化工学报 ›› 2021, Vol. 72 ›› Issue (1): 14-26.DOI: 10.11949/0438-1157.20200863
陈润道1( ),郑芳1,郭立东1,杨启炜1,2,张治国1,2,杨亦文1,2,任其龙1,2,鲍宗必1,2(
),郑芳1,郭立东1,杨启炜1,2,张治国1,2,杨亦文1,2,任其龙1,2,鲍宗必1,2( )
)
收稿日期:2020-07-01
修回日期:2020-07-16
出版日期:2021-01-05
发布日期:2021-01-05
通讯作者:
鲍宗必
作者简介:陈润道(1998—),男,博士研究生,基金资助:
CHEN Rundao1( ),ZHENG Fang1,GUO Lidong1,YANG Qiwei1,2,ZHANG Zhiguo1,2,YANG Yiwen1,2,REN Qilong1,2,BAO Zongbi1,2(
),ZHENG Fang1,GUO Lidong1,YANG Qiwei1,2,ZHANG Zhiguo1,2,YANG Yiwen1,2,REN Qilong1,2,BAO Zongbi1,2( )
)
Received:2020-07-01
Revised:2020-07-16
Online:2021-01-05
Published:2021-01-05
Contact:
BAO Zongbi
摘要:
稀有气体Xe/Kr的高效捕集分离是气体工业、核环境监测和乏燃料处理等领域的重要分离过程之一。氙与氪结构与极化率相似,传统低温精馏方法借助氙与氪的沸点差异实现二者分离,能耗巨大,吸附分离是较为理想的替代分离技术。以金属有机框架材料为代表的新型多孔材料具有结构多样性与高度可设计性,通过调节材料微孔表面的极化环境与孔道窗口结构,借助氙与氪极化率的微小差异,可实现对二者的精准辨识,有良好的吸附分离性能与应用前景。重点综述了金属有机框架材料在氙氪分离中的研究进展,归纳了材料的极化环境、孔道结构、框架柔性等因素对氙氪吸附分离性能的影响规律,探讨了金属有机框架材料在氙氪吸附分离研究中存在的问题和局限,并对未来发展方向进行了展望。
中图分类号:
陈润道, 郑芳, 郭立东, 杨启炜, 张治国, 杨亦文, 任其龙, 鲍宗必. 稀有气体Xe/Kr吸附分离研究进展[J]. 化工学报, 2021, 72(1): 14-26.
CHEN Rundao, ZHENG Fang, GUO Lidong, YANG Qiwei, ZHANG Zhiguo, YANG Yiwen, REN Qilong, BAO Zongbi. Advancements in adsorption separation of Xe/Kr noble gases[J]. CIESC Journal, 2021, 72(1): 14-26.
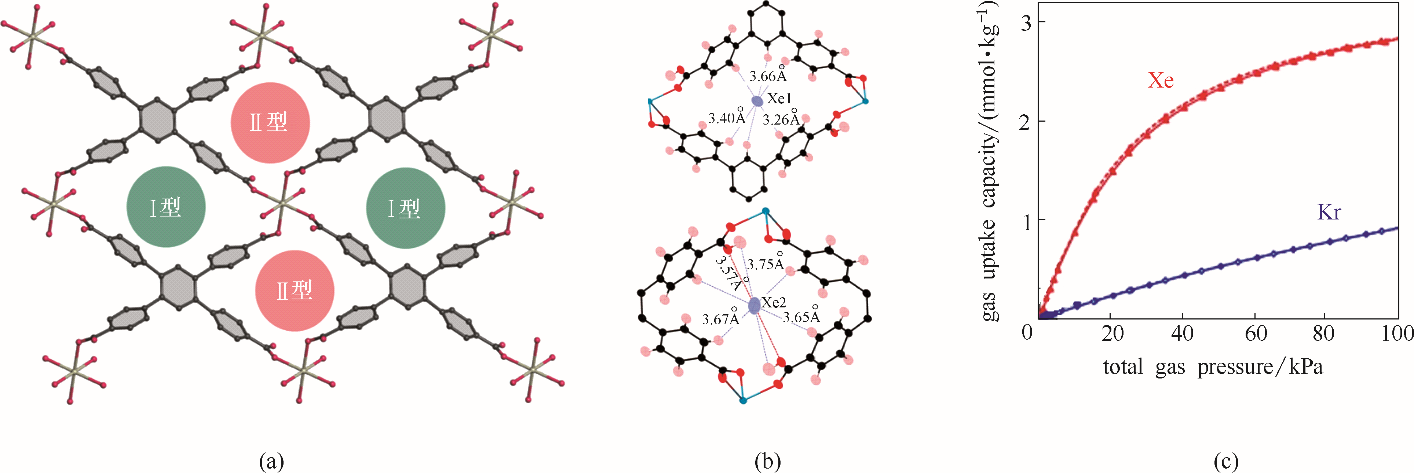
图2 SBMOF-2孔道示意图(a);Xe在SBMOF-2 Ⅰ型孔道(上)与Ⅱ型孔道(下)中的结合位点(b);SBMOF-2 Xe/Kr吸附等温线(298 K)(c)
Fig.2 Pore structure of SBMOF-2(a); Binding sites of Xe with pore Ⅰ (upper) and pore II (lower) in SBMOF-2 (b); Isotherms of Xe/Kr in SBMOF-2 collected at 298 K (c)
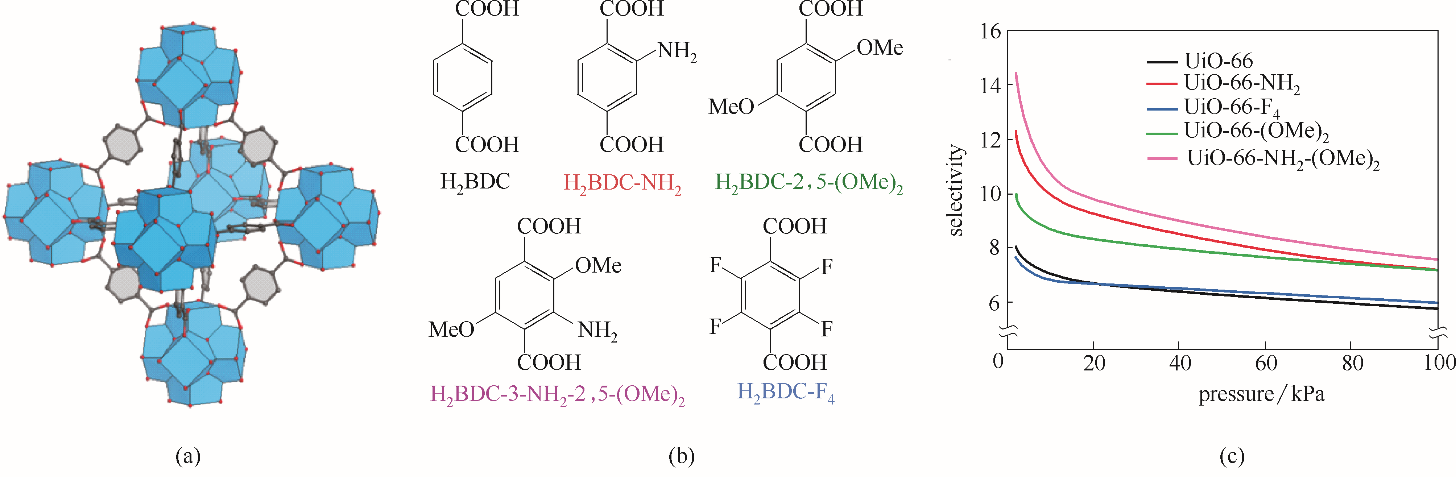
图3 UiO-66材料结构(a);UiO-66功能化修饰配体(b);UiO-66系列材料Xe/Kr IAST选择性(283 K,Xe/Kr=20∶80)(c)
Fig.3 Structure of UiO-66(a); Functionalized ligands for UiO-66 (b); IAST-predicted Xe/Kr selectivity at 283 K for pristine and functionalized UiO-66 materials (Xe/Kr=20∶80) (c)

图6 方酸钴框架材料结构及孔内羟基示意(a);方酸钴孔道内Xe结合位点(b);298 K材料Xe吸附等温线低压区对比(c);298 K材料IAST选择性对比(Xe/Kr=20∶80)(d)
Fig.6 Co squarate structure with -OH groups decorated in channels (a); Xe binding site in Co squarate (b); Xe adsorption isotherms of Co squarate at low pressure at 298 K and comparison with other materials (c); Comparison of the IAST selectivity of Co squarate versus other materials for Xe/Kr (20/80) mixtures at 298 K (d)
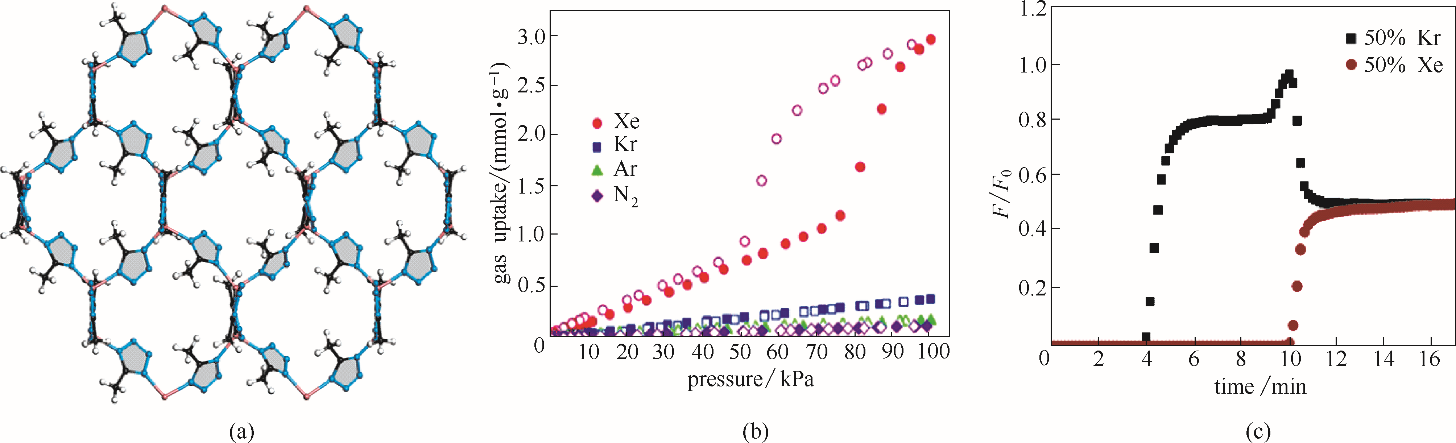
图7 UTSA-49材料结构 (a); 材料298 K吸附等温线 (b); 材料296 K、100 kPa穿透曲线(Xe/Kr=50∶50)(c)
Fig.7 UTSA-49 structure (a); Adsorption isotherms at 298 K (b); Column breakthrough curve of UTSA-49 at 296 K and 100 kPa (Xe/Kr=50∶50) (c)
| 吸附剂 | Xe吸附容量/ (mmol·g-1) | IAST选择性 (Xe/Kr=20∶80) | Xe等量吸附热Qst/ (kJ·mol-1) | Xe Henry系数/(mmol·g-1·bar-1) | Henry选择性 |
|---|---|---|---|---|---|
| MOF-5 | 1.98 | 3 | 13.75 | — | 8.56 |
| HKUST-1 | 3.3 | 2.6 | 17.5 | 12.2 | 8.5 |
| Ni-MOF-74 | 4.2 | 4 | 22 | 8.4 | 5.8 |
| Ag@Ni-MOF-74 | 4.6 | 11.5 | 23.6 | — | — |
