化工学报 ›› 2020, Vol. 71 ›› Issue (10): 4733-4749.DOI: 10.11949/0438-1157.20191318
罗雪1( ),荆川1(
),荆川1( ),黄海军1,李红茹1,王治永1,王震强1,2,高放1(
),黄海军1,李红茹1,王治永1,王震强1,2,高放1( ),张胜涛1
),张胜涛1
收稿日期:2019-11-11
修回日期:2020-03-28
出版日期:2020-10-05
发布日期:2020-10-05
通讯作者:
高放
作者简介:罗雪(1994—),女,硕士研究生,基金资助:
Xue LUO1( ),Chuan JING1(
),Chuan JING1( ),Haijun HUANG1,Hongru LI1,Zhiyong WANG1,Zhenqiang WANG1,2,Fang GAO1(
),Haijun HUANG1,Hongru LI1,Zhiyong WANG1,Zhenqiang WANG1,2,Fang GAO1( ),Shengtao ZHANG1
),Shengtao ZHANG1
Received:2019-11-11
Revised:2020-03-28
Online:2020-10-05
Published:2020-10-05
Contact:
Fang GAO
摘要:
通过多步法合成了离子型含双苯并三氮唑环的目标分子,4,4'-{苯-1,3-二基二[(1E)-3-羰基丙-1-烯-1,3-二基]}二[2-(2H-苯并三唑-2-基)苯醇酸]二钾。在室温条件下,目标分子在3.5%(质量)NaCl/DMSO(二甲基亚枫)混合溶液 (体积比:40/60) 中能够发生分子自组装产生纳-微米级的自聚集体。通过傅里叶变换红外光谱 (FT-IR)、拉曼光谱和X射线光电子能谱 (XPS) 的表征,证实了所形成的目标分子自聚集体能够对铜表面产生强烈的化学吸附作用,在铜表面形成自组装膜。利用电化学方法测定了目标分子自聚集体吸附在铜表面形成自组装膜后,在3.5%(质量)NaCl溶液中的缓蚀性能。结果表明目标分子自聚集体在NaCl溶液中能高效地抑制铜腐蚀。
中图分类号:
罗雪, 荆川, 黄海军, 李红茹, 王治永, 王震强, 高放, 张胜涛. 规整有机分子自聚集体对铜的高效缓蚀的研究[J]. 化工学报, 2020, 71(10): 4733-4749.
Xue LUO, Chuan JING, Haijun HUANG, Hongru LI, Zhiyong WANG, Zhenqiang WANG, Fang GAO, Shengtao ZHANG. Study on highly efficient corrosion inhibition of copper by regular self-aggregates of organic molecule[J]. CIESC Journal, 2020, 71(10): 4733-4749.
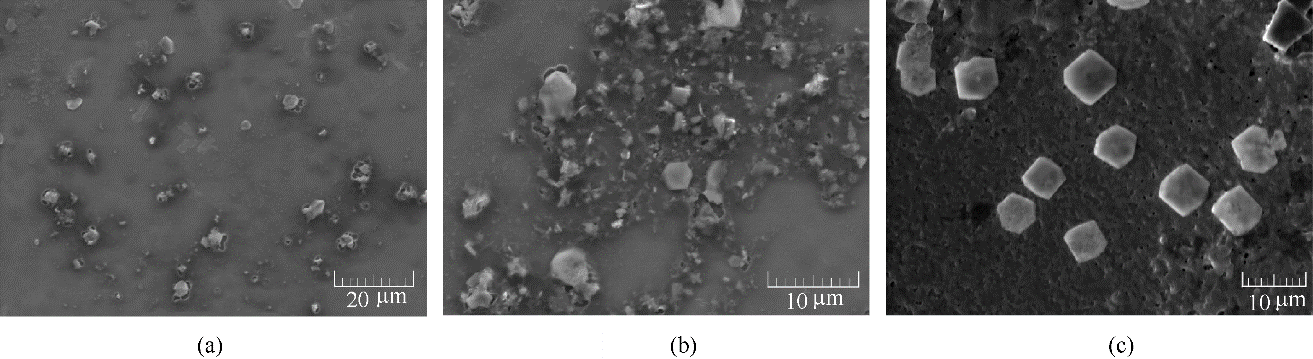
图3 目标分子BDBD自聚集体在浓度为5×10-4 mol/L条件下,在混合溶剂3.5% NaCl/DMSO溶液中 (DMSO体积含量40%) 聚集20 min后的扫描电镜图像(a);1 h后的扫描电镜图像(b);2 h后的扫描电镜图像(c)
Fig.3 SEM images of BDBD aggregates at 5×10-4 mol/L in the mixed 3.5% NaCl solution/DMSO (40% volume ratio of DMSO) at aggregation time course of 20 min (a), 1 h (b), 2 h (c), respectively
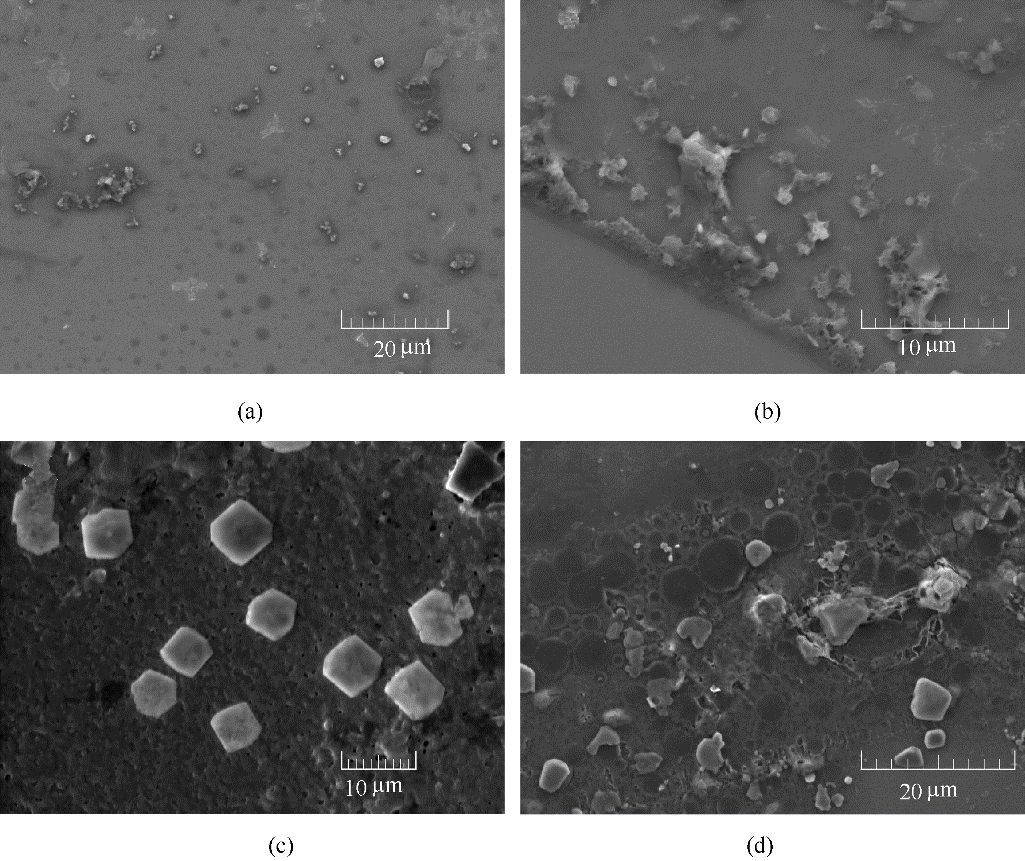
图4 自聚集时间2 h,在3.5% NaCl/ DMSO混合水溶液 (DMSO体积比为40%) 中,BDBD自聚集体浓度分别为1.0×10-4 mol/L(a)、3.0×10-4 mol/L(b)、5.0×10-4 mol/L(c)、7.0×10-4 mol/L(d)时, 样品的扫描电镜图
