化工学报 ›› 2021, Vol. 72 ›› Issue (1): 320-333.DOI: 10.11949/0438-1157.20201089
收稿日期:2020-08-03
修回日期:2020-10-24
出版日期:2021-01-05
发布日期:2021-01-05
通讯作者:
周景文
作者简介:王炼(1992—),男,博士研究生,基金资助:
WANG Lian( ),WU Di,ZHOU Jingwen(
),WU Di,ZHOU Jingwen( )
)
Received:2020-08-03
Revised:2020-10-24
Online:2021-01-05
Published:2021-01-05
Contact:
ZHOU Jingwen
摘要:
木脂素是一类分布于植物中的次级代谢产物,由两分子苯丙素衍生物聚合而成。木脂素具有抗细菌、抗病毒和抗真菌活性,被广泛用于药物和食品中。目前,木脂素的生产主要依赖于植物提取。植物生长周期长、木脂素含量低等问题,限制了木脂素的商业应用。随着木脂素合成路径和关键酶的不断解析,木脂素的生物催化合成过程受到了越来越多的关注。本文总结了典型木脂素的生物活性、生物合成路径和微生物法生产的研究进展,可以为深入研究木脂素的微生物合成提供参考。
中图分类号:
王炼, 吴迪, 周景文. 木脂素的生物合成及其微生物法生产的研究进展[J]. 化工学报, 2021, 72(1): 320-333.
WANG Lian, WU Di, ZHOU Jingwen. Research progress of lignans biosynthesis and their microbial production[J]. CIESC Journal, 2021, 72(1): 320-333.
| 木脂素类别 | 典型化合物 | 天然来源 | 生物活性 | 其他合成方式 |
|---|---|---|---|---|
| 芳基萘 | 山荷叶素 | 丘生闭花木 | 治疗胃癌 | 无报道 |
| 赛菊芋黄素 | 台湾杉 | 抑制脑肿瘤转移 | 无报道 | |
| 芳基四氢萘 | 鬼臼毒素 | 桃儿七、足叶草 | 抗肿瘤药物的前体 | 内生菌TW5 |
| 南烛木树脂酚 | 小果南烛 | 皮肤美白剂 | 植物细胞培养 | |
| 双并四氢呋喃 | 松脂素 | 木本植物、纤维植物 | 抗细菌和真菌、改善记忆障碍 | 植物细胞培养、微生物发酵 |
| 芝麻素 | 芝麻 | 抑制和阿尔兹海默症相关的部分症状 | 微生物发酵 | |
| 二苄基丁内酯 | 罗汉松脂素 | 种子、蔬菜和水果 | 抗氧化、抗雌激素、抗骨质疏松 | 微生物发酵、化学合成 |