| MOF-505 | 6.31 | 9~10 | — | 10.26 | 6.8 |
| Co3(HCOO)6 | 2 | 12 | 28 | 9.9 | 8.7 |
| MOF-Cu-H | 3.19 | 15.8 | 33.4 | 39.74 | 15.8 |
| UiO-66 | 1.58 | ~7 | ~22 | — | — |
| NU-403-PSDH | 2.23 | ~9 | ~26 | — | — |
| CPM-6 | 2.89 | 7.3 | 25.1 | 6.15 | 7.2 |
| Co2+-CPM-6 | 3.20 | 9.3 | 25.9 | 6.28 | 7.8 |
| SBMOF-2 | 2.83 | 10 | 26.4 | 10.5 | 8.6 |
| SBMOF-1 | 1.38 | 16① | 35 | 38.42 | 16.2 |
| CROFOUR-1-Ni | 1.8 | 22 | 37.4 | 18.73 | 24.3 |
| CROFOUR-2-Ni | 1.5 | 15.5 | 30.5 | 15.95 | 18.5 |
| Co squarate | 1.35 | 69.7 | 43.6 | 192.06 | 51.4 |
| UTSA-49 | 3 | 9.2 | 23.53 | — | — |
| ZU-62 | 3.76 | 9.72② | 35.2③ | — | — |
| FMOF-Cu | 0.8 | 2④ | 15 | — | — |
| NaA | 1.52~2.28 | 4.5⑤ | — | — | — |
| AgZ-PAN | 0.46⑥ | 4.6⑦ | — | — | — |
| Ag@ZSM-5 | ~1.2 | ~40 | 65 | — | ~3 |
| activated carbon | 4.2 | 2.9 | 28.2 | 16.2 | 9.1 |
| Z11CBF-1000-2 | 4.87 | 13.0 | 32.1 | 80.0 | 19.7 |
| CC3 | 2.69 | 20.4 | 31.3 | ~19 | ~2 |
| Noria | 1.55 | 9.4 | 24.5~26.9 | ~9 | ~9.5 |
表1 Xe/Kr吸附分离材料性能对比(298 K,100 kPa)
Table 1 Xe adsorption capacity and Xe/Kr selectivity for various materials at 298 K and 100 kPa
| 吸附剂 | Xe吸附容量/ (mmol·g-1) | IAST选择性 (Xe/Kr=20∶80) | Xe等量吸附热Qst/ (kJ·mol-1) | Xe Henry系数/(mmol·g-1·bar-1) | Henry选择性 |
|---|---|---|---|---|---|
| MOF-5 | 1.98 | 3 | 13.75 | — | 8.56 |
| HKUST-1 | 3.3 | 2.6 | 17.5 | 12.2 | 8.5 |
| Ni-MOF-74 | 4.2 | 4 | 22 | 8.4 | 5.8 |
| Ag@Ni-MOF-74 | 4.6 | 11.5 | 23.6 | — | — |
| MOF-505 | 6.31 | 9~10 | — | 10.26 | 6.8 |
| Co3(HCOO)6 | 2 | 12 | 28 | 9.9 | 8.7 |
| MOF-Cu-H | 3.19 | 15.8 | 33.4 | 39.74 | 15.8 |
| UiO-66 | 1.58 | ~7 | ~22 | — | — |
| NU-403-PSDH | 2.23 | ~9 | ~26 | — | — |
| CPM-6 | 2.89 | 7.3 | 25.1 | 6.15 | 7.2 |
| Co2+-CPM-6 | 3.20 | 9.3 | 25.9 | 6.28 | 7.8 |
| SBMOF-2 | 2.83 | 10 | 26.4 | 10.5 | 8.6 |
| SBMOF-1 | 1.38 | 16① | 35 | 38.42 | 16.2 |
| CROFOUR-1-Ni | 1.8 | 22 | 37.4 | 18.73 | 24.3 |
| CROFOUR-2-Ni | 1.5 | 15.5 | 30.5 | 15.95 | 18.5 |
| Co squarate | 1.35 | 69.7 | 43.6 | 192.06 | 51.4 |
| UTSA-49 | 3 | 9.2 | 23.53 | — | — |
| ZU-62 | 3.76 | 9.72② | 35.2③ | — | — |
| FMOF-Cu | 0.8 | 2④ | 15 | — | — |
| NaA | 1.52~2.28 | 4.5⑤ | — | — | — |
| AgZ-PAN | 0.46⑥ | 4.6⑦ | — | — | — |
| Ag@ZSM-5 | ~1.2 | ~40 | 65 | — | ~3 |
| activated carbon | 4.2 | 2.9 | 28.2 | 16.2 | 9.1 |
| Z11CBF-1000-2 | 4.87 | 13.0 | 32.1 | 80.0 | 19.7 |
| CC3 | 2.69 | 20.4 | 31.3 | ~19 | ~2 |
| Noria | 1.55 | 9.4 | 24.5~26.9 | ~9 | ~9.5 |
| 1 | Bussiahn R, Gortchakov S, Lange H, et al. Experimental and theoretical investigations of a low-pressure He-Xe discharge for lighting purpose[J]. Journal of Applied Physics, 2004, 95(9): 4627-4634. |
| 2 | Tsuchiya A, Obara N, Miwa M, et al. Hematoporphyrin derivative and laser photoradiation in the diagnosis and treatment of bladder cancer[J]. Journal of Urology, 1983, 130(1): 79-82. |
