化工学报 ›› 2020, Vol. 71 ›› Issue (12): 5530-5540.DOI: 10.11949/0438-1157.20200304
刘帅1( ),李学雷2,3,李启朦1,王彦娟1,张健1(
),李学雷2,3,李启朦1,王彦娟1,张健1( ),封瑞江1,胡绍争1
),封瑞江1,胡绍争1
收稿日期:2020-03-23
修回日期:2020-07-18
出版日期:2020-12-05
发布日期:2020-12-05
通讯作者:
张健
作者简介:刘帅 (1996— ),男,硕士研究生,基金资助:
LIU Shuai1( ),LI Xuelei2,3,LI Qimeng1,WANG Yanjuan1,ZHANG Jian1(
),LI Xuelei2,3,LI Qimeng1,WANG Yanjuan1,ZHANG Jian1( ),FENG Ruijiang1,HU Shaozheng1
),FENG Ruijiang1,HU Shaozheng1
Received:2020-03-23
Revised:2020-07-18
Online:2020-12-05
Published:2020-12-05
Contact:
ZHANG Jian
摘要:
采用一步热解法制备了木棉纤维(KF)改性的石墨相氮化碳(g-C3N4)催化剂,并考察了催化剂光催化降解有机污染物的性能。采用XRD、UV-Vis DRS、FT-IR、TEM、XPS、N2吸附-脱附、PL表征对催化剂进行了结构、形貌、光学性能测试。结果表明,KF改性可以提高催化剂的比表面积,更大的比表面积可以提供更多的活性位点来参与光催化降解过程。UV-Vis DRS结果表明KF改性可以缩小催化剂的禁带宽度,提高催化剂对光能的吸收。在可见光下,KF改性的g-C3N4基催化剂对苯酚降解速率常数为0.259 h-1,是纯g-C3N4的4.2倍,且具有优异的催化稳定性和结构稳定性。
中图分类号:
刘帅,李学雷,李启朦,王彦娟,张健,封瑞江,胡绍争. 木棉纤维改性氮化碳光催化降解有机污染物[J]. 化工学报, 2020, 71(12): 5530-5540.
LIU Shuai,LI Xuelei,LI Qimeng,WANG Yanjuan,ZHANG Jian,FENG Ruijiang,HU Shaozheng. Kapok fiber modified carbon nitride photocatalytic degradation of organic pollutants[J]. CIESC Journal, 2020, 71(12): 5530-5540.
| 浓度/(μg·g-1) | 吸光度 |
|---|---|
| 0 | 0 |
| 1 | 0.136 |
| 1.5 | 0.208 |
| 2 | 0.278 |
| 2.5 | 0.352 |
| 3 | 0.419 |
| 3.5 | 0.49 |
| 4 | 0.563 |
表1 不同苯酚溶液浓度所对应的吸光度
Table 1 The absorbance of different concentrations of phenol
| 浓度/(μg·g-1) | 吸光度 |
|---|---|
| 0 | 0 |
| 1 | 0.136 |
| 1.5 | 0.208 |
| 2 | 0.278 |
| 2.5 | 0.352 |
| 3 | 0.419 |
| 3.5 | 0.49 |
| 4 | 0.563 |
| Sample | C/% (mass) | N/% (mass) | H/% (mass) | C/N ratio |
|---|---|---|---|---|
| CN(600) | 38.3 | 60.5 | 1.2 | 0.740 |
| KF(1%)-CN(600) | 38.8 | 59.9 | 1.3 | 0.755 |
| KF(5%)-CN(600) | 39.7 | 58.4 | 1.9 | 0.792 |
| KF(10%)-CN(600) | 41.3 | 56.3 | 2.4 | 0.858 |
| KF(5%)-CN(550) | 39.8 | 58.4 | 1.8 | 0.795 |
| KF(5%)-CN(650) | 40.6 | 57.4 | 2.0 | 0.826 |
表2 制备催化剂的元素含量
Table 2 Elemental composition of as-prepared catalysts
| Sample | C/% (mass) | N/% (mass) | H/% (mass) | C/N ratio |
|---|---|---|---|---|
| CN(600) | 38.3 | 60.5 | 1.2 | 0.740 |
| KF(1%)-CN(600) | 38.8 | 59.9 | 1.3 | 0.755 |
| KF(5%)-CN(600) | 39.7 | 58.4 | 1.9 | 0.792 |
| KF(10%)-CN(600) | 41.3 | 56.3 | 2.4 | 0.858 |
| KF(5%)-CN(550) | 39.8 | 58.4 | 1.8 | 0.795 |
| KF(5%)-CN(650) | 40.6 | 57.4 | 2.0 | 0.826 |

图9 催化剂的苯酚降解性能