Fig.4 SEM images of the BDBD aggregates in the mixed 3.5% NaCl DMSO aqueous solution (40% DMSO volume ratio) at 2 h evolving time with diflerent BDBD concentration: 1.0×10-4 mol/L (a), 3.0×10-4 mol/L (b), 5.0×10-4 mol/L (c), 7.0×10-4 mol/L (d)

图5 铜表面的SEM表面形貌分析: 打磨好的铜试样的表面形貌 (a); 铜试样在3.0×10-4 mol/L(b)、5.0×10-4 mol/L(c)和7.0×10-4 mol/L(d)的BDBD自聚集体、3.5% NaCl/DMSO溶液浸泡3 h后的表面形貌
Fig.5 SEM micrographs of the studied Cu specimen surfaces: before the immersion in the 3.5% NaCl/DMSO aqueous solution containing the stable BDBD aggregates (a); after the immersion in the 3.5% NaCl/DMSO aqueous solution with 3.0×10-4 mol/L of the stable BDBD aggregates for 3 h (b); after the immersion in the 3.5% NaCl/DMSO aqueous solution with 5.0×10-4 mol/L of the stable BDBD aggregates for 3 h (c); after the immersion in the 3.5% NaCl/DMSO aqueous solution with 7.0×10-4 mol/L of the stable BDBD aggregates for 3 h (d)
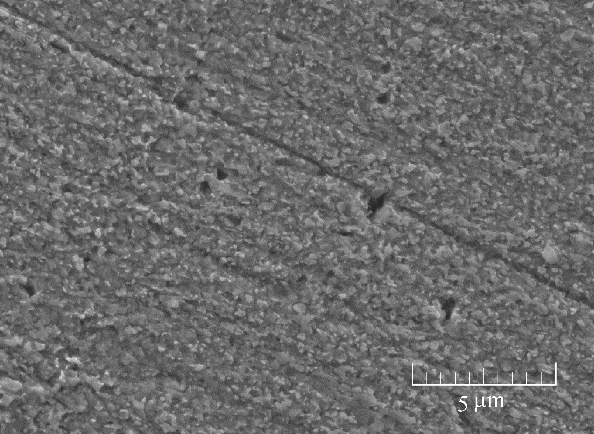
图6 铜试样在5.0×10-4 mol/L稳定的BDBD自聚集体的3.5% NaCl/ DMSO溶液浸泡3 h后,取出浸泡于3.5% NaCl溶液14 d后的表面形貌图
Fig.6 SEM micrographs of the studied Cu specimen surface absorbed with 5.0×10-4 mol/L of the stable BDBD aggregates for 3 h in 3.5% NaCl/ DMSO, which was take out and immersed in the 3.5% NaCl for 14 d

图7 BDBD粉末的FT-IR光谱图(a); 稳定的BDBD自聚集体吸附在铜表面后的FT-IR光谱图(b); 稳定的BDBD自聚集体吸附在铜表面后的拉曼光谱图(c)
Fig.7 FT-IR spectrum of the BDBD powder (a); FT-IR spectrum of the stable BDBD aggregates adsorbed on the studied copper specimen surfaces (b); Raman spectra of stable BDBD aggregates adsorbed on the studied copper specimen surfaces (c)

图8 在3.5% NaCl/DMSO (DMSO/H2O,体积比40/60) 混合溶液中浸泡3 h后铜表面的Cu 2p (a); O 1s (b); C 1s (c) XPS谱图
Fig.8 Cu 2p (a), O 1s (b), C 1s (c) XPS spectra and the fitted curves measured on the studied copper specimens that were immersion in the mixed 3.5% NaCl DMSO aqueous solution for 3 h (DMSO/H2O: 40/60, volume ratio)