| 3 | Cullen S C, Gross E G. The anesthetic properties of xenon in animals and human beings, with additional observations on krypton[J]. Science, 1951, 113(2942): 580-582. |
| 4 | Albert M S, Cates G D, Driehuys B, et al. Biological magnetic resonance imaging using laser-polarized 129Xe[J]. Nature, 1994, 370(6486): 199-201. |
| 5 | 孙小兵. 核电在中国中长期能源供应体系中的作用[J]. 南方能源建设, 2016, 3(3): 6-15. |
| Sun X B. The role of nuclear power in China's medium and long term energy supply system[J]. Southern Energy Construction, 2016, 3(3): 6-15. | |
| 6 | Blue Ribbon Commission on America's Energy Future. Report to the secretary of energy[R]. Washington D C: U.S. Department of Energy, 2012. |
| 7 | Blue Ribbon Commission on America's Energy Future. Strategy for the management and disposal of used nuclear fuel and high-level radioactive waste[R]. Washington D C: U.S. Department of Energy, 2013. |
| 8 | Auer M, Kumberg T, Sartorius H, et al. Ten years of development of equipment for measurement of atmospheric radioactive xenon for the verification of the CTBT[J]. Pure and Applied Geophysics, 2010, 167(4): 471-486. |
| 9 | Kerry F G. Industrial Gas Handbook: Gas Separation and Purification[M]. Boca Raton, FL: CRC Press, 2007. |
| 10 | Jameson C J, Jameson A K, Lim H M. Competitive adsorption of xenon and krypton in zeolite NaA: 129Xe nuclear magnetic resonance studies and grand canonical Monte Carlo simulations[J]. The Journal of Chemical Physics, 1997, 107(11): 4364-4372. |
| 11 | Munakata K, Yamatsuki S, Tanaka K, et al. Screening test of adsorbents for recovery of krypton[J]. Journal of Nuclear Science and Technology, 2000, 37(1): 84-89. |
| 12 | Munakata K, Kanjo S, Yamatsuki S, et al. Adsorption of noble gases on silver-mordenite[J]. Journal of Nuclear Science and Technology, 2003, 40(9): 695-697. |
| 13 | Garn T G, Law J D, Greenhalgh M R, et al. Composite media for fluid stream processing, a method of forming the composite media, and a related method of processing a fluid stream: US8686083[P]. 2014-04-01. |
| 14 | Garn T G, Law J D, Greenhalgh M R. FY-12 INL krypton capture activities supporting the off-gas sigma team[R]. Idaho Falls, Idaho, USA: Idaho National Laboratory, 2012. |
| 15 | Daniel C, Elbaraoui A, Aguado S, et al. Xenon capture on silver-loaded zeolites: characterization of very strong adsorption sites[J]. Journal of Physical Chemistry C, 2013, 117(29): 15122-15129. |
| 16 | Deliere L, Coasne B, Topin S, et al. Breakthrough in xenon capture and purification using adsorbent-supported silver nanoparticles[J]. Chemistry-A European Journal, 2016, 22(28): 9660-9666. |
| 17 | Bazan R E, Bastos-Neto M, Moeller A, et al. Adsorption equilibria of O2, Ar, Kr and Xe on activated carbon and zeolites: single component and mixture data[J]. Adsorption-Journal of the International Adsorption Society, 2011, 17(2): 371-383. |
