化工学报 ›› 2020, Vol. 71 ›› Issue (4): 1646-1656.DOI: 10.11949/0438-1157.20191050
焦昭杰1( ),陈立功1,柳云骐2,张贤明1,龚海峰1,高旭3(
),陈立功1,柳云骐2,张贤明1,龚海峰1,高旭3( )
)
收稿日期:2019-09-23
修回日期:2019-12-09
出版日期:2020-04-05
发布日期:2020-04-05
通讯作者:
高旭
作者简介:焦昭杰(1981—),男,博士,助理研究员,基金资助:
Zhaojie JIAO1( ),Ligong CHEN1,Yunqi LIU2,Xianming ZHANG1,Haifeng GONG1,Xu GAO3(
),Ligong CHEN1,Yunqi LIU2,Xianming ZHANG1,Haifeng GONG1,Xu GAO3( )
)
Received:2019-09-23
Revised:2019-12-09
Online:2020-04-05
Published:2020-04-05
Contact:
Xu GAO
摘要:
为解决Fenton法存在活性组分流失及通常在pH 2~3条件下运行的局限性,采用柠檬酸络合法制备了CuCe氧化物催化剂,建立了双酚A非均相催化湿式过氧化氢氧化(CWPO)反应体系。考察了焙烧温度、Cu/Ce摩尔比、H2O2用量、双酚A初始浓度和pH对催化剂物化结构和CWPO性能的影响。并分析了可能的降解路径。结果表明:催化剂具有良好的高温稳定性和pH适应性,在pH 1.6~7.9范围内对双酚A都具有较高的降解性能,不需要调节pH。在焙烧温度450℃、Cu/Ce摩尔比1.0、催化剂用量1 g·L-1、H2O2用量196 mmol·L-1、BPA浓度152 mg·L-1、pH 6.6、反应温度75℃、反应95 min后,BPA和TOC去除率分别为91.8%和84.5%,Cu2+析出浓度为19.3 mg·L-1。推测了双酚A可能的降解路径。
中图分类号:
焦昭杰, 陈立功, 柳云骐, 张贤明, 龚海峰, 高旭. CuCe氧化物催化剂的制备及CWPO降解双酚A废水研究[J]. 化工学报, 2020, 71(4): 1646-1656.
Zhaojie JIAO, Ligong CHEN, Yunqi LIU, Xianming ZHANG, Haifeng GONG, Xu GAO. Preparation of CuCe oxide catalyst for CWPO degradation of bisphenol A[J]. CIESC Journal, 2020, 71(4): 1646-1656.
CuCe 催化剂 | 结晶度①/% | 平均晶粒尺寸/nm | 晶胞参数 | ||
|---|---|---|---|---|---|
| a (α=90°)/? | b (β=90°)/? | c (γ=90°)/? | |||
| CC450 | 42.55 | 8.0 | 5.4045 | 5.4001 | 5.3241 |
| CC550 | 45.43 | 9.6 | 5.4054 | 5.4219 | 5.4065 |
| CC650 | 62.17 | 17.9 | 5.4032 | 5.4111 | 5.4071 |
| CC750 | 63.14 | 32.5 | 5.4095 | 5.4096 | 5.4096 |
| Cu2Ce1 | 63.81 | 20.7 | 5.3988 | 5.4100 | 5.4003 |
| Cu1Ce2 | 56.57 | 15.8 | 5.4031 | 5.4167 | 5.4085 |
| Cu1Ce3 | 52.58 | 12.5 | 5.4097 | 5.4133 | 5.4081 |
表1 制得的CuCe氧化物催化剂参数
Table 1 Parameters of CuCe oxide catalysts
CuCe 催化剂 | 结晶度①/% | 平均晶粒尺寸/nm | 晶胞参数 | ||
|---|---|---|---|---|---|
| a (α=90°)/? | b (β=90°)/? | c (γ=90°)/? | |||
| CC450 | 42.55 | 8.0 | 5.4045 | 5.4001 | 5.3241 |
| CC550 | 45.43 | 9.6 | 5.4054 | 5.4219 | 5.4065 |
| CC650 | 62.17 | 17.9 | 5.4032 | 5.4111 | 5.4071 |
| CC750 | 63.14 | 32.5 | 5.4095 | 5.4096 | 5.4096 |
| Cu2Ce1 | 63.81 | 20.7 | 5.3988 | 5.4100 | 5.4003 |
| Cu1Ce2 | 56.57 | 15.8 | 5.4031 | 5.4167 | 5.4085 |
| Cu1Ce3 | 52.58 | 12.5 | 5.4097 | 5.4133 | 5.4081 |