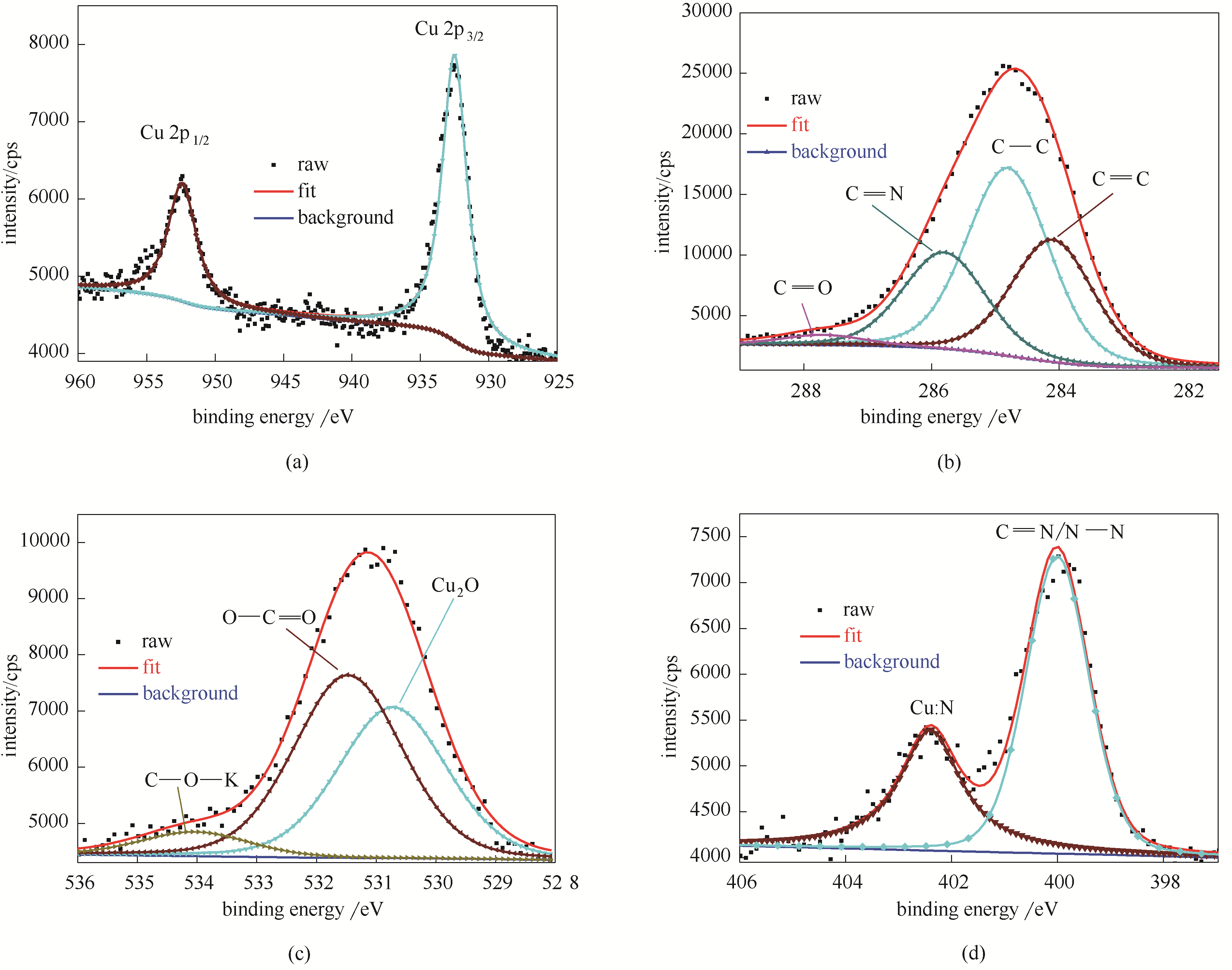
图9 铜片在含有浓度为5.0×10-4 mol/L稳定的BDBD自聚集体的3.5% NaCl/ DMSO溶液 (DMSO/H2O,体积比40/60) 中浸泡3 h后的铜表面的Cu 2p (a); C 1s (b); O 1s (c)和N 1s (d) XPS谱图
Fig.9 Cu 2p (a), C 1s (b); O 1s (c), N 1s (d) XPS spectra and the fitted curves measured on the studied copper specimens after 3 h of immersion in the mixed 3.5% NaCl DMSO aqueous solution (DMSO/H2O: 40/60, volume ratio) containing the stable BDBD aggregates of 5.0 ×10-4 mol/L
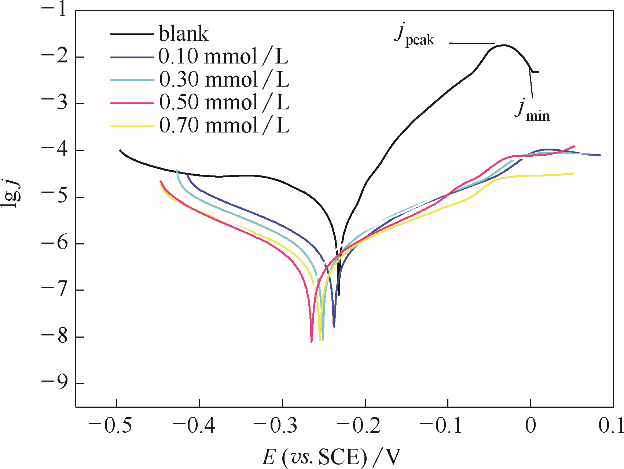
图10 空白铜片和吸附了不同浓度的稳定BDBD自聚集体的铜片在3.5% NaCl溶液中的动电位极化曲线jpeak——Tafel曲线腐蚀峰的最大电流密度;jmin—Tafel曲线腐蚀峰最小电流密度
Fig.10 Potentiodynamic polarization curves in 3.5 % NaCl solution for the studied naked copper electrodes, and for the studied stable BDBD-aggregates of different concentrations covered copper electrodes
| 缓蚀剂 | 极化曲线参数 | |||||
|---|---|---|---|---|---|---|
| c (mol/L) | Ecorr(SCE)/ V | jcorr/(A/cm2) | βc/(V/dec) | βa/(V/dec) | ηj /% | |
| 空白 | — | -0.221 | 5.233×10-6 | -0.1667 | 0.04305 | — |
| BDBD自聚集体 | 1.0×10-4 | -0.237 | 1.349×10-6 | -0.1331 | 0.1153 | 74.22 |
| 3.0×10-4 | -0.265 | 9.35×10-7 | -0.1382 | 0.1192 | 82.14 | |
| 5.0×10-4 | -0.269 | 2.22×10-7 | -0.1471 | 0.2025 | 95.76 | |
| 7.0×10-4 | -0.251 | 4.78×10-7 | -0.1425 | 0.1393 | 90.87 | |
表1 空白铜和吸附了不同浓度的稳定的BDBD自组装体的铜在3.5% NaCl溶液中的极化曲线参数
Table 1 Polarization parameters for the studied copper specimens covered without and with the stable BDBD aggregates of different concentrations in 3.5% NaCl solution
| 缓蚀剂 | 极化曲线参数 | |||||
|---|---|---|---|---|---|---|
| c (mol/L) | Ecorr(SCE)/ V | jcorr/(A/cm2) | βc/(V/dec) | βa/(V/dec) | ηj /% | |
| 空白 | — | -0.221 | 5.233×10-6 | -0.1667 | 0.04305 | — |
| BDBD自聚集体 | 1.0×10-4 | -0.237 | 1.349×10-6 | -0.1331 | 0.1153 | 74.22 |
| 3.0×10-4 | -0.265 | 9.35×10-7 | -0.1382 | 0.1192 | 82.14 | |
| 5.0×10-4 | -0.269 | 2.22×10-7 | -0.1471 | 0.2025 | 95.76 | |