Fig.9 Phenol degradation performance of catalyst(reaction conditions: 0.1 g catalyst, 30℃, atmospheric pressure, oxygen intake was 80 ml·min-1, 300 min)
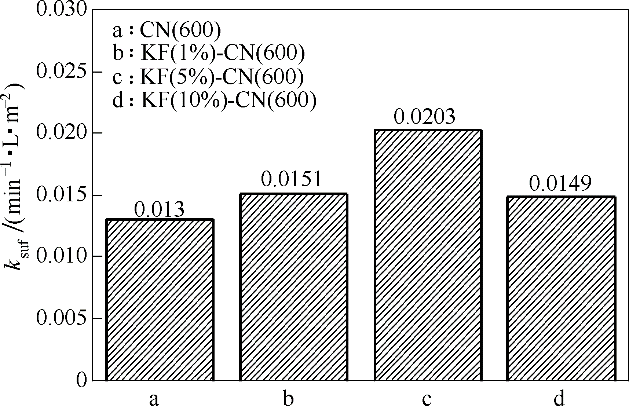
图10 苯酚降解的比表面积归一化速率常数ksuf
Fig.10 The normalized rate constant ksuf of specific surface area degraded by phenol(reaction conditions: 0.1 g catalyst, 30℃, atmospheric pressure, oxygen intake was 80 ml·min-1, 300 min)
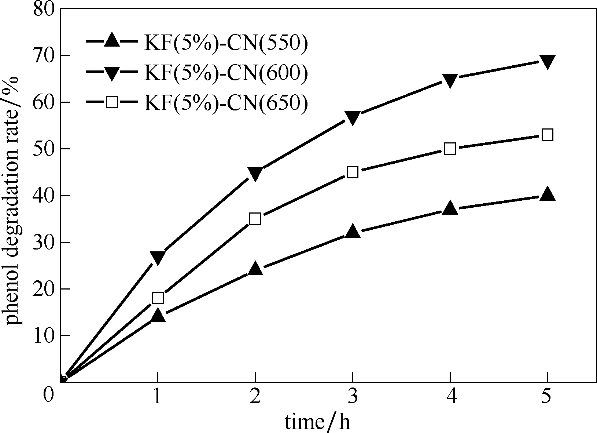
图11 催化剂焙烧温度对催化剂降解苯酚性能的影响
Fig.11 The influence of calcination temperature of catalysts on the phenol degradation performance(reaction conditions: 0.1 g catalyst, 30℃, atmospheric pressure, oxygen intake was 80 ml·min-1, 300 min)

图12 CN(600)和KF(5%)-CN(600)对儿茶酚和2-氯苯酚的降解性能对比
Fig.12 Photocatalytic degradation rate of catechol and 2-chlorophenol over CN(600) and KF(5%)-CN(600)(reaction conditions: 0.1 g catalyst, 30℃, atmospheric pressure, oxygen intake was 80 ml·min-1, 300 min)
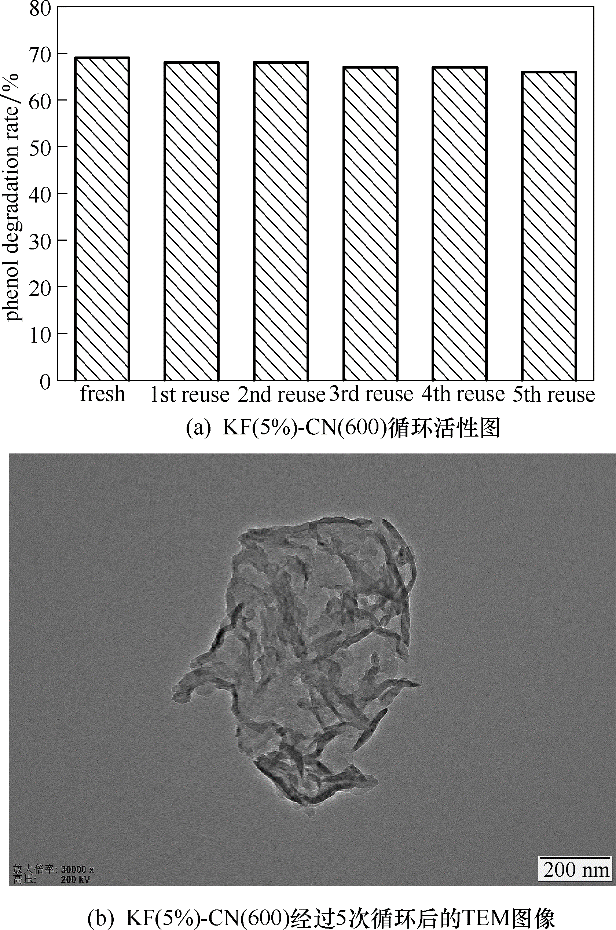
图13 KF(5%)-CN(600)的循环实验
Fig.13 Cyclic performance of KF(5%)-CN(600)(reaction conditions: 0.1 g catalyst, 30℃, atmospheric pressure, oxygen intake was 80 ml·min-1, 300 min)

图14 捕获剂对光降解苯酚的影响
Fig.14 Capture agent’s effect on photodegradation of phenol(reaction conditions: 0.1 g catalyst, 30℃, atmospheric pressure, oxygen intake was 80 ml·min-1, 300 min)
| 1 | Pawar R, Khare V, Lee S C. Hybrid photocatalysts using graphitic carbon nitride/cadmium sulfide/reduced graphene oxide (g-C3N4/CdS/RGO) for superior photodegradation of organic pollutants under UV and visible light[J]. Dalton Trans., 2014, 43: 12514-12527. |
| 2 | Dong G H, Jacobs D, Zang L, et al. Carbon vacancy regulated photoreduction of NO to N2 over ultrathin g-C3N4 nanosheets[J]. Appl. Catal. B: Environ., 2017, 218: 515-524. |
| 24 | Hu P, Chen C J, Zeng R, et al. Facile synthesis of bimodal porous graphitic carbon nitride nanosheets as efficient photocatalysts for hydrogen evolution[J]. Nano Energy, 2018, 50: 376-382. |
| 25 | Li C M, Yu S Y, Dong H J, et al. Mesoporous ferriferrous oxide nanoreactors modified on graphitic carbon nitride towards improvement of physical, photoelectrochemical properties and photocatalytic performance[J]. J. Colloid. Interf. Sci., 2018, 531: 331-342. |
| 26 | Yan H J, Chen Y, Xu S M. Synthesis of graphitic carbon nitride by directly heating sulfuric acid treated melamine for enhanced photocatalytic H2 production from water under visible light[J]. Int. J. Hydrogen Energ., 2012, 37: 125. |