| CuCe催化剂 | 晶格氧/% | 吸附氧/% | 羟基氧/% | Ce/%(质量分数) | Cu/%(质量分数) | O/%(质量分数) |
|---|---|---|---|---|---|---|
| Cu1Ce3 | 62.71 | 28.04 | 9.25 | 23.68 | 8.92 | 67.39 |
| Cu1Ce2 | 69.16 | 18.07 | 12.77 | 25.22 | 8.49 | 66.30 |
| Cu2Ce1 | 64.10 | 22.75 | 13.14 | 2.44 | 18.90 | 78.67 |
| CC450 | 68.32 | 20.00 | 11.69 | 23.54 | 12.02 | 64.44 |
| CC550 | 66.44 | 20.56 | 13.00 | 21.62 | 12.83 | 65.55 |
| CC650 | 65.49 | 20.38 | 14.13 | 19.75 | 13.95 | 66.31 |
| CC750 | 68.73 | 18.47 | 12.80 | 19.78 | 14.14 | 66.08 |
表2 制备的CuCe氧化物催化剂XPS参数
Table 2 XPS parameters of CuCe oxide catalysts
| CuCe催化剂 | 晶格氧/% | 吸附氧/% | 羟基氧/% | Ce/%(质量分数) | Cu/%(质量分数) | O/%(质量分数) |
|---|---|---|---|---|---|---|
| Cu1Ce3 | 62.71 | 28.04 | 9.25 | 23.68 | 8.92 | 67.39 |
| Cu1Ce2 | 69.16 | 18.07 | 12.77 | 25.22 | 8.49 | 66.30 |
| Cu2Ce1 | 64.10 | 22.75 | 13.14 | 2.44 | 18.90 | 78.67 |
| CC450 | 68.32 | 20.00 | 11.69 | 23.54 | 12.02 | 64.44 |
| CC550 | 66.44 | 20.56 | 13.00 | 21.62 | 12.83 | 65.55 |
| CC650 | 65.49 | 20.38 | 14.13 | 19.75 | 13.95 | 66.31 |
| CC750 | 68.73 | 18.47 | 12.80 | 19.78 | 14.14 | 66.08 |
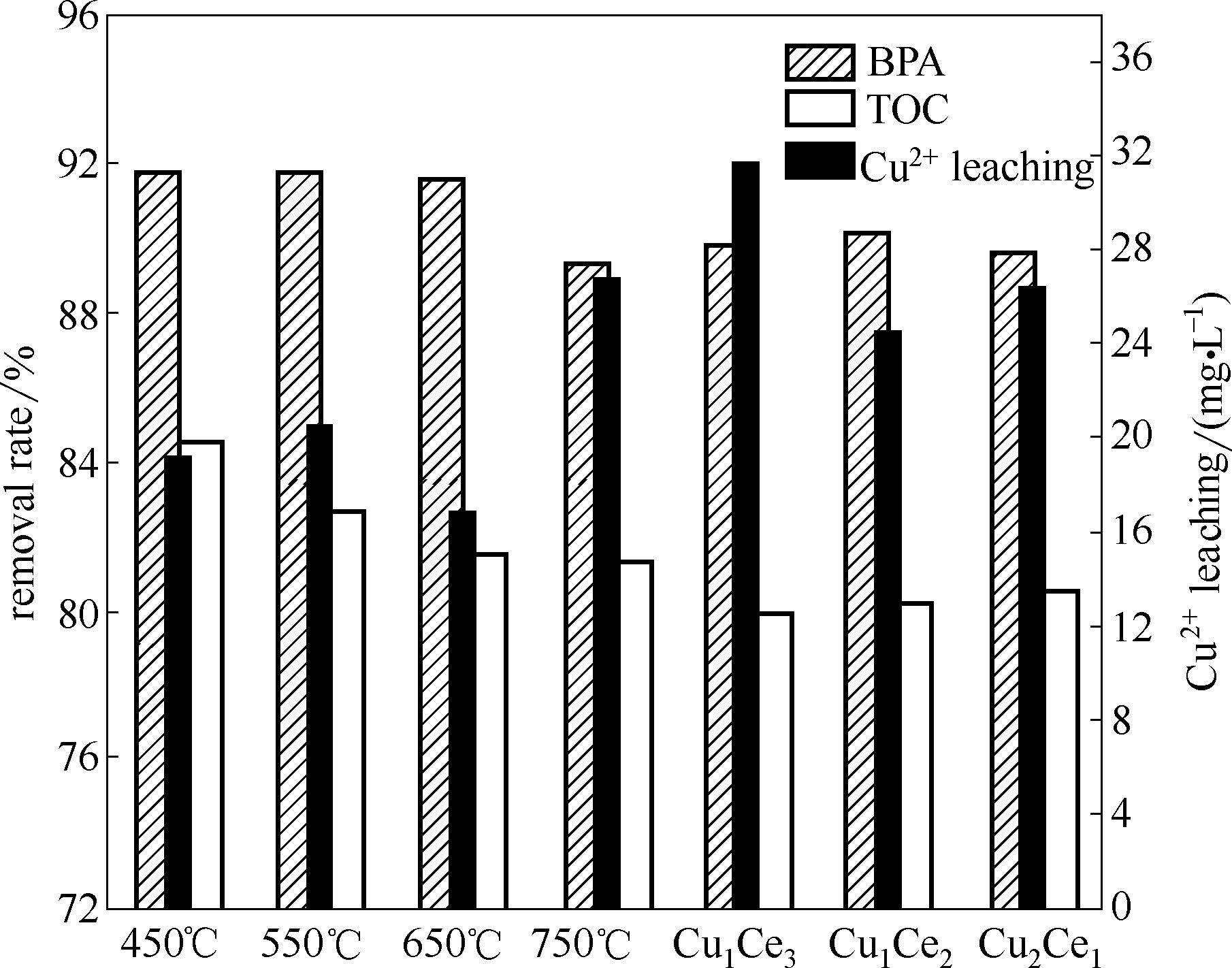
图5 制备条件对CWPO性能的影响
Fig.5 Effects of preparations on heterogeneous CWPO performance of bisphenol A (催化剂=1 g·L-1, BPA=152 mg·L-1, H2O2=196 mmol·L-1, pH=6.6, t=95 min, T=75℃)
| Compound | Molecular weight | Tentative structure |
|---|---|---|
| benzaldehyde, 4-ethyl- | 134 |  |
| p-benzoquinone | 108 |  |
| phenol | 94 |  |
| styrene | 104 |  |
| p-isopropenylphenol | 134 |  |
| p-xylene | 106 |  |
| p-hydroxytoluene | 108 |  |
| o-cresol | 108 |  |
| phenol,-2,4-bis(1,1-dimethylethyl)- | 206 |  |
| phenol, 2,5-bis(1,1-dimethylethyl)- | 206 |  |
表3 BPA降解过程中可能的主要中间产物
Table 3 Possible major intermediates in BPA degradation process
| Compound | Molecular weight | Tentative structure |
|---|---|---|
| benzaldehyde, 4-ethyl- | 134 |  |
| p-benzoquinone | 108 |  |
| phenol | 94 |  |
| styrene | 104 |  |
| p-isopropenylphenol | 134 |  |
| p-xylene | 106 |  |
| p-hydroxytoluene | 108 |  |
| o-cresol | 108 |  |
| phenol,-2,4-bis(1,1-dimethylethyl)- | 206 |  |
| phenol, 2,5-bis(1,1-dimethylethyl)- | 206 |  |
| 34 | 王光毅, 李金钗. CuO纳米线的热氧化制备及其表面的ZnO纳米颗粒修饰[J]. 武汉大学学报(理学版), 2011, 57(3): 220-224. |