| 7.0×10-4 | -0.251 | 4.78×10-7 | -0.1425 | 0.1393 | 90.87 | |
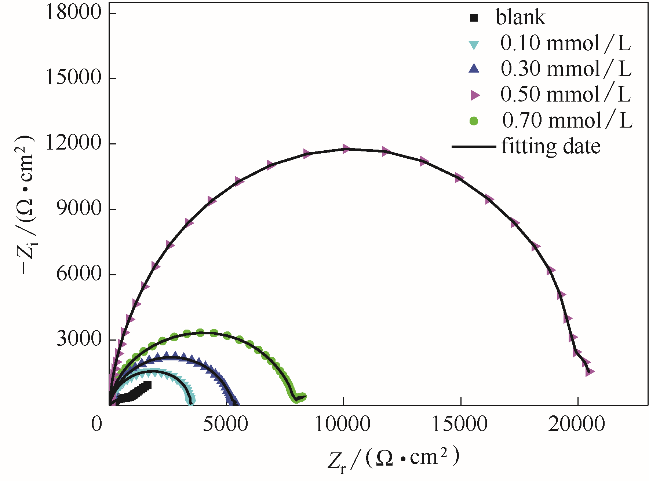
图11 空白铜片和吸附了不同浓度的稳定的BDBD自聚集体的铜片在3.5% NaCl溶液中的Nyquist图
Fig.11 Nyquist plots for the studied naked copper electrodes and the stable BDBD aggregates of different concentrations covered copper electrodes in 3.5% NaCl solution
| 方法 | Kads/ (L/mol) | 吸附能/ (J/mol) |
|---|---|---|
| Polarization | 4.9×104 | -6730 |
| EIS | 4.5×104 | -36510 |
表3 在298 K下,稳定的BDBD自聚集体于3.5% NaCl溶液中的吸附热力学参数
Table 3 Thermodynamic parameters for the adsorption of stable BDBD aggregates in 3.5% NaCl solution at 298 K
| 方法 | Kads/ (L/mol) | 吸附能/ (J/mol) |
|---|---|---|
| Polarization | 4.9×104 | -6730 |
| EIS | 4.5×104 | -36510 |

图A2 目标分子BDBD自聚集体在浓度为5×10-4 mol/L条件下在混合溶剂3.5% NaCl 溶液/DMSO (DMSO体积占比为40%)中聚集6 h后的扫描电镜图像
Fig.A2 SEM images of the BDBD aggregates of 5.0×10-4 mol/L in the mixed 3.5% NaCl DMSO aqueous solution (40% DMSO volume ratio) at 6 h evolving time
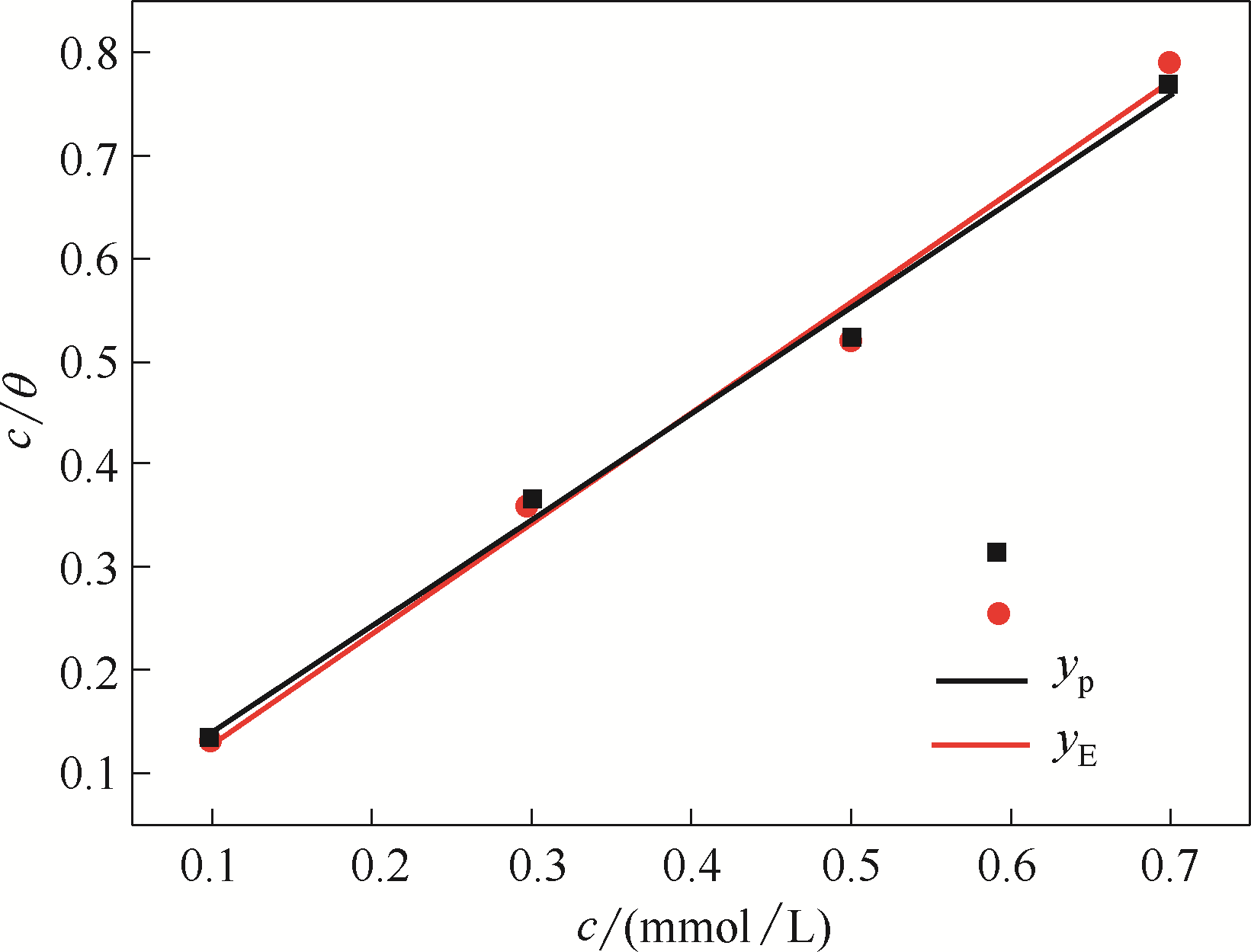
图A4 吸附了稳定的BDBD自聚集体的铜在3.5% NaCl溶液中的Langmuir吸附等温线
Fig.A4 Langmuir adsorption isotherms of the stable BDBD aggregates covered on the studied copper specimen surfaces in 3.5 % NaCl solution (yp to potentiodynamic polarization and yE to electrochemical impedance spectroscopy

图A5 经结构优化后目标分子BDBD的稳定构型、HOMO和LUMO前线轨道以及Mulliken电荷分布
Fig.A5 Optimized geometric structure, electron cloudy density distribution of HOMO and LUMO and Mulliken charge of the target molecule BDBD
| 1 | Fan H, Li S, Zhao Z, et al. Inhibition of brass corrosion in sodium chloride solutions by self-assembled silane films[J]. Corrosion Science, 2011, 53: 4273-4281. |
| 2 | Lyon S B, Bingham R, Mills Douglas J. Advances in corrosion protection by organic coatings: what we know and what we would like to know[J]. Progress in Organic Coatings, 2017, 102: 2-7. |
