化工学报 ›› 2020, Vol. 71 ›› Issue (5): 2352-2362.DOI: 10.11949/0438-1157.20191505
莫官海1( ),谢水波1,2,曾涛涛1(
),谢水波1,2,曾涛涛1( ),刘迎九1,蔡萍莉1
),刘迎九1,蔡萍莉1
收稿日期:2019-12-12
修回日期:2020-02-20
出版日期:2020-05-05
发布日期:2020-05-05
通讯作者:
曾涛涛
作者简介:莫官海(1994—),男,硕士研究生,基金资助:
Guanhai MO1( ),Shuibo XIE1,2,Taotao ZENG1(
),Shuibo XIE1,2,Taotao ZENG1( ),Yingjiu LIU1,Pingli CAI1
),Yingjiu LIU1,Pingli CAI1
Received:2019-12-12
Revised:2020-02-20
Online:2020-05-05
Published:2020-05-05
Contact:
Taotao ZENG
摘要:
通过城市污泥(SS)慢速热解制备污泥基生物炭(SSB),并研究初始pH、投加量、共存离子、吸附时间和温度等因素对SSB去除U(Ⅵ)的影响,探讨吸附动力学和吸附等温线特征。通过元素分析、扫描电镜(SEM)、傅里叶红外光谱(FTIR)、X射线衍射(XRD)和X射线光电子能谱(XPS)分析U(Ⅵ)吸附去除的机理。结果表明SSB去除U(Ⅵ)的适宜条件为:pH=3、投加量1 g/L、吸附时间240 min;在此条件下,在温度30℃时最大吸附量为34.51 mg/g。吸附动力学符合拟二级动力学模型;Langmuir吸附等温模型能更好描述生物炭对U(Ⅵ)的吸附行为。U(Ⅵ)吸附去除机理主要包括静电作用,与Si—O—Si的n-π相互作用,与羟基(—OH)、羧基(—COOH)的配位络合。通过5次吸附-解吸试验发现,U(Ⅵ)去除率和SSB再生率均在80%以上。本研究表明污泥基生物炭具备处理与修复酸性含U(Ⅵ)废水污染的潜力。
中图分类号:
莫官海, 谢水波, 曾涛涛, 刘迎九, 蔡萍莉. 污泥基生物炭处理酸性含U(Ⅵ)废水的效能与机理[J]. 化工学报, 2020, 71(5): 2352-2362.
Guanhai MO, Shuibo XIE, Taotao ZENG, Yingjiu LIU, Pingli CAI. The efficiency and mechanism of U(Ⅵ) removal from acidic wastewater by sewage sludge-derived biochar[J]. CIESC Journal, 2020, 71(5): 2352-2362.
| 理化性质 | SS | SSB |
|---|---|---|
| 产率/% | / | 77.95±1.47 |
| 灰分/% | 61.03±0.32 | 70.46±0.28 |
| 挥发分/% | 37.94±0.55 | 27.10±0.86 |
| pH | 6.09±0.21 | 7.75±0.14 |
| 微孔孔径/nm | 1.34 | 1.00 |
| 介孔孔径/nm | 27.10 | 16.60 |
| 孔体积/(cm3/g) | 0.12 | 0.36 |
| 比表面积/(m2/g) | 11.88 | 59.81 |
| C/% | 12.20 | 10.98 |
| H/% | 2.64 | 1.66 |
| N/% | 2.01 | 1.37 |
| O/% | 22.20 | 15.53 |
| H/C | 0.22 | 0.15 |
| O/C | 1.85 | 1.41 |
表1 污泥及污泥基生物炭的理化性质
Table 1 Physicochemical properties of sewage sludge and sewage sludge-derived biochar
| 理化性质 | SS | SSB |
|---|---|---|
| 产率/% | / | 77.95±1.47 |
| 灰分/% | 61.03±0.32 | 70.46±0.28 |
| 挥发分/% | 37.94±0.55 | 27.10±0.86 |
| pH | 6.09±0.21 | 7.75±0.14 |
| 微孔孔径/nm | 1.34 | 1.00 |
| 介孔孔径/nm | 27.10 | 16.60 |
| 孔体积/(cm3/g) | 0.12 | 0.36 |
| 比表面积/(m2/g) | 11.88 | 59.81 |
| C/% | 12.20 | 10.98 |