| Wang G Y, Li J C. Syntheses of CuO nanowires by thermal oxidation and surface modification by ZnO nanoparticles[J]. Journal of Wuhan University (Natural Science Edition), 2011, 57(3): 220-224. | |
| 35 | Yao H C, Yao Y Y F. Ceria in automotive exhaust catalysts (Ⅰ): Oxygen storage[J]. Journal of Catalysis, 1984, 86: 254-265. |
| 36 | Zeng J, Zhou G L, Ai Y M, et al. Catalytic wet peroxide oxidation of chlorophenol over a Ce0.86Cu0.14-xO2 catalyst[J]. International Journal of Chemical Reactor Engineering, 2013, 11(1): 1-9. |
| 37 | He C, Yu Y C, Chen C W, et al. Facile preparation of 3D ordered mesoporous CuOx-CeO2 with notably enhanced efficiency for the low temperature oxidation of heteroatom-containing volatile organic compounds[J]. RSC Advances, 2013, 3: 19639-19656. |
| 38 | Zhu J K, Gao Q M, Chen Z. Preparation of mesoporous copper cerium bimetal oxides with high performance for catalytic oxidation of carbon monoxide[J]. Applied Catalysis B: Environmental, 2008, 81(2): 236-243. |
| 39 | Zeng S H, Wang Y, Liu K W, et al. CeO2 nanoparticles supported on CuO with petal-like and sphere-flower morphologies for preferential CO oxidation[J]. International Journal of Hydrogen Energy, 2012, 37(16): 11640-11649. |
| 40 | Song Z X, Ning P, Zhang Q L, et al. The role of surface properties of silicotungstic acid doped CeO2 for selective catalytic reduction of NOx by NH3: effect of precipitant[J]. Journal of Molecular Catalysis A: Chemical, 2016, 413: 15-23. |
| 41 | Dai Q G, Huang H, Zhu Y, et al. Catalysis oxidation of 1, 2-dichloroethane and ethyl acetate over ceria nanocrystals with well-defined crystal planes[J]. Applied Catalysis B: Environmental, 2012, 117/118: 360-368. |
| 42 | Sun M, Yu L, Yu J, et al. Catalytic combustion of dimethyl ether over Ce-doped cryptomelane type manganese oxide[J]. Journal of Fuel Chemistry and Technology, 2010, 38(1): 108-115. |
| 43 | Huang M, Xu C, Wu Z, et al. Photocatalytic discolorization of methyl orange solution by Pt modified TiO2 loaded on natural zeolite[J]. Dyes and Pigments, 2008, 77(2): 327-334. |
| 1 | Bhatnagar A, Anastopoulos I. Adsorptive removal of bisphenol A (BPA) from aqueous solution: a review[J]. Chemosphere, 2017, 168: 885-902. |