| 3 | Kokalj A, Peljhan S. Density functional theory study of ATA, BTAH, and BTAOH as copper corrosion inhibitors: adsorption onto Cu(111) from gas phase[J]. Langmuir, 2010, 26: 14582-14593. |
| 4 | Finsgar M. 2-Mercaptobenzimidazole as a copper corrosion inhibitor: Part I. Long-term immersion, 3D-profilometry, and electrochemistry[J]. Corrosion Science, 2013, 72: 82-89. |
| 5 | Finsgar M. EQCM and XPS analysis of 1,2,4-triazole and 3-amino-1,2,4-triazole as copper corrosion inhibitors in chloride solution[J]. Corrosion Science, 2013, 77: 350-359. |
| 6 | Wang Z, Gong Y, Jing C, et al. Synthesis of dibenzotriazole derivatives bearing alkylene linkers as corrosion inhibitors for copper in sodium chloride solution: a new thought for the design of organic inhibitors[J]. Corrosion Science, 2016, 113: 64-77. |
| 7 | Sherif M E S. Effects of 2-amino-5-(ethylthio)-1,3,4-thiadiazole on copper corrosion as a corrosion inhibitor in 3% NaCl solutions[J]. Applied Surface Science, 2006, 252: 8615-8623. |
| 8 | Hong S, Chen W, Zhang Y, et al. Investigation of the inhibition effect of trithiocyanuric acid on corrosion of copper in 3.0wt.% NaCl[J]. Corrosion Science, 2013, 66: 308-314. |
| 9 | Izquierdo J, Santana J J, González S, et al. Uses of scanning electrochemical microscopy for the characterization of thin inhibitor films on reactive metals: The protection of copper surfaces by benzotriazole[J]. Electrochimica Acta, 2010, 55: 8791-8800. |
| 10 | Jafari A H, Hosseini S M A, Jamalizadeh E. Investigation of smart nanocapsules containing inhibitors for corrosion protection of copper[J]. Electrochimica Acta, 2010, 55: 9004-9009. |
| 11 | Khaled K F. Studies of the corrosion inhibition of copper in sodium chloride solutions using chemical and electrochemical measurements[J]. Materials Chemistry and Physics, 2011, 125: 427-433. |