| 18 | Thallapally P K, Grate J W, Motkuri R K. Facile xenon capture and release at room temperature using a metal-organic framework: a comparison with activated charcoal[J]. Chemical Communications, 2012, 48(3): 347-349. |
| 19 | Gong Y J, Tang Y M, Mao Z H, et al. Metal-organic framework derived nanoporous carbons with highly selective adsorption and separation of xenon[J]. Journal of Materials Chemistry A, 2018, 6(28): 13696-13704. |
| 20 | 冯淑娟, 周崇阳, 周国庆, 等. 氙在活性炭和碳分子筛上的动态吸附性能[J]. 核化学与放射化学, 2010, 32(5): 274-279. |
| Feng S J, Zhou C Y, Zhou G Q, et al. Dynamic adsorption property of xenon on activated carbon and carbon molecular sieves[J]. Journal of Nuclear and Radiochemistry, 2010, 32(5): 274-279. | |
| 21 | 刘孟, 张莉, 王茜. 碳分子筛和活性炭吸附氙气性能的研究[J]. 湘潭大学自然科学学报, 2015, 37(2): 27-32. |
| Liu M, Zhang L, Wang Q. Adsorption properties of xenon by carbon molecular sieve and activated charcoal[J]. Natural Science Journal of Xiangtan University, 2015, 37(2): 27-32. | |
| 22 | Zhou H C, Long J R, Yaghi O M. Introduction to metal-organic frameworks[J]. Chemical Reviews, 2012, 112(2): 673-674. |
| 23 | Banerjee D, Cairns A J, Liu J, et al. Potential of metal-organic frameworks for separation of xenon and krypton[J]. Accounts of Chemical Research, 2015, 48(2): 211-219. |
| 24 | Furukawa H, Ko N, Go Y B, et al. Ultrahigh porosity in metal-organic frameworks[J]. Science, 2010, 329(5990): 424-428. |
| 25 | Grzesiak A L, Uribe F J, Ockwig N W, et al. Polymer-induced heteronucleation for the discovery of new extended solids[J]. Angewandte Chemie International Edition, 2006, 45(16): 2553-2556. |
| 26 | Cohen S M. Modifying MOFs: new chemistry, new materials[J]. Chemical Science, 2010, 1(1): 32-36. |
| 27 | Wang B, Xie L H, Wang X Q, et al. Applications of metal-organic frameworks for green energy and environment: new advances in adsorptive gas separation, storage and removal[J]. Green Energy & Environment, 2018, 3(3): 191-228. |
| 28 | Lv X L, Wang K C, Wang B, et al. A base-resistant metalloporphyrin metal-organic framework for C—H bond halogenation[J]. Journal of the American Chemical Society, 2017, 139(1): 211-217. |
| 29 | Yang F, Xu G, Dou Y B, et al. A flexible metal-organic framework with a high density of sulfonic acid sites for proton conduction[J]. Nature Energy, 2017, 2(11): 877-883. |
| 30 | Horcajada P, Chalati T, Serre C, et al. Porous metal-organic framework nanoscale carriers as a potential platform for drug delivery and imaging[J]. Nature Materials, 2010, 9(2): 172-178. |
| 31 | Li J R, Yu J M, Lu W G, et al. Porous materials with pre-designed single-molecule traps for CO2 selective adsorption[J]. Nature Communications, 2013, 4(1): 1538. |