| 2 | Im J, Ffler F L. Fate of bisphenol A in terrestrial and aquatic environments[J]. Environmental Science & Technology, 2016, 16(50): 8403-8416. |
| 44 | Chen W J, Zou C J, Liu Y, et al. The experimental investigation of bisphenol A degradation by Fenton process with different types of cyclodextrins[J]. Journal of Industrial and Engineering Chemistry, 2017, 56: 428-434. |
| 3 | Vom Saal F S, Nagel S C, Coe B L, et al. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity[J]. Molecular and Cellular Endocrinology, 2012, 354(1/2): 74-84. |
| 4 | Wang H, Ding Z, Shi Q M, et al. Anti-androgenic mechanisms of bisphenol A involve androgen receptor signaling pathway[J]. Toxicology, 2017, 387: 10-16. |
| 5 | Rodriguez-Mozaz S, López De Alda M J, Barceló D. Monitoring of estrogens, pesticides and bisphenol A in natural waters and drinking water treatment plants by solid-phase extraction–liquid chromatography-mass spectrometry[J]. Journal of Chromatography A, 2004, 1045(1/2): 85-92. |
| 6 | Yu L, Wang C P, Ren X H, et al. Catalytic oxidative degradation of bisphenol A using an ultrasonic-assisted tourmaline-based system: influence factors and mechanism study[J]. Chemical Engineering Journal, 2014, 252: 346-354. |
| 7 | Flint S, Markle T, Thompson S, et al. Bisphenol A exposure, effects, and policy: a wildlife perspective[J]. Journal of Environmental Management, 2012, 104: 19-34. |
| 8 | Fang L, Hong R, Gao J, et al. Degradation of bisphenol A by nano-sized manganese dioxide synthesized using montmorillonite as templates[J]. Applied Clay Science, 2016, 132/133: 155-160. |
| 9 | Mercogliano R, Santonicola S. Investigation on bisphenol A levels in human milk and dairy supply chain: a review[J]. Food and Chemical Toxicology, 2018, 114: 98-107. |
| 10 | Heidari H, Sedighi M, Zamir S M, et al. Bisphenol A degradation by Ralstonia eutropha in the absence and presence of phenol[J]. International Biodeterioration & Biodegradation, 2017, 119: 37-42. |
| 11 | Yang X J, Tian P F, Zhang C X, et al. Au/carbon as Fenton-like catalysts for the oxidative degradation of bisphenol A[J]. Applied Catalysis B: Environmental, 2013, 134(5): 145-152. |