| 27 | Liu J Y, Fang W J, Wei Z D, et al. Efficient photocatalytic hydrogen evolution on N-deficient g-C3N4 achieved by a molten salt post-treatment approach[J]. Appl. Catal. B: Environ., 2018, 238(15): 465-470. |
| 3 | Hu S Z, Jin R R, Lu G, et al. The properties and photocatalytic performance comparison of Fe3+-doped g-C3N4 and Fe2O3/g-C3N4 composite catalysts[J]. RSC Adv., 2014, 4: 24863-24869. |
| 4 | Hu S Z, Qu X Y, Li P, et al. Photocatalytic oxygen reduction to hydrogen peroxide over copper doped graphitic carbon nitride hollow microsphere: the effect of Cu(I)-N active sites[J]. Chem. Eng. J., 2018, 334: 410-418. |
| 5 | Lan Z A, Zhang G G, Wang X C. A facile synthesis of Br-modified g-C3N4 semiconductors for photoredox water splitting[J]. Appl. Catal. B: Environ., 2016, 192: 116-125. |
| 6 | Liu D X, Zhang J, Li C, et al. In-situ fabrication of atomic charge transferring path for constructing heterojunction photocatalysts with hierarchical structure[J]. Appl. Catal. B: Environ., 2019, 248: 459-465. |
| 28 | Li X F, Zhang J, Shen L H, et al. Preparation and characterization of graphitic carbon nitride through pyrolysis of melamine[J]. Appl. Phys. A: Mater., 2009, 94(2): 387-392. |
| 29 | Wang S M, Li D L, Sun C, et al. Synthesis and characterization of g-C3N4/Ag3VO4 composites with significantly enhanced visible-light photocatalytic activity for triphenylmethane dye degradation[J]. Appl. Catal. B: Environ., 2014, 144: 885-892. |
| 30 | Tong Z W, Yang D, Xiao T X, et al. Biomimetic fabrication of g-C3N4/TiO2 nanosheets with enhanced photocatalytic activity toward organic pollutant degradation[J]. Chem. Eng. J., 2015, 260: 117-125. |
| 31 | Ma Y J, Li X L, Yang Z, et al. Morphology control and photocatalysis enhancement by in situ hybridization of cuprous oxide with nitrogen-doped carbon quantum dots[J]. Langmuir., 2016, 32(37): 9418-9427. |
| 7 | Dong G H, Ho W K, Wang C Y. Selective photocatalytic N2 fixation dependent on g-C3N4 induced by nitrogen vacancies[J]. J. Mater. Chem. A, 2015, 3: 23435-23441. |
| 8 | Li H, Shang J, Ai Z H. Efficient visible light nitrogen fixation with BiOBr nanosheets of oxygen vacancies on the exposed {001} facets[J]. J. Am. Chem. Soc., 2015, 137: 6393-6399. |
