Metabolic engineering for the synthesis of polyesters: a 100-year journey from polyhydroxyalkanoates to non-natural microbial polyesters[J]. Metabolic Engineering, 2019, 52(19): 30088-30093. |
| 17 | 王景昌, 杨昌盛, 万泽韬, 等. 生物医用脂肪族聚酯开环聚合的研究进展[J]. 高分子通报, 2018, 236(12): 34-39. |
| Wang J C, Yang C S, Wang Z T, et al. Research progress of ring-opening polymerization for biomedical aliphatic polyster[J]. Polymer Bulletin, 2018, 236(12): 34-39. | |
| 18 | Hu L, Sun Y, Wu Y. Advances in chitosan-based drug delivery vehicles[J]. Nanoscale, 2013, 5(8): 3103-3111. |
| 19 | Li P, Yang Z, Wang Y, et al. Microencapsulation of coupled folate and chitosan nanoparticles for targeted delivery of combination drugs to colon[J]. Journal of Microencapsulation, 2015, 32(1): 40-45. |
| 20 | Wang Y, Li P, Chen L, et al. Targeted delivery of 5-fluorouracil to HT-29 cells using high efficient folic acid-conjugated nanoparticles[J]. Drug Delivery, 2015, 22(2): 191-198. |
| 21 | Wang K, Nune K C, Misra R D K. The functional response of alginate-gelatin-nanocrystalline cellulose injectable hydrogels toward delivery of cells and bioactive molecules[J]. Acta Biomaterialia, 2016, 36(5): 143-151. |
| 22 | Biswas S, Chattopadhyay M, Sen K K, et al. Development and characterization of alginate coated low molecular weight chitosan nanoparticles as new carriers for oral vaccine delivery in mice[J]. Carbohydrate Polymers, 2015, 121(5): 403-410. |
| 23 | Haq F, Yu H, Wang L, et al. Advances in chemical modifications of starches and their applications[J]. Carbohydrate Polymers, 2019, 476(4): 12-35. |
| 24 | Myint A A, Lee H W, Seo B, et al. One pot synthesis of environmentally friendly lignin nanoparticles with compressed liquid carbon dioxide as an antisolvent[J]. Green Chemistry, 2016, 18(7): 2129-2146. |
| 25 | 于坤, 韩晓东, 何丽华, 等. 用于药物载体系统的多糖材料的修饰方法[J]. 材料导报, 2019, 33(3): 141-147. |
| Yu K, Han X D, He L H, et al. A survey on modification methods of polysaccharides used for drug carrier systems[J]. Materials Reports, 2019, 33(3): 141-147. | |
| 26 | Rosenblum D, Joshi N, Tao W, et al. Progress and challenges towards targeted delivery of cancer therapeutics[J]. Nature Communications, 2018, 9(1): 1410-1421. |
| 27 | Brunner G. Gas extraction: an introduction to fundamentals of supercritical fluids and the application to separation processes[M]//Topics in Physical Chemistry. New York: Springer, 1994. |
| 28 | Ž Knez, Markočič E, Leitgeb M, et al. Industrial applications of supercritical fluids: a review[J]. Energy, 2014, 77(12): 235-243. |
| 29 | Kankala R K, Zhang Y S, Wang S B, et al. Supercritical fluid technology: an emphasis on drug delivery and related biomedical applications[J]. Advanced Healthcare Materials, 2017, 6(16): 1700433. |
| 30 | Ž Knez, Knez-Hrnčič M, Škerget M. Particle formation and product formulation using supercritical fluids[J]. Annual Review of Chemical and Biomolecular Engineering, 2015, 6(1): 379-407. |
| 31 | 陈震, 周进莉, 陶钰婷, 等. 超临界CO2抗溶剂法制备卡维地洛固体分散体[J]. 中国新药杂志, 2019, 28(7): 256-262. |
| Chen Z, Zhou J L, Tao Y T, et al. Preparation of carvedilol solid dispersions by supercritical CO2 anti-solvent technology [J]. Chinese Journal of New Drugs, 2019, 28(7): 256-262. | |