| 12 | Li C C, Guo X. Y, Shen S,et al. Adsorption and corrosion inhibition of phytic acid calcium on the copper surface in 3wt% NaCl solution[J]. Corrosion Science, 2014, 83: 147-154. |
| 13 | Liu Y, Li S, Zhang J, et al. Corrosion inhibition of biomimetic super-hydrophobic electrodeposition coatings on copper substrate[J]. Corrosion Science, 2015, 94: 190-196. |
| 14 | Khiati Z, Othman A A, Sanchez-Moreno M, et al. Corrosion inhibition of copper in neutral chloride media by a novel derivative of 1,2,4-triazole[J]. Corrosion Science, 2011, 53: 3092-3099. |
| 15 | Wang B, Gao F, Ma H. Preparation and XPS studies of macromolecule mixed-valent Cu(I, II) and Fe(II, III) complexes[J]. Journal of Hazardous Materials, 2007, 144: 363-368. |
| 16 | Doong R A, Liao C Y. Enhanced visible-light-responsive photodegradation of bisphenol A by Cu, N-codoped titanate nanotubes prepared by microwave-assisted hydrothermal method[J]. Journal of Hazardous Materials, 2017, 322: 254-262. |
| 17 | Huang H, Fu Y, Wang X, et al. Nano- to micro-self-aggregates of new bisimidazole-based copoly(ionic liquid)s for protecting copper in aqueous sulfuric acid solution[J]. ACS Applied Materials & Interfaces, 2019, 11: 10135-10145. |
| 18 | Zhang D Q, Joo H G, Lee K Y. Investigation of molybdate-benzotriazole surface treatment against copper tarnishing[J]. Surface and Interface Analysis, 2009, 41: 164-169. |
| 19 | Lou W, Cai W, Li J P, et al. Additives-assisted electrodeposition of fine spherical copper powder from sulfuric acid solution[J]. Powder Technology, 2018, 326: 84-88. |
| 20 | Sherif E S M, Erasmus R M, Comins J D. In situ Raman spectroscopy and electrochemical techniques for studying corrosion and corrosion inhibition of iron in sodium chloride solutions[J]. Electrochimica Acta, 2010, 55: 3657-3663. |