| H/% | 2.64 | 1.66 |
| N/% | 2.01 | 1.37 |
| O/% | 22.20 | 15.53 |
| H/C | 0.22 | 0.15 |
| O/C | 1.85 | 1.41 |
| 样品 | Ba | Cr | Cd | Cu | Pb | Zn |
|---|---|---|---|---|---|---|
| SS | 5.32 | 0.82 | 0.08 | 1.10 | 0.66 | 6.82 |
| SSB | 7.14 | 1.08 | 0.09 | 1.37 | 0.81 | 8.79 |
| 《危险废物鉴别标准 浸出毒性鉴别》(GB 5085.3—2007) | 100 | 15 | 1 | 100 | 5 | 100 |
表2 污泥及其生物炭中重金属含量/(mg/L)
Table 2 Heavy metal concentrations of sewage sludge and sewage sludge-derived biochar/(mg/L)
| 样品 | Ba | Cr | Cd | Cu | Pb | Zn |
|---|---|---|---|---|---|---|
| SS | 5.32 | 0.82 | 0.08 | 1.10 | 0.66 | 6.82 |
| SSB | 7.14 | 1.08 | 0.09 | 1.37 | 0.81 | 8.79 |
| 《危险废物鉴别标准 浸出毒性鉴别》(GB 5085.3—2007) | 100 | 15 | 1 | 100 | 5 | 100 |
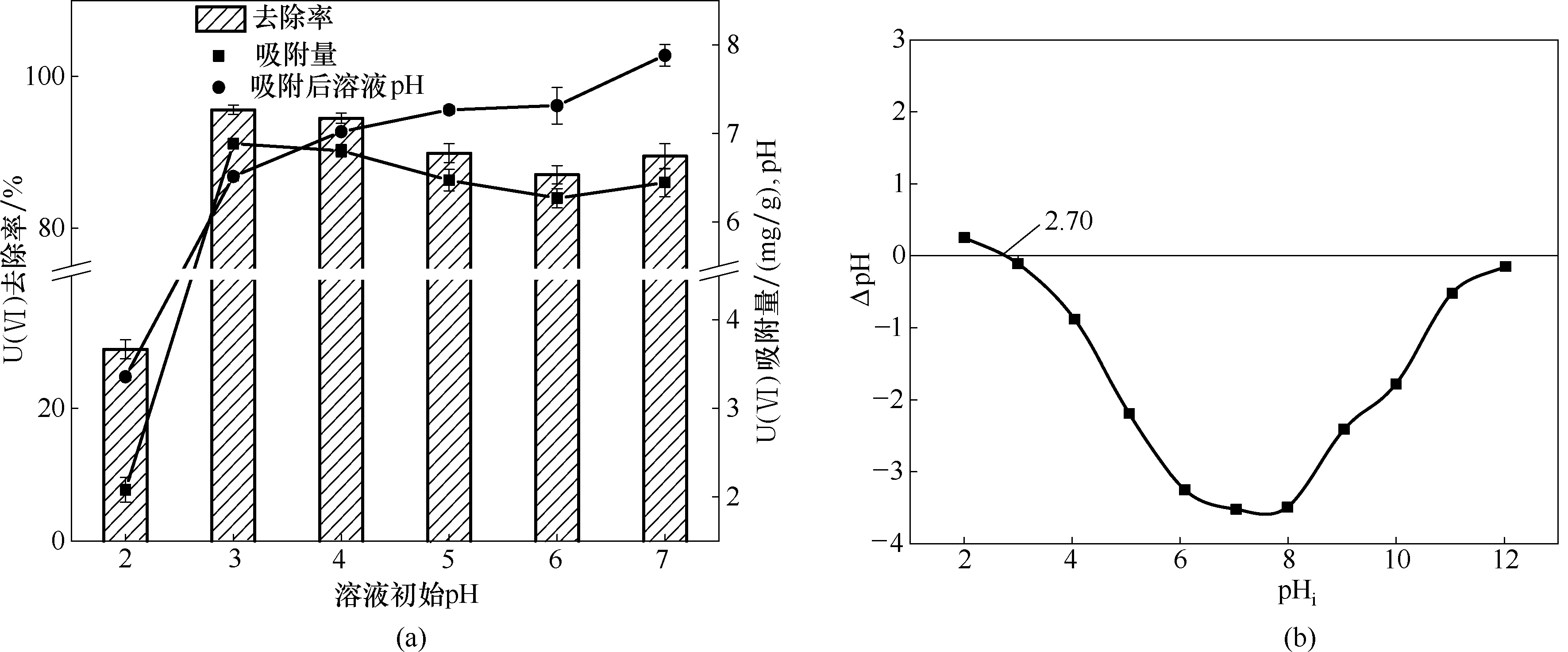
图2 初始pH对U(Ⅵ)吸附去除的影响(a)以及污泥基生物炭的零电势点(b)
Fig. 2 Effect of initial pH on U(Ⅵ) adsorption removal (a) and pH of point zero charge (pHPZC) of sewage sludge-derived biochar (b)
| 拟一级动力学方程 | 拟二级动力学方程 | ||||||
|---|---|---|---|---|---|---|---|
| qe/(mg/g) | k1/min-1 | R2 | qe/(mg/g) | k2/(g/(mg·min)) | R2 | ||
| 6.67 | 0.115 | 0.601 | 6.88 | 0.039 | 0.934 | ||
| 颗粒内扩散模型 | |||||||
| kd1/(mg/(g·min1/2)) | C1 | R2 | kd2/(mg/(g·min1/2)) | C2 | R2 | ||
| 0.165 | 4.951 | 0.956 | 0.004 | 6.726 | 0.966 | ||
表3 SSB对U(Ⅵ)的吸附动力学参数
Table 3 Kinetic parameters of U(Ⅵ) adsorption by SSB
| 拟一级动力学方程 | 拟二级动力学方程 | ||||||
|---|---|---|---|---|---|---|---|
| qe/(mg/g) | k1/min-1 | R2 | qe/(mg/g) | k2/(g/(mg·min)) | R2 | ||
| 6.67 | 0.115 | 0.601 | 6.88 | 0.039 | 0.934 | ||
| 颗粒内扩散模型 | |||||||
| kd1/(mg/(g·min1/2)) | C1 | R2 | kd2/(mg/(g·min1/2)) | C2 | R2 | ||
| 0.165 | 4.951 | 0.956 | 0.004 | 6.726 | 0.966 | ||
| 温度/℃ | Langmuir等温线方程 | Freundlich等温线方程 | ||||
|---|---|---|---|---|---|---|
| qmax/(mg/g) | Kb/ (L/mg) | R12 | Kf/ (mg1+n/(Ln·g)) | n | R22 | |
| 20 | 33.22 | 1.018 | 0.996 | 14.58 | 3.66 | 0.902 |
| 30 | 34.51 | 1.144 | 0.995 | 15.48 | 3.68 | 0.909 |
| 40 | 35.65 | 1.287 | 0.996 | 16.47 | 3.72 | 0.906 |
表4 SSB对U(Ⅵ)的吸附等温线拟合参数