| 32 | Škerget M, Ž Knez, Knez-Hrnčič M. Solubility of solids in sub- and supercritical fluids: a review[J]. Journal of Chemical and Engineering Data, 2011, 56(4): 694-719. |
| 33 | Zhang X, Heinonen S, Levänen E. Applications of supercritical carbon dioxide in materials processing and synthesis[J]. RSC Advances, 2014, 4(105): 61137-61152. |
| 34 | Frerich S C. Biopolymer foaming with supercritical CO2-thermodynamics, foaming behaviour and mechanical characteristics[J]. Journal of Supercritical Fluids, 2015, 96(1): 349-358. |
| 35 | Chauvet M, Sauceau M, Fages J. Extrusion assisted by supercritical CO2: a review on its application to biopolymers[J]. Journal of Supercritical Fluids, 2017, 120(2): 408-420. |
| 36 | Barros A A, Silva J, Craveiro R, et al. Green solvents for enhanced impregnation processes in biomedicine[J]. Current Opinion Green and Sustainable Chemistry, 2017, 5(6): 82-87. |
| 37 | Tsivintzelis I, Sanxaridou G, Pavlidou E, et al. Foaming of polymers with supercritical fluids: a thermodynamic investigation[J]. Journal of Supercritical Fluids, 2016, 110(4): 240-250. |
| 38 | Smith R D, Udseth H R. Mass spectrometry with direct supercritical fluid injection[J]. Analytical Chemistry, 1983, 55(14): 2266-2272. |
| 39 | Matson D W, Petersen R C, Smith R D. Production of powders and films by the rapid expansion of supercritical solutions[J]. Journal of Materials Science, 1987, 22(6): 1919-1928. |
| 40 | Pasquali I, Bettini R. Are pharmaceutics really going supercritical?[J]. International Journal of Pharmaceutics, 2008, 364(2): 176-187. |
| 41 | Türk M, Hils P, Helfgen B, et al. Micronization of pharmaceutical substances by the rapid expansion of supercritical solutions (RESS): a promising method to improve bioavailability of poorly soluble pharmaceutical agents[J]. Journal of Supercritical Fluids, 2002, 22(1): 75-84. |
| 42 | Paisana M C, Müllers K C, Wahl M A, et al. Production and stabilization of olanzapine nanoparticles by rapidexpansion of supercritical solutions (RESS)[J]. Journal of Supercritical Fluids, 2016, 109(3): 124-133. |
| 43 | Türk M. Manufacture of submicron drug particles with enhanced dissolution behaviour by rapid expansion processes[J]. Journal of Supercritical Fluids, 2009, 47(3): 537-545. |
| 44 | Debenedetti P G. Homogeneous nucleation in supercritical fluids[J]. AIChE Journal, 1990, 36(9): 1289-1298. |
| 45 | Pasquali I, Bettini R, Giordano F. Supercritical fluid technologies: an innovative approach for manipulating the solid-state of pharmaceuticals[J]. Advanced Drug Delivery Reviews, 2008, 60(3): 399-410. |
| 46 | Pando C, Cabañas A, Cuadra I A. Preparation of pharmaceutical co-crystals through sustainable processes using supercritical carbon dioxide: a review[J]. RSC Advances, 2016, 6(75): 71134-71150. |
| 47 | Wolff S, Beuermann S, Türk M. Impact of rapid expansion of supercritical solution process conditions on the crystallinity of poly(vinylidene fluoride) nanoparticles[J]. Journal of Supercritical Fluids, 2016, 117(11): 18-25. |