| 32 | Chen K J, Madden D G, Pham T, et al. Tuning pore size in square-lattice coordination networks for size-selective sieving of CO2[J]. Angewandte Chemie International Edition, 2016, 55(35): 10268-10272. |
| 33 | Chen B L, Ockwig N W, Millward A R, et al. High H2 adsorption in a microporous metal-organic framework with open metal sites[J]. Angewandte Chemie International Edition, 2005, 44(30): 4745-4749. |
| 34 | Dinca M, Long J R. Hydrogen storage in microporous metal-organic frameworks with exposed metal sites[J]. Angewandte Chemie International Edition, 2008, 47(36): 6766-6779. |
| 35 | Wood C D, Tan B, Trewin A, et al. Microporous organic polymers for methane storage[J]. Advanced Materials, 2008, 20(10): 1916-1921. |
| 36 | Niu Z, Cui X L, Pham T, et al. A metal-organic framework based methane nano-trap for the capture of coal-mine methane[J]. Angewandte Chemie International Edition, 2019, 58(30): 10138-10141. |
| 37 | Nijem N, Wu H, Canepa P, et al. Tuning the gate opening pressure of metal-organic frameworks (MOFs) for the selective separation of hydrocarbons[J]. Journal of the American Chemical Society, 2012, 134(37): 15201-15204. |
| 38 | Li B, Cui X L, Daniel O'Nolan, et al. An ideal molecular sieve for acetylene removal from ethylene with record selectivity and productivity[J]. Advanced Materials, 2017, 29(47): 1704210. |
| 39 | Bao Z B, Wang J W, Zhang Z G, et al. Molecular sieving of ethane from ethylene through the molecular cross-section size differentiation in gallate-based metal-organic frameworks[J]. Angewandte Chemie International Edition, 2018, 57(49): 16020-16025. |
| 40 | Mueller U, Schubert M, Teich F, et al. Metal-organic frameworks-prospective industrial application[J]. Journal of Materials Chemistry, 2006, 16(7): 626-636. |
| 41 | Li J R, Kuppler R J, Zhou H C. Selective gas adsorption and separation in metal-organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1477-1504. |
| 42 | Liu J, Strachan D M, Thallapally P K. Enhanced noble gas adsorption in Ag@MOF-74Ni[J]. Chemical Communications, 2013, 50(4): 466-468. |
| 43 | Chui S Y, Lo M F, Charmant J P H, et al. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n[J]. Science, 1999, 283(5405): 1148-1150. |
| 44 | Liu J, Thallapally P K, Strachan D. Metal-organic frameworks for removal of Xe and Kr from nuclear fuel reprocessing plants[J]. Langmuir, 2012, 28(31): 11584-11589. |
| 45 | Hulvey Z, Lawler K V, Qiao Z, et al. Noble gas adsorption in copper trimesate, HKUST-1: an experimental and computational study[J]. The Journal of Physical Chemistry C, 2013, 117(39): 20116-20126. |