Table 4 Isotherms parameters of U(Ⅵ) adsorption by SSB
| 温度/℃ | Langmuir等温线方程 | Freundlich等温线方程 | ||||
|---|---|---|---|---|---|---|
| qmax/(mg/g) | Kb/ (L/mg) | R12 | Kf/ (mg1+n/(Ln·g)) | n | R22 | |
| 20 | 33.22 | 1.018 | 0.996 | 14.58 | 3.66 | 0.902 |
| 30 | 34.51 | 1.144 | 0.995 | 15.48 | 3.68 | 0.909 |
| 40 | 35.65 | 1.287 | 0.996 | 16.47 | 3.72 | 0.906 |
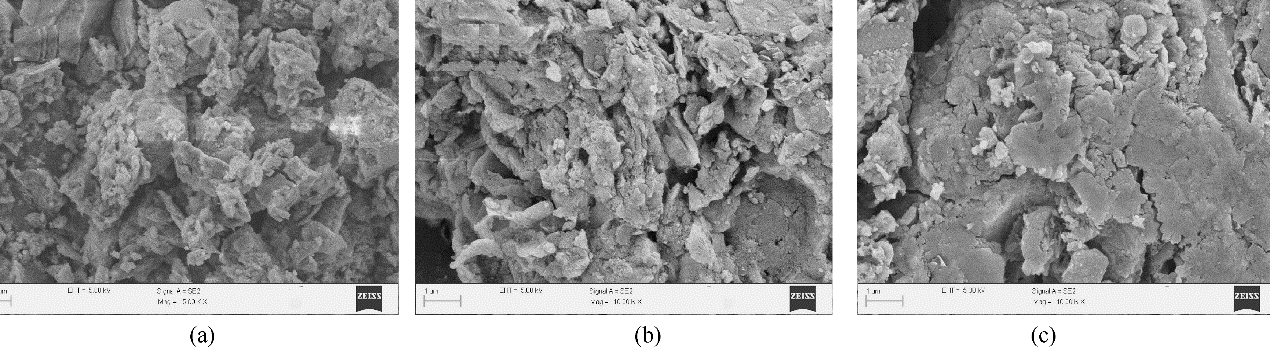
图7 干污泥(a)及污泥基生物炭吸附U(Ⅵ)前(b)、后(c)的微观形态结构
Fig.7 Micromorphology of sludge sewage (a), sludge sewage-derived biochar before (b) and after (c) U(Ⅵ) adsorption
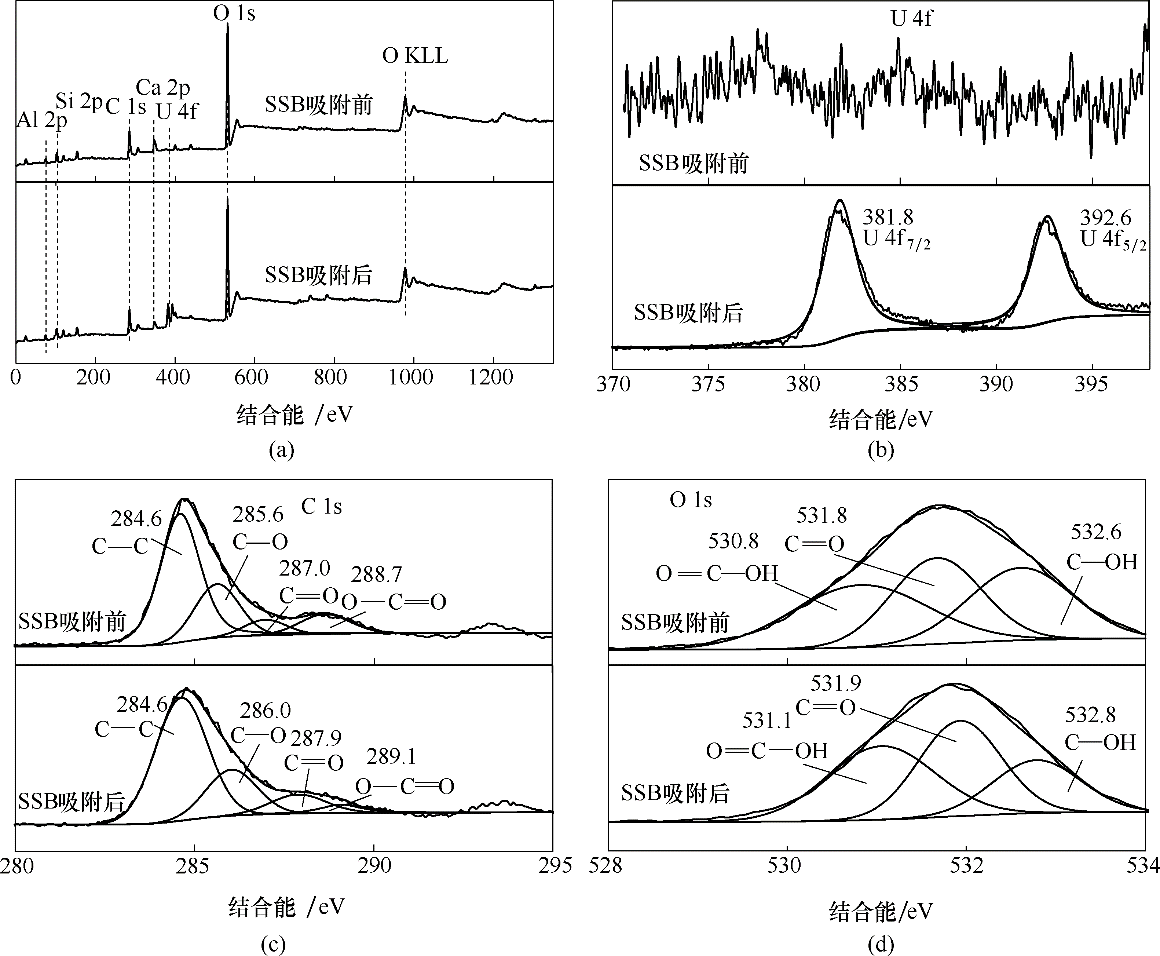
图9 污泥基生物炭吸附U(Ⅵ)前、后的XPS光谱图
Fig.9 XPS spectra of sewage sludge-derived biochar before and after U(Ⅵ) adsorption(a) overall spectra; (b) U 4f; (c) C 1s; (d) O 1s
| 1 | 郭栋清, 李静, 张利波, 等. 核工业含铀废水处理技术进展[J]. 工业水处理, 2019, 39(1): 22-28. |
| Guo D Q, Li J, Zhang L B, et al. Progress in the treatment technology of uranium-bearing wastewater in nuclear industry[J]. Industrial Water Treatment, 2019, 39(1): 22-28. | |
| 2 | 汪萍, 吕彩霞, 盛青, 等. 含铀废水处理技术的研究进展[J]. 现代化工, 2016, 36(12): 23-27. |
| Wang P, Lyu C X, Sheng Q, et al. Research development of uranium-containing wastewater treatment technologies[J]. Modern Chemical Industry, 2016, 36(12): 23-27. | |
| 3 | 张晨璐, 冷然, 张一阚, 等. 锰铝二元水滑石和锰铁铝三元水滑石对U(Ⅵ)的高效去除及其机理研究[J].中国科学: 化学, 2019, 49(1): 133-144. |
| Zhang C L, Leng R, Zhang Y K, et al. Interaction of U(Ⅵ) with MnAl and MnFeAl layered double hydroxides: batch and spectroscopy study[J]. Scientia Sinica Chimica, 2019, 49(1): 133-144. | |
| 4 | 梁宇, 顾鹏程, 姚文, 等. 碳基纳米材料对水环境中放射性元素铀的吸附[J]. 化学进展, 2017, 29(9): 1062-1071. |
| Liang Y, Gu P C, Yao W, et al. Adsorption of radionuclide uranium onto carbon-based nanomaterials from aqueous systems[J]. Progress of Chemistry, 2017, 29(9): 1062-1071. | |
| 5 | 刘红娟, 谢水波, 张希晨, 等. 氧化石墨烯复合材料吸附铀的研究进展[J]. 材料工程, 2018, 46(5): 11-21. |
| Liu H J, Xie S B, Zhang X C, et al. Research progress of graphene oxide composite materials for uranium adsorption[J]. Journal of Materials Engineering, 2018, 46(5): 11-21. | |