| 9 | Bao N, Hu X, Zhang Q, et al. Synthesis of porous carbon-doped g-C3N4 nanosheets with enhanced visible-light photocatalytic activity[J]. Appl. Surf. Sci., 2017, 403: 682-690. |
| 32 | Li X Y, Wang D S, Cheng G X, et al. Preparation of polyaniline-modified TiO2 nanoparticles and their photocatalytic activity under visible light illumination[J]. Appl. Catal. B: Environ., 2008, 81: 267-273. |
| 33 | Ikeda S, Sugiyama N, Murakami S, et al. Quantitative analysis of defective sites in titanium (Ⅳ) oxide photocatalyst powders[J]. Phys. Chem. Chem. Phys., 2003, 5: 778-783. |
| 10 | Tong Z W, Yang D, Zhao X Y, et al. Bio-inspired synthesis of three-dimensional porous g-C3N4@carbon microflowers with enhanced oxygen evolution reactivity[J]. Chem. Eng. J., 2018, 337: 312-321. |
| 11 | Jin Z, Chen J, Huang S, et al. A facile approach to fabricating carbonaceous material/g-C3N4 composites with superior photocatalytic activity[J]. Catal. Today, 2018, 315: 149-154. |
| 12 | Liu J Y, Xu H, Xu Y G, et al. Graphene quantum dots modified mesoporous graphite carbon nitride with significant enhancement of photocatalytic activity[J]. Appl. Catal. B: Environ., 2017, 207: 429-437. |
| 13 | Wang F L, Chen P, Feng Y P, et al. Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin[J]. Appl. Catal. B: Environ., 2017, 207: 103-113. |
| 14 | Fang S, Xia Y, Lv K L, et al. Effect of carbon-dots modification on the structure and photocatalytic activity of g-C3N4[J]. Appl. Catal. B: Environ., 2016, 185: 225-232. |
| 15 | Cao Y F, Xie L J, Sun G H, et al. Hollow carbon microtubes from kapok fiber: structural evolution and energy storage performance[J]. Sustain. Energy Fuels, 2018, 2: 455-465. |
| 16 | Wang Y, Wang X C, Antonietti M. Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: from photochemistry to multipurpose catalysis to sustainable chemistry[J]. Angew. Chem. Int. Ed., 2012, 51: 68-89. |
| 17 | 鲍勇霖. 高光催化活性类石墨相氮化碳的制备与优化[D]. 沈阳: 辽宁大学, 2019. |
| Bao Y L. Preparation and optimization of graphite-like carbon nitride with high photocatalytic activity[D]. Shenyang: Liaoning University, 2019. | |
| 18 | Wang X C, Chen X F, Thomas A, et al. Metal-containing carbon nitride compounds: a new functional organic-metal hybrid material[J]. Adv. Mater., 2009, 21: 1609-1612. |
| 19 | 高龙. 活性炭负载氮化碳活化过一硫酸氢盐降解酸性橙7的研究[D]. 武汉: 武汉纺织大学, 2017. |