| 46 | Böhlmann W, Pöppl A, Sabo M, et al. Characterization of the metal-organic framework compound Cu3(benzene1,3,5-tricarboxylate)2 by means of 129Xe nuclear magnetic and electron paramagnetic resonance spectroscopy[J]. The Journal of Physical Chemistry B, 2006, 110(41): 20177-20181. |
| 47 | Liu Z, Wu Y, Liu B, et al. Tuning the adsorption and separation properties of noble gases and N2 in CuBTC by ligand functionalization[J]. RSC Advances, 2016, 6(94): 91093-91101. |
| 48 | Chen X, Plonka A M, Banerjee D, et al. Direct observation of Xe and Kr adsorption in a Xe-selective microporous metal-organic framework[J]. Journal of the American Chemical Society, 2015, 137(22): 7007-7010. |
| 49 | Dan-Hardi M, Serre C, Frot T, et al. A new photoactive crystalline highly porous titanium (Ⅳ) dicarboxylate[J]. Journal of the American Chemical Society, 2009, 131(31): 10857-10859. |
| 50 | Fu Y, Sun D, Chen Y, et al. An amine-functionalized titanium metal-organic framework photocatalyst with visible-light-induced activity for CO2 reduction[J]. Angewandte Chemie International Edition, 2012, 51(14): 3364-3367. |
| 51 | Wang Z, Cohen S M. Postsynthetic covalent modification of a neutral metal-organic framework[J]. Journal of the American Chemical Society, 2007, 129(41): 12368-12369. |
| 52 | Tanabe K K, Wang Z, Cohen S M. Systematic functionalization of a metal-organic framework via a postsynthetic modification approach[J]. Journal of the American Chemical Society, 2008, 130(26): 8508-8517. |
| 53 | Yaghi O M, Kalmutzki M J, Diercks C S. Introduction to Reticular Chemistry[M]. Weinheim, Germany: Wiley-VCH, 2019: 145-173. |
| 54 | Chen R, Zhang J, Chelora J, et al. Ruthenium(Ⅱ) complex incorporated UiO-67 metal-organic framework nanoparticles for enhanced two-photon fluorescence imaging and photodynamic cancer therapy[J]. ACS Applied Materials & Interfaces, 2017, 9(7): 5699-5708. |
| 55 | Kim M, Cahill J F, Fei H, et al. Postsynthetic ligand and cation exchange in robust metal-organic frameworks[J]. Journal of the American Chemical Society, 2012, 134(43): 18082-18088. |
| 56 | Kim M, Cahill J F, Su Y, et al. Postsynthetic ligand exchange as a route to functionalization of ‘inert' metal-organic frameworks[J]. Chemical Science, 2011, 3(1): 126-130. |
| 57 | Lee S J, Kim S, Kim E J, et al. Adsorptive separation of xenon/krypton mixtures using ligand controls in a zirconium-based metal-organic framework[J]. Chemical Engineering Journal, 2018, 335: 345-351. |
| 58 | Sikora B J, Wilmer C E, Greenfield M L, et al. Thermodynamic analysis of Xe/Kr selectivity in over 137000 hypothetical metal-organic frameworks[J]. Chemical Science, 2012, 3(7): 2217-2223. |
| 59 | Wang H, Yao K X, Zhang Z J, et al. The first example of commensurate adsorption of atomic gas in a MOF and effective separation of xenon from other noble gases[J]. Chemical Science, 2014, 5(2): 620-624. |