| 6 | Mishra V, Sureshkumar M K, Gupta N, et al. Study on sorption characteristics of uranium onto biochar derived from eucalyptus wood[J]. Water Air & Soil Pollution, 2017, 228(8): 309-323. |
| 7 | Hu H, Zhang X, Wang T, et al. Bamboo (Acidosasa longiligula) shoot shell biochar: its potential application to isolation of uranium(Ⅵ) from aqueous solution[J]. Journal of Radioanalytical & Nuclear Chemistry, 2018, 11: 1-14. |
| 8 | Jin J, Li S W, Peng X Q, et al. HNO3 modified biochars for uranium(Ⅵ) removal from aqueous solution[J]. Bioresource Technology, 2018, 256: 247-253. |
| 9 | Alam M S, Gorman-Lewis D, Chen N, et al. Mechanisms of the removal of U(Ⅵ) from aqueous solution using biochar: a combined spectroscopic and modeling approach[J]. Environmental Science & Technology, 2018, 52(55): 13057-13067. |
| 10 | Chen T, Zhou Z, Han R, et al. Adsorption of cadmium by biochar derived from municipal sewage sludge: impact factors and adsorption mechanism[J]. Chemosphere, 2015, 134: 286-293. |
| 11 | Wang S J, Guo W, Gao F, et al. Characterization and Pb(Ⅱ) removal potential of corn straw- and municipal sludge-derived biochars[J]. Royal Society Open Science, 2017, 4(9): 170402. |
| 12 | Nguyen N T, Lee S Y, Chen S S, et al. Preparation of Zn-doped biochar from sewage sludge for chromium ion removal[J]. Journal of Nanoscience and Nanotechnology, 2018, 18: 5520-5527. |
| 13 | Zhang W H, Zheng J, Zheng P P, et al. Sludge-derived biochar for arsenic(Ⅲ) immobilization: effects of solution chemistry on sorption behavior[J]. Journal of Environmental Quality, 2015, 44: 1119-1126. |
| 14 | 郜礼阳, 邓金环, 唐国强, 等. 不同温度桉树叶生物炭对Cd2+的吸附特性及机制[J]. 中国环境科学, 2018, 38(3): 1001-1009. |
| Gao L Y, Deng J H, Tang G Q, et al. Adsorption characteristics and mechanism of Cd2+ on biochar with different pyrolysis temperatures produced from eucalyptus leaves[J]. China Environmental Science, 2018, 38(3): 1001-1009. | |
| 15 | 木质活性炭试验方法 灰分含量的测定: GB/T 12496.3—1999[S]. 北京: 中国标准出版社, 1999. |