| 21 | Sudheer M A Q. Electrochemical and theoretical investigation of triazole derivatives on corrosion inhibition behavior of copper in hydrochloric acid medium[J]. Corrosion Science, 2013, 70: 161-169. |
| 22 | Mihajlovic M B P, Radovanovic M B, Tasic Z Z, et al. Imidazole based compounds as copper corrosion inhibitors in seawater[J]. Journal of Molecular Liquids, 2017, 225: 127-136. |
| 23 | Qafsaoui W, Kendig M W, Perrot H, et al. Coupling of electrochemical techniques to study copper corrosion inhibition in 0.5 mol·L-1 NaCl by 1-pyrrolidine dithiocarbamate[J]. Electrochimica Acta, 2013, 87: 348-360. |
| 24 | 张景玲. 苯并三氮唑复配体系对铜的协同缓蚀性能的研究[D]. 长沙:湖南大学, 2008. |
| Zhang J L. Investigation of the synergistic effect between BTA and its composite corrosion inhibitiors on copper[D]. Changsha:Hunan University, 2008. | |
| 25 | Zhang D Q, Gao L X, Zhou G D. Inhibition of copper corrosion by bis-(1-benzotriazolymethylene)-(2,5-thiadiazoly)-disulfide in chloride media[J]. Applied Surface Science, 2004, 225: 287-293. |
| 26 | Singh M M, Rastogi R B, Upadhyay B N, et al. Thiosemicarbazide, phenyl isothiocyanate and their condensation product as corrosion inhibitors of copper in aqueous chloride solutions[J]. Materials Chemistry and Physics, 2003, 80: 283-293. |
| 27 | Hu L, Zhang S, Li W, et al. Electrochemical and thermodynamic investigation of diniconazole and triadimefon as corrosion inhibitors for copper in synthetic seawater[J]. Corrosion Science, 2010, 52: 2891-2896. |
| 28 | Qiang Y, Zhang S, Yan S, et al. Three indazole derivatives as corrosion inhibitors of copper in a neutral chloride solution[J]. Corrosion Science, 2017, 126: 295-304. |
| 29 | Solomon M M, Umoren S A. In-situ preparation, characterization and anticorrosion property of polypropylene glycol/silver nanoparticles composite for mild steel corrosion in acid solution[J]. Journal of Colloid and Interface Science, 2016, 462: 29-41. |