| 60 | Xiong S S, Gong Y J, Hu S L, et al. A microporous metal-organic framework with commensurate adsorption and highly selective separation of xenon[J]. Journal of Materials Chemistry A, 2018, 6(11): 4752-4758. |
| 61 | Li T, Kozlowski M T, Doud E A, et al. Stepwise ligand exchange for the preparation of a family of mesoporous MOFs[J]. Journal of the American Chemical Society, 2013, 135(32): 11688-11691. |
| 62 | Idrees K B, Chen Z, Zhang X, et al. Tailoring pore aperture and structural defects in zirconium-based metal-organic frameworks for krypton/xenon separation[J]. Chemistry of Materials, 2020, 32(9): 3776-3782. |
| 63 | 刘博煜, 龚有进, 刘强, 等. 新型多孔材料在惰性气体Xe/Kr分离中的应用[J]. 材料导报, 2017, 31(19): 54-62. |
| Liu B Y, Gong Y J, Liu Q, et al. Application of novel porous materials in noble gas Xe/Kr separation[J]. Materials Reports, 2017, 31(19): 54-62. | |
| 64 | Liu B Y, Gong Y J, Wu X N, et al. Enhanced xenon adsorption and separation with an anionic indium-organic framework by ion exchange with Co2+[J]. RSC Advances, 2017, 7(87): 55012-55019. |
| 65 | Banerjee D, Simon C M, Plonka A M, et al. Metal-organic framework with optimally selective xenon adsorption and separation[J]. Nature Communications, 2016, 7: ncomms11831. |
| 66 | Mohamed M H, Elsaidi S K, Pham T, et al. Hybrid ultra-microporous materials for selective xenon adsorption and separation[J]. Angewandte Chemie International Edition, 2016, 55(29): 8285-8289. |
| 67 | Li L Y, Guo L D, Zhang Z G, et al. A robust squarate-based metal-organic framework demonstrates record-high affinity and selectivity for xenon over krypton[J]. Journal of the American Chemical Society, 2019, 141(23): 9358-9364. |
| 68 | Witman M, Ling S, Jawahery S, et al. The influence of intrinsic framework flexibility on adsorption in nanoporous materials[J]. Journal of the American Chemical Society, 2017, 139(15): 5547-5557. |
| 69 | Xiong S S, Liu Q, Wang Q, et al. A flexible zinc tetrazolate framework exhibiting breathing behaviour on xenon adsorption and selective adsorption of xenon over other noble gases[J]. Journal of Materials Chemistry A, 2015, 3(20): 10747-10752. |
| 70 | Wang Q J, Ke T,Yang L F, et al. Separation of Xe from Kr with record selectivity and productivity in anion-pillared ultramicroporous materials by inverse size-sieving effect[J]. Angewandte Chemie International Edition, 2020, 59(9): 3423-3428. |
| 71 | Fernandez C A, Liu J, Thallapally P K, et al. Switching Kr/Xe selectivity with temperature in a metal-organic framework[J]. Journal of the American Chemical Society, 2012, 134(22): 9046-9049. |
| 72 | Sun X D, Yao S, Li G H, et al. A flexible doubly interpenetrated metal-organic framework with breathing behavior and tunable gate opening effect by introducing Co2+ into Zn4O clusters[J]. Inorganic Chemistry, 2017, 56(11): 6645-6651. |