| Test methods of wooden activated carbon—determination of ash content: GB/T 12496.3—1999[S]. Beijing: Standards Press of China, 1999. | |
| 16 | 焦炭工业分析测定方法 挥发分含量的测定: GB/T 2001—91[S]. 北京: 中国标准出版社, 2001. |
| Coke determination of proximate analysis—determination of volatile components: GB/T 2001—91[S]. Beijing: Standards Press of China, 2001. | |
| 17 | Jin J W, Li Y A, Zhang J Y, et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge[J]. Journal of Hazardous Materials, 2016, 320: 417-426. |
| 18 | 危险废物鉴别标准 浸出毒性鉴别: GB 5085.3—2007 [S]. 北京: 中国环境科学出版社, 2007. |
| Identification standards for hazardous waste—identification for extraction toxicity: GB 5085.3—2007[S]. Beijing: China Environmental Science Press, 2007. | |
| 19 | 高凯芳, 简敏菲, 余厚平, 等. 裂解温度对稻杆和稻壳制备生物炭表面官能团的影响[J]. 环境化学, 2016, 35(8): 1663-1669. |
| Gao K F, Jian M F, Yu H P, et al. Effects of pyrolysis temperatures on the biochars and its surface functional groups made from rice straw and rice husk[J]. Environmental Chemistry, 2016, 35(8): 1663-1669. | |
| 20 | Ofomaja A E, Naidoo E B, Modise S J. Removal of copper(Ⅱ) from aqueous solution by pine and base modified pine cone powder as biosorbent[J]. Journal of Hazardous Materials, 2009, 168: 909-917. |
| 21 | Zhang H Y, Yue X P, Li F, et al. Preparation of rice straw-derived biochar for efficient cadmium removal by modification of oxygen-containing functional groups[J]. Science of the Total Environment, 2018, 631/632: 795-802. |
| 22 | 谢华, 苏伟, 王烈林, 等. 地质水泥对U(Ⅵ)的吸附行为及其机理研究[J]. 原子能科学技术, 2018, 52(3): 390-396. |
| Xie H, Su W, Wang L L, et al. Study on adsorption behavior and mechanism of U(Ⅵ) by geological cement[J]. Atomic Energy Science and Technology, 2018, 52(3): 390-396. | |
| 23 | 环境样品中微量铀的分析方法: HJ 840—2017[S]. 北京: 中国标准出版社, 2017. |
| Analytical method for trace uranium in environmental samples: HJ 840—2017[S]. Beijing: Standards Press of China, 2017. | |