| 48 | Jiao Z, Wang X, Han S, et al. Preparation of vitamin C liposomes by rapid expansion of supercritical solution process: experiments and optimization[J]. Journal of Drug Delivery Science and Technology, 2019, 51(6): 1-6. |
| 49 | Satvati H R, Lotfollahi M N. Effects of extraction temperature, extraction pressure and nozzle diameter on micronization of cholesterol by RESS process[J]. Powder Technology, 2011, 210(2): 109-114. |
| 50 | Gholamhossein S, Ali S S, Sahar D. Preparation of aprepitant, nanoparticles (efficient drug for coping with the effects of cancer treatment) by rapid expansion of supercritical solution with solid cosolvent (RESS-SC)[J]. Journal of Supercritical Fluids, 2018, 140(10): 72-84. |
| 51 | Prosapio V, De Marco I, Reverchon E. Supercritical antisolvent coprecipitation mechanisms[J]. Journal of Supercritical Fluids, 2018, 138(8): 247-258. |
| 52 | Jin H, Hemingway M, Gupta R B, et al. Preparation of thalidomide nano-flakes by supercritical antisolvent with enhanced mass transfer[J]. Particuology, 2012, 10(1): 17-23. |
| 53 | Prosapio V, Reverchon E, De Marco I. Antisolvent micronization of BSA using supercritical mixtures carbon dioxide+organic solvent[J]. Journal of Supercritical Fluids, 2014, 94(10): 189-197. |
| 54 | Rodrigues M A, Li J, Padrela L, et al. Anti-solvent effect in the production of lysozyme nanoparticles by supercritical fluid-assisted atomization processes[J]. Journal of Supercritical Fluids, 2009, 48(3): 253-260. |
| 55 | US Food and Drug Administration (FDA), Generally Recognized as Safe (GRAS)[EB/OL].[2019-7-20]. . |
| 56 | International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), Harmonised Guideline ICH, Impurities: Guideline for Residual Solvents Q3C(R6)[EB/OL].[2019-7-20]. . |
| 57 | Duta Lestari S, Machmudah S, Winardi S, et al. Particle micronization of curcuma mangga rhizomes ethanolic extract/biopolymer PVP using supercritical antisolvent process[J]. Journal of Supercritical Fluids, 2019, 146(4): 226-239. |
| 58 | De Almeida M, Da Rocha B A, Francisco C R L, et al. Evaluation of the in vivo acute antiinflammatory response of curcumin-loaded nanoparticles[J]. Food & Function, 2018, 9(1): 440-449. |
| 59 | De Marco I, Knauer O, Cice F, et al. Interactions of phase equilibria, jet fluid dynamics and mass transfer during supercritical antisolvent micronization: the influence of solvents[J]. Chemical Engineering Journal, 2012, 203(9): 71-80. |
| 60 | Cuadra I A, Zahran F, Martín D, et al. Preparation of 5-fluorouracil microparticles and 5-fluorouracil/poly(L-lactide) composites by a supercritical CO2 antisolvent process[J]. Journal of Supercritical Fluids, 2019, 143(1): 64-71. |
| 61 | Campardelli R, Reverchon E. α-Tocopherol nanosuspensions produced using a supercritical assisted process[J]. Journal of Food Engineering, 2015, 149(3): 131-136. |
| 62 | Di Capua A, Adami R, Cosenza E, et al. β-Carotene/PVP microspheres produced by supercritical assisted atomization[J]. Powder Technology, 2019, 346(3): 228-236. |
| 63 | Peng H H, Hong D X, Guan Y X, et al. Preparation of pH-responsive DOX-loaded chitosan nanoparticles using supercritical assisted atomization with an enhanced mixer[J]. International Journal of Pharmaceutics, 2019, 558(3): 82-90. |