| 12 | Noszczyńska M, Piotrowska-Seget Z. Bisphenols: application, occurrence, safety, and biodegradation mediated by bacterial communities in wastewater treatment plants and rivers[J]. Chemosphere, 2018, 201: 214-223. |
| 13 | Vijayalakshmi V, Senthilkumar P, Mophin-Kani K, et al. Bio-degradation of bisphenol A by Pseudomonas aeruginosa PAb1 isolated from effluent of thermal paper industry: kinetic modeling and process optimization[J]. Journal of Radiation Research and Applied Sciences, 2018, 11(1): 56-65. |
| 14 | Žerjav G, Kaplan R, Pintar A. Catalytic wet air oxidation of bisphenol A aqueous solution in trickle-bed reactor over single TiO2 polymorphs and their mixtures[J]. Journal of Environmental Chemical Engineering, 2018, 6(2): 2148-2158. |
| 15 | Kaplan R, Erjavec B, Senila M, et al. Catalytic wet air oxidation of bisphenol A solution in a batch-recycle trickle-bed reactor over titanate nanotube-based catalysts[J]. Environmental Science and Pollution Research, 2014, 21(19): 11313-11319. |
| 16 | Mena I F, Diaz E, Rodriguez J J, et al. CWPO of bisphenol A with iron catalysts supported on microporous carbons from grape seeds activation[J]. Chemical Engineering Journal, 2017, 318: 153-160. |
| 17 | Yang S S, Wu P X, Yang Q L, et al. Regeneration of iron-montmorillonite adsorbent as an efficient heterogeneous Fenton catalytic for degradation of bisphenol A: structure, performance and mechanism[J]. Chemical Engineering Journal, 2017, 328: 737-747. |
| 18 | Cleveland V, Bingham J, Kan E. Heterogeneous Fenton degradation of bisphenol A by carbon nanotube-supported Fe3O4[J]. Separation and Purification Technology, 2014, 133: 388-395. |
| 19 | Zhang L L, Xu D, Hu C, et al. Framework Cu-doped AlPO4 as an effective Fenton-like catalyst for bisphenol A degradation[J]. Applied Catalysis B: Environmental, 2017, 207: 9-16. |
| 20 | Chang Z, Zhao N, Liu J F, et al. Cu-Ce-O mixed oxides from Ce-containing layered double hydroxide precursors: controllable preparation and catalytic performance[J]. Journal of Solid State Chemistry, 2011, 184(12): 3232-3239. |
| 21 | Pachamuthu M P, Karthikeyan S, Maheswari R, et al. Fenton-like degradation of bisphenol A catalyzed by mesoporous Cu/TUD-1[J]. Applied Surface Science, 2017, 393: 67-73. |