| 24 | Gascó G, Paz-Ferreiro J, Méndez A. Thermal analysis of soil amended with sewage sludge and biochar from sewage sludge pyrolysis[J]. Journal of Thermal Analysis and Calorimetry, 2012, 108(2): 769-775. |
| 25 | Agrafioti E, Kalderis D, Diamadopoulos E. Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge[J]. Journal of Environmental Management, 2014, 133: 309-314. |
| 26 | 王兴栋, 张斌, 余广炜, 等. 不同粒径污泥热解制备生物炭及其特性分析[J]. 化工学报, 2016, 67(11): 4808-4816. |
| Wang X D, Zhang B, Yu G W, et al. Preparation of biochar with different particle sized sewage sludge and its characteristics[J]. CIESC Journal, 2016, 67(11): 4808-4816. | |
| 27 | Ahmed B, Rachid R, Hocine H, et al. The removal of uranium (Ⅵ) from aqueous solutions onto activated carbon developed from grinded used tire[J]. Environmental Science and Pollution Research International, 2014, 21(1): 684-694. |
| 28 | 高翔, 谢水波, 刘迎久, 等. 壳聚糖-生物炭复合材料对U(Ⅵ)的吸附性能试验研究[J]. 原子能科学技术, 2019, 53(8): 1350-1358. |
| Gao X, Xie S B, Liu Y J, et al. Experimental investigation on adsorption of U(Ⅵ) by chitosan-biochar composite[J]. Atomic Energy Science and Technology, 2019, 53(8): 1350-1358. | |
| 29 | 陈华柏, 谢水波, 刘金香, 等. 粉末活性污泥处理含铀废水的特性[J]. 环境工程学报, 2015, 9(3): 1141-1147. |
| Chen H B, Xie S B, Liu J X, et al. Adsorption characteristics of uranium by powdered activated sludge[J]. Chinese Journal of Environmental Engineering, 2015, 9(3): 1141-1147. | |
| 30 | Gondhalekar S C, Shukla S R. Equilibrium and kinetics study of uranium(Ⅵ) from aqueous solution by Citrus limetta peels[J]. Journal of Radioanalytical & Nuclear Chemistry, 2014, 302(1): 451-457. |
| 31 | 马锋锋, 赵保卫, 刁静茹, 等. 磁性生物炭对水体中对硝基苯酚的吸附特性[J]. 中国环境科学, 2019, 39(1): 170-178 |
| Ma F F, Zhao B W, Diao J R, et al. Adsorption characteristics of p-nitrophenol removal by magnetic biochar [J]. China Environmental Science, 2019, 39(1): 170-178. | |
| 32 | Xiao B Y, Dai Q, Yu X, et al. Effects of sludge thermal-alkaline pretreatment on cationic red X-GRL adsorption onto pyrolysis biochar of sewage sludge[J]. Journal of Hazardous Materials, 2018, 343: 347-355. |