| 73 | Zhu A X, Yang Q Y, Mukherjee S, et al. Tuning the gate-opening pressure in a switching pcu coordination network, X-pcu-5-Zn, by pillar-ligand substitution[J]. Angewandte Chemie International Edition, 2019, 58(50): 18212-18217. |
| 74 | Elsaidi S K, Mohamed M H, Banerjee D, et al. Flexibility in metal-organic frameworks: a fundamental understanding[J]. Coordination Chemistry Reviews, 2018, 358: 125-152. |
| 75 | Kong G Q, Wu C D. Four novel coordination polymers based on a flexible zwitterionic ligand and their framework dependent luminescent properties[J]. Crystal Growth & Design, 2010, 10(10): 4590-4595. |
| 76 | Holst J R, Trewin A, Cooper A I. Porous organic molecules[J]. Nature Chemistry, 2010, 2(11): 915-920. |
| 77 | Chen L J, Reiss P S, Chong S Y, et al. Separation of rare gases and chiral molecules by selective binding in porous organic cages[J]. Nature Materials, 2014, 13(10): 954-960. |
| 78 | Patil R S, Banerjee D, Simon C M, et al. Noria: a highly Xe-selective nanoporous organic solid[J]. Chemistry-A European Journal, 2016, 22(36): 12618-12623. |
| 79 | Tozawa T, Jones J T A, Swamy S I, et al. Porous organic cages[J]. Nature Materials, 2009, 8(12): 973-978. |
| 80 | Bezzu C G, Helliwell M, Warren J E, et al. Heme-like coordination chemistry within nanoporous molecular crystals[J]. Science, 2010, 327(5973): 1627-1630. |
| [1] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [2] | 李沐紫, 贾国伟, 赵砚珑, 张鑫, 李建荣. 金属有机框架材料对非二氧化碳温室气体捕捉研究进展[J]. 化工学报, 2023, 74(1): 365-379. |
| [3] | 毛恒, 王月, 王森, 刘伟民, 吕静, 陈甫雪, 赵之平. APTES改性ZIF-L/PEBA混合基质膜强化渗透汽化分离苯酚研究[J]. 化工学报, 2022, 73(3): 1389-1402. |
| [4] | 李媛, 张飞飞, 王丽, 杨江峰, 李立博, 李晋平. MIL-101Cr-F/Cl用于N2O的捕集研究[J]. 化工学报, 2021, 72(9): 4759-4767. |
| [5] | 韩笑,陈雨亭,苏宝根,鲍宗必,张治国,杨亦文,任其龙,杨启炜. 己烷异构体吸附分离材料研究进展[J]. 化工学报, 2021, 72(7): 3445-3465. |
| [6] | 李建惠, 兰天昊, 陈杨, 杨江峰, 李立博, 李晋平. MOF复合材料在气体吸附分离中的研究进展[J]. 化工学报, 2021, 72(1): 167-179. |
| [7] | 马佳欢, 杨微微, 白羽, 孙克宁. 二维金属有机框架及其衍生物用于电催化分解水的研究进展[J]. 化工学报, 2020, 71(9): 4006-4030. |
| [8] | 宋渤,杨敏,安然,王晓坡. 单原子气体第二介电维里系数准确计算[J]. 化工学报, 2019, 70(S2): 50-53. |
| [9] | 安珂, 杨冬, 赵展烽, 任汉杰, 陈瑶, 周致远, 姜忠义. 金属有机框架光催化剂微环境调控研究进展[J]. 化工学报, 2019, 70(10): 3776-3790. |
| [10] | 崔希利, 邢华斌. 金属有机框架材料分离低碳烃的研究进展[J]. 化工学报, 2018, 69(6): 2339-2352. |
| [11] | 盖月庭,顾昊辉,梁战桥,刘中勋,周震寰. 对二乙苯生产技术评述[J]. 化工进展, 2014, 33(03): 538-541. |
| [12] | 郝广平,李文翠,陆安慧. 纳米结构多孔固体在二氧化碳吸附分离中的应用[J]. 化工进展, 2012, 31(11): 2493-2510. |
| [13] | 李云东,易红宏,唐晓龙,李芬容,何 丹. 吸附剂特性对CO2/CH4吸附分离的影响分析[J]. 化工进展, 2012, 31(05): 974-980. |
| [14] | 席芳,林文胜,顾安忠,刘薇,齐研科. 煤层气在活性炭和炭分子筛上变压吸附分离 [J]. CIESC Journal, 2010, 61(S2): 54-57. |
| [15] | 张英, 苏宏业, 褚健. 基于支持向量基的关联规则挖掘及其在模拟移动床PX吸附分离过程中的应用[J]. CIESC Journal, 2005, 13(6): 751-757. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号