| Gao L. Degradation of acid orange 7 by activated mono-bisulfate with carbon nitride on activated carbon[D]. Wuhan: Wuhan Textile University, 2017. | |
| 20 | Kudo A, Miseki Y. Heterogeneous photocatalyst materials for water splitting[J]. Chem. Soc. Rev., 2009, 38(1): 253-278. |
| 21 | Lei W W, Portenhault D, Dimova R, et al. Boron carbon nitride nanostructures from salt melts: tunable water-soluble phosphors[J]. J. Am. Chem. Soc., 2011, 133(18): 5300-5303. |
| 22 | Zhao Z W, Sun Y J, Dong F, et al. Template synthesis of carbon self-doped g-C3N4 with enhanced visible to near-infrared absorption and photocatalytic performance[J]. RSC. Adv., 2015, 5: 39549-39556. |
| 23 | Hu S Z, Chen X, Li Q, et al. Fe3+ doping promoted N2 photofixation ability of honeycombed graphitic carbon nitride: the experimental and density functional theory simulation analysis[J]. Appl. Catal. B: Environ., 2017, 201: 58-69. |
| 34 | Zhou L, Song W, Chen Z Q, et al. Degradation of organic pollutants in wastewater by bicarbonate-activated hydrogen peroxide with a supported cobalt catalyst[J]. Environ. Sci. Technol., 2013, 47: 3833-3839. |
| 35 | Cao S W, Low J X, Yu J G, et al. Polymeric photocatalysts based on graphitic carbon nitride[J]. Adv. Mater., 2015, 27: 2150-2176. |
| 36 | Chang F, Zhang J, Xie Y C, et al. Fabrication, characterization, and photocatalytic performance of exfoliated g-C3N4–TiO2 hybrids[J]. Appl. Surf. Sci., 2014, 311: 574-581. |
| [1] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [2] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [3] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [4] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [5] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [6] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [7] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| [8] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [9] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [10] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [11] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [12] | 吴文涛, 褚良永, 张玲洁, 谭伟民, 沈丽明, 暴宁钟. 腰果酚生物基自愈合微胶囊的高效制备工艺研究[J]. 化工学报, 2023, 74(7): 3103-3115. |
| [13] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [14] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [15] | 张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号