| 30 | Scendo M. Inhibition of copper corrosion in sodium nitrate solutions with nontoxic inhibitors[J]. Corrosion Science, 2008, 50: 1584-1592. |
| 31 | Scendo M. The effect of purine on the corrosion of copper in chloride solutions[J]. Corrosion Science, 2007, 49: 373-390. |
| 32 | Mendonça G L F, Costa S N, Freire V N, et al. Understanding the corrosion inhibition of carbon steel and copper in sulphuric acid medium by amino acids using electrochemical techniques allied to molecular modelling methods[J]. Corrosion Science, 2017, 115: 41-55. |
| 33 | Zhang J, Liu Z, Han G C, et al. Inhibition of copper corrosion by the formation of Schiff base self-assembled monolayers[J]. Applied Surface Science, 2016, 389: 601-608. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 康飞, 吕伟光, 巨锋, 孙峙. 废锂离子电池放电路径与评价研究[J]. 化工学报, 2023, 74(9): 3903-3911. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [5] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| [6] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [7] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [8] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [9] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [10] | 邢美波, 张中天, 景栋梁, 张洪发. 磁调控水基碳纳米管协同多孔材料强化相变储/释能特性[J]. 化工学报, 2023, 74(7): 3093-3102. |
| [11] | 李艳辉, 丁邵明, 白周央, 张一楠, 于智红, 邢利梅, 高鹏飞, 王永贞. 非常规服役超临界锅炉的微纳尺度腐蚀动力学模型建立及应用[J]. 化工学报, 2023, 74(6): 2436-2446. |
| [12] | 朱理想, 罗默也, 张晓东, 龙涛, 余冉. 醌指纹法指示三氯乙烯污染土功能微生物活性应用研究[J]. 化工学报, 2023, 74(6): 2647-2654. |
| [13] | 张艳梅, 袁涛, 李江, 刘亚洁, 孙占学. 高效SRB混合菌群构建及其在酸胁迫条件下的性能研究[J]. 化工学报, 2023, 74(6): 2599-2610. |
| [14] | 胡南, 陶德敏, 杨照岚, 王学兵, 张向旭, 刘玉龙, 丁德馨. 铁炭微电解与硫酸盐还原菌耦合修复铀尾矿库渗滤水的研究[J]. 化工学报, 2023, 74(6): 2655-2667. |
| [15] | 王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号