| 33 | Zhao Y J, Feng D D, Yu Z, et al. Effect of pyrolysis temperature on char structure and chemical speciation of alkali and alkaline earth metallic species in biochar[J]. Fuel Processing Technology, 2016, 141(2): 54-60. |
| 34 | Chen T, Zhang Y X, Wang H T, et al. Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge[J]. Bioresource Technology, 2014, 164(7): 47-54. |
| 35 | Zielińska A, Oleszczuk P, Charmas B, et al. Effect of sewage sludge properties on the biochar characteristic[J]. Journal of Analytical & Applied Pyrolysis, 2015, 112: 201-213. |
| 36 | Saleh A S, Lee J Y, Jo Y, et al. Uranium(Ⅵ) sorption complexes on silica in the presence of calcium and carbonate[J]. Journal of Environmental Radioactivity, 2018, 182: 63-69. |
| 37 | Khamphe P T, Zhang H, Shao L M, et al. Leaching characteristics and phytotoxic effects of sewage sludge biochar[J]. Journal of Material Cycles and Waste Management, 2018, 20: 2089-2099. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [3] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [4] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [5] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [6] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [7] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [8] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [9] | 杨峥豪, 何臻, 常玉龙, 靳紫恒, 江霞. 生物质快速热解下行式流化床反应器研究进展[J]. 化工学报, 2023, 74(6): 2249-2263. |
| [10] | 朱理想, 罗默也, 张晓东, 龙涛, 余冉. 醌指纹法指示三氯乙烯污染土功能微生物活性应用研究[J]. 化工学报, 2023, 74(6): 2647-2654. |
| [11] | 张艳梅, 袁涛, 李江, 刘亚洁, 孙占学. 高效SRB混合菌群构建及其在酸胁迫条件下的性能研究[J]. 化工学报, 2023, 74(6): 2599-2610. |
| [12] | 胡南, 陶德敏, 杨照岚, 王学兵, 张向旭, 刘玉龙, 丁德馨. 铁炭微电解与硫酸盐还原菌耦合修复铀尾矿库渗滤水的研究[J]. 化工学报, 2023, 74(6): 2655-2667. |
| [13] | 王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588. |
| [14] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [15] | 李辰鑫, 潘艳秋, 何流, 牛亚宾, 俞路. 基于碳微晶结构的炭膜模型及其气体分离模拟[J]. 化工学报, 2023, 74(5): 2057-2066. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号