| 22 | Zhang X Y, Ding Y B, Tang H Q, et al. Degradation of bisphenol A by hydrogen peroxide activated with CuFeO2 microparticles as a heterogeneous Fenton-like catalyst: efficiency, stability and mechanism[J]. Chemical Engineering Journal, 2014, 236: 251-262. |
| 23 | Abdullah W N W, Bakar W A W A, Ali R, et al. Catalytic oxidative desulfurization technology of supported ceria based catalyst: physicochemical and mechanistic studies[J]. Journal of Cleaner Production, 2017, 162: 1455-1464. |
| 24 | Zhao B X, Shi B C, Zhang X L, et al. Catalytic wet hydrogen peroxide oxidation of H-acid in aqueous solution with TiO2-CeO2 and Fe/TiO2-CeO2 catalysts[J]. Desalination, 2011, 268(1/2/3): 55-59. |
| 25 | 谢鹏, 聂天明, 邓黎丹, 等. 新型铜铈复合材料的制备及其脱硝性能的研究[J]. 华中师范大学学报(自然科学版), 2017, 51(3): 317-323. |
| Xie P, Nie T M, Deng L D, et al. Preparation of novel Cu-Ce composited material and its performance of de NOx[J]. Journal of Huazhong Normal University(Natural Sciences), 2017, 51(3): 317-323. | |
| 26 | Hossain S T, Azeeva E, Zhang K, et al. A comparative study of CO oxidation over Cu-O-Ce solid solutions and CuO/CeO2 nanorods catalysts[J]. Applied Surface Science, 2018, 455: 132-143. |
| 27 | Zedan A F, Polychronopoulou K, Asif A, et al. Cu-Ce-O catalyst revisited for exceptional activity at low temperature CO oxidation reaction[J]. Surface and Coatings Technology, 2018, 354: 313-323. |
| 28 | Liu Z, He C, Chen B, et al. CuO-CeO2 mixed oxide catalyst for the catalytic decomposition of N2O in the presence of oxygen[J]. Catalysis Today, 2017, 297: 78-83. |
| 29 | 甘蓉丽, 罗光前, 许洋, 等. 低温等离子体协同铜铈催化剂脱除甲苯[J]. 化工进展, 2018, 37(9): 3416-3423. |
| Gan R L, Luo G Q, Xu Y, et al. Removal of toluene by non-thermal plasma coupled with Cu-Ce catalysts[J]. Chemical Industry and Engineering Progress, 2018, 37(9): 3416-3423. | |
| 30 | 单文娟.铜-铈固溶体催化剂的制备、表征及甲醇氧化重整制氢的研究[D].大连: 中国科学院大连化学物理研究所, 2004. |
| Shan W J. The preparation and characterization of Ce-Cu-O solid solution catalyst and the study on oxidative steam reforming of methanol[D]. Dalian: Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 2004. | |