| 64 | Silva A S, Sousa A M, Cabral R P, et al. Aerosolizable gold nano-in-micro dry powder formulations for theragnosis and lung delivery[J]. International Journal of Pharmaceutics, 2017, 519(1/2): 240-249. |
| 65 | Reverchon E, Porta G D. Micronization of antibiotics by supercritical assisted atomization[J]. Journal of Supercritical Fluids, 2003, 26(3): 243-252. |
| 66 | Di Capua A, Adami R, Izzo L, et al. Luteolin/dextran-FITC fluorescent microspheres produced by supercritical assisted atomization[J]. Journal of Supercritical Fluids, 2017, 130(12): 97-104. |
| 67 | Reátegui J L P, Fernandes F P, Dos Santos P, et al. Production of copaiba (Copaifera officinalis) oleoresin particles by supercritical fluid extraction of emulsions[J]. Journal of Supercritical Fluids, 2018, 140(10): 364-371. |
| 68 | Aguiar A C D, Silva L P S, Rezende C A D, et al. Encapsulation of pepper oleoresin by supercritical fluid extraction of emulsions[J]. Journal of Supercritical Fluids, 2016, 112(6): 37-43. |
| 69 | Escobedo-Flores Y, Chavez-Flores D, Salmeron I, et al. Optimization of supercritical fluid extraction of polyphenols from oats (Avena sativa L.) and their antioxidant activities[J]. Journal of Cereal Science, 2018, 80(3): 198-204. |
| 70 | Guamán-Balcázar M C, Montesa A, Pereyra C, et al. Production of submicron particles of the antioxidants of mango leaves/PVP by supercritical antisolvent extraction process[J]. Journal of Supercritical Fluids, 2019, 143(1): 294-304. |
| 71 | Páulia M C, Reis L, Mezzomo N, et al. Ultrasound-assisted emulsion of laurel leaves essential oil (Laurus nobilis L.) encapsulated by SFEE[J]. Journal of Supercritical Fluids, 2019, 147(5): 284-292. |
| [1] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [2] | 刘杰, 吴立盛, 李锦锦, 罗正鸿, 周寅宁. 含乙烯基胺酯键聚醚类可逆交联聚合物的制备及性能研究[J]. 化工学报, 2023, 74(7): 3051-3057. |
| [3] | 龙臻, 王谨航, 任俊杰, 何勇, 周雪冰, 梁德青. 离子液体协同PVCap抑制天然气水合物生成实验研究[J]. 化工学报, 2023, 74(6): 2639-2646. |
| [4] | 杨琴, 秦传鉴, 李明梓, 杨文晶, 赵卫杰, 刘虎. 用于柔性传感的双形状记忆MXene基水凝胶的制备及性能研究[J]. 化工学报, 2023, 74(6): 2699-2707. |
| [5] | 陈韶云, 徐东, 陈龙, 张禹, 张远方, 尤庆亮, 胡成龙, 陈建. 单层聚苯胺微球阵列结构的制备及其吸附性能[J]. 化工学报, 2023, 74(5): 2228-2238. |
| [6] | 张建华, 陈萌萌, 孙雅雯, 彭永臻. 部分短程硝化同步除磷耦合Anammox实现生活污水高效脱氮除磷[J]. 化工学报, 2023, 74(5): 2147-2156. |
| [7] | 姚晓宇, 沈俊, 李健, 李振兴, 康慧芳, 唐博, 董学强, 公茂琼. 流体气液临界参数测量方法研究进展[J]. 化工学报, 2023, 74(5): 1847-1861. |
| [8] | 罗来明, 张劲, 郭志斌, 王海宁, 卢善富, 相艳. 1~5 kW高温聚合物电解质膜燃料电池堆的理论模拟与组装测试[J]. 化工学报, 2023, 74(4): 1724-1734. |
| [9] | 龙臻, 王谨航, 何勇, 梁德青. 离子液体与动力学抑制剂作用下混合气体水合物生成特性研究[J]. 化工学报, 2023, 74(4): 1703-1711. |
| [10] | 吴学红, 栾林林, 陈亚南, 赵敏, 吕财, 刘勇. 可降解柔性相变薄膜的制备及其热性能[J]. 化工学报, 2023, 74(4): 1818-1826. |
| [11] | 吕阳光, 左培培, 杨正金, 徐铜文. 三嗪框架聚合物膜用于有机纳滤甲醇/正己烷分离[J]. 化工学报, 2023, 74(4): 1598-1606. |
| [12] | 刘海芹, 李博文, 凌喆, 刘亮, 俞娟, 范一民, 勇强. 羟基-炔点击化学改性半乳甘露聚糖薄膜的制备及性能研究[J]. 化工学报, 2023, 74(3): 1370-1378. |
| [13] | 徐东, 田杜, 陈龙, 张禹, 尤庆亮, 胡成龙, 陈韶云, 陈建. 聚苯胺/二氧化锰/聚吡咯复合纳米球的制备及其电化学储能性[J]. 化工学报, 2023, 74(3): 1379-1389. |
| [14] | 刘润竹, 储甜甜, 张孝阿, 王成忠, 张军营. α,ω-端羟基亚苯基氟硅聚合物的合成及性能[J]. 化工学报, 2023, 74(3): 1360-1369. |
| [15] | 胡月, 马守骏, 蹇锡高, 翁志焕. 新型聚芳醚腈固化邻苯二甲腈树脂的研究[J]. 化工学报, 2023, 74(2): 871-882. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号