| 31 | Liu Z G, Zhou R X, Zheng X M. Comparative study of different methods of preparing CuO-CeO2 catalysts for preferential oxidation of CO in excess hydrogen[J]. Journal of Molecular Catalysis A: Chemical, 2007, 267(1/2): 137-142. |
| 32 | 王桂燕, 夏笠, 迂君. 有机硅功能化碳纳米球: 合成及可见光催化活性[J]. 无机化学学报, 2017, 33(2): 299-306. |
| Wang G Y, Xia L, Yu J. Organosilane-functionalized carbon nanospheres: synthesis and visible light photocatalytic activity[J]. Chinese Journal of Inorganic Chemistry, 2017, 33(2): 299-306. | |
| 33 | 曹亚亚, 黄少斌, 尹佳芝. 不同煅烧温度制备的n-p型CeO2/BiOBr光催化性能研究[J]. 分子催化, 2016, 30(2): 159-168. |
| Cao Y Y, Huang S B, Yin J Z. Study on the photocatalytic activities of n-p type CeO2/BiOBr composite prepared at different calcination temperatures[J]. Journal of Molecular Catalysis(China), 2016, 30(2): 159-168. |
| [1] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [2] | 李瑞康, 何盈盈, 卢维鹏, 王园园, 丁皓东, 骆勇名. 电化学强化钴基阴极活化过一硫酸盐的研究[J]. 化工学报, 2023, 74(5): 2207-2216. |
| [3] | 吴学红, 栾林林, 陈亚南, 赵敏, 吕财, 刘勇. 可降解柔性相变薄膜的制备及其热性能[J]. 化工学报, 2023, 74(4): 1818-1826. |
| [4] | 谢煜, 张民, 胡卫国, 王玉军, 骆广生. 利用膜分散微反应器高效溶解D-7-ACA的研究[J]. 化工学报, 2023, 74(2): 748-755. |
| [5] | 李彩风, 王晓, 李岗建, 林军章, 汪卫东, 束青林, 曹嫣镔, 肖盟. 嗜烃乳化菌SL-1与内源菌协同驱油的菌群作用关系研究[J]. 化工学报, 2022, 73(9): 4095-4102. |
| [6] | 靳文章, 张玉玲, 贾晓宇. 电化学高级氧化对HEDP的降解效能研究[J]. 化工学报, 2022, 73(9): 4062-4069. |
| [7] | 许贤伦, 钱旸, 张兴旺, 雷乐成. 高压脉冲介质阻挡放电降解土壤中芘的研究[J]. 化工学报, 2022, 73(9): 4025-4033. |
| [8] | 徐振和, 李泓江, 高雨, 礼峥, 张含烟, 徐宝彤, 丁茯, 孙亚光. In2O3/Ag:ZnIn2S4“Type Ⅱ”型异质结构材料的制备及可见光催化性能[J]. 化工学报, 2022, 73(8): 3625-3635. |
| [9] | 黄仕元, 邓简, 袁瀚钦, 王国华, 吴兴良. 钴强化铁磁体活化过一硫酸盐的实验研究[J]. 化工学报, 2022, 73(7): 3045-3056. |
| [10] | 贾艳萍, 丁雪, 刚健, 佟泽为, 张海丰, 张兰河. Mn强化Fe/C微电解工艺条件优化及降解油墨废水机理[J]. 化工学报, 2022, 73(5): 2183-2193. |
| [11] | 韩雪, 高生旺, 王国英, 夏训峰. 铈掺杂强化碳纳米管活化过一硫酸盐实验研究[J]. 化工学报, 2022, 73(4): 1743-1753. |
| [12] | 万丽, 梁德青. 一种可生物降解水合物动力学抑制剂的研究[J]. 化工学报, 2022, 73(2): 894-903. |
| [13] | 侯晓松, 刘晨星, 任爱玲, 郭斌, 郭渊明. 超声雾化/表面活性剂强化吸收耦合生物洗涤净化甲苯废气[J]. 化工学报, 2022, 73(10): 4692-4706. |
| [14] | 朱振林, 王松林, 姜冰雪, 李家旭, 邓维, 吴海强, 杨轩, 刘平伟, 王文俊. 聚酯生物降解及评价方法研究[J]. 化工学报, 2022, 73(1): 110-121. |
| [15] | 黄莉婷, 韩昫身, 金艳, 马强, 于建国. 煤化工反渗透浓水的高效降解菌株筛选、鉴定及应用研究[J]. 化工学报, 2021, 72(9): 4881-4891. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号