| 牛蒡酚 | 牛蒡 | 降低收缩血压 | 无报道 | |
| 二苯并环辛二烯 | 五加前胡素 | 剑叶菊 | 未用于药物治疗 | 化学合成 |
| 二芳基丁烷 | 叶下珠脂素 | 叶下珠 | 抑制HepG2的增殖 | 无报道 |
| 二苄基丁酸乳酯 | 荜澄茄素 | 木材、根和树脂 | 镇痛、消炎 | 无报道 |
| 呋喃 | 落叶松脂素 | 菲律宾茜草 | 抗菌、抗氧化 | 微生物发酵 |
表1 木脂素的种类及其典型化合物的基本性质
Table 1 The categories of lignans and the basic characters of typical compounds
| 木脂素类别 | 典型化合物 | 天然来源 | 生物活性 | 其他合成方式 |
|---|---|---|---|---|
| 芳基萘 | 山荷叶素 | 丘生闭花木 | 治疗胃癌 | 无报道 |
| 赛菊芋黄素 | 台湾杉 | 抑制脑肿瘤转移 | 无报道 | |
| 芳基四氢萘 | 鬼臼毒素 | 桃儿七、足叶草 | 抗肿瘤药物的前体 | 内生菌TW5 |
| 南烛木树脂酚 | 小果南烛 | 皮肤美白剂 | 植物细胞培养 | |
| 双并四氢呋喃 | 松脂素 | 木本植物、纤维植物 | 抗细菌和真菌、改善记忆障碍 | 植物细胞培养、微生物发酵 |
| 芝麻素 | 芝麻 | 抑制和阿尔兹海默症相关的部分症状 | 微生物发酵 | |
| 二苄基丁内酯 | 罗汉松脂素 | 种子、蔬菜和水果 | 抗氧化、抗雌激素、抗骨质疏松 | 微生物发酵、化学合成 |
| 牛蒡酚 | 牛蒡 | 降低收缩血压 | 无报道 | |
| 二苯并环辛二烯 | 五加前胡素 | 剑叶菊 | 未用于药物治疗 | 化学合成 |
| 二芳基丁烷 | 叶下珠脂素 | 叶下珠 | 抑制HepG2的增殖 | 无报道 |
| 二苄基丁酸乳酯 | 荜澄茄素 | 木材、根和树脂 | 镇痛、消炎 | 无报道 |
| 呋喃 | 落叶松脂素 | 菲律宾茜草 | 抗菌、抗氧化 | 微生物发酵 |
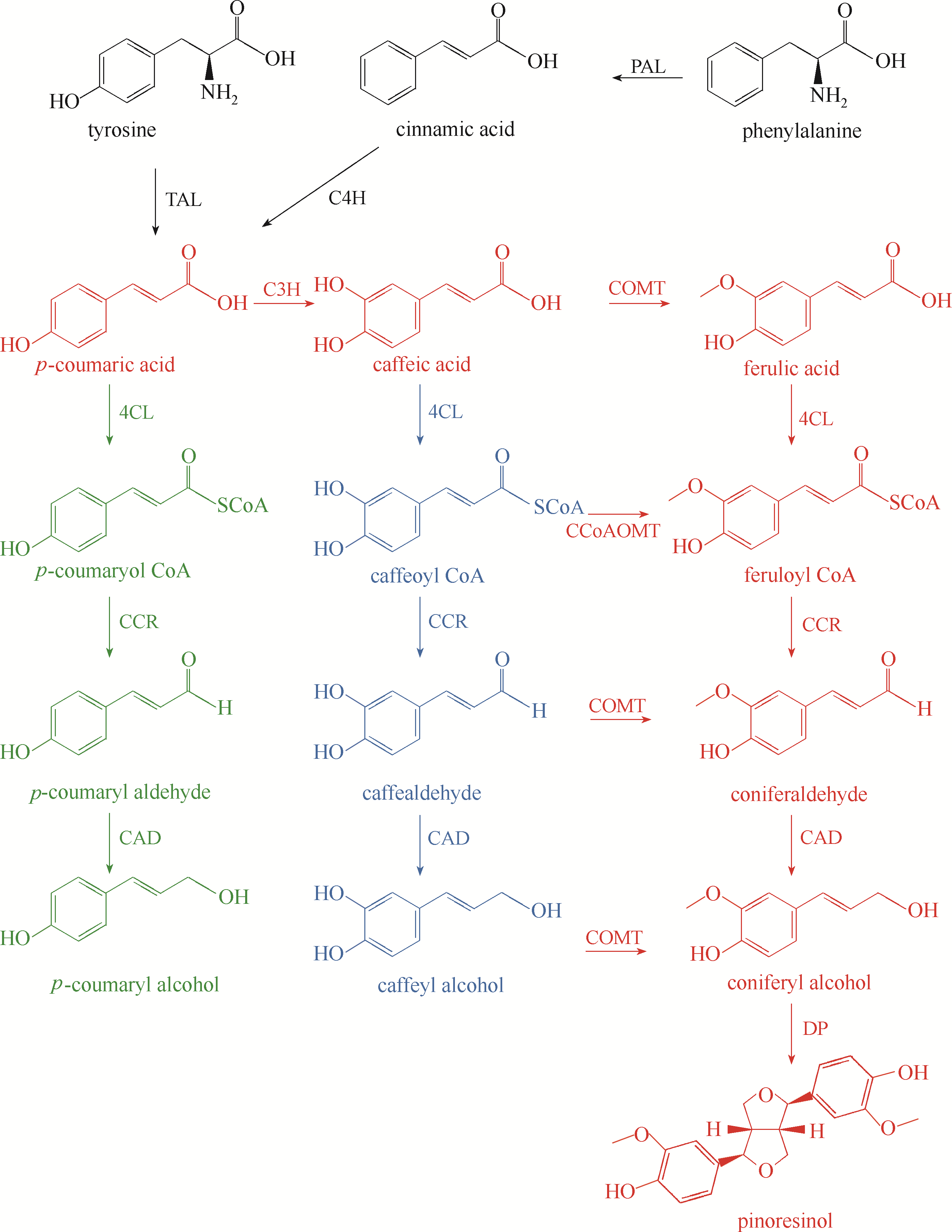
图3 植物松脂素合成途径松脂素、对香豆醇和咖啡醇的合成路径分别用红色、绿色和蓝色表示;PAL—苯丙氨酸解氨酶(phenylalanine ammonia-lyase);C4H—肉桂酸-4-羟化酶(cinnamate 4-hydroxylase);TAL—酪氨酸解氨酶(tyrosine ammonia-lyase);C3H—香豆酸羟化酶(coumarate3-hydroxylase);COMT—咖啡酸/5-羟基-阿魏酸 O-甲基转移酶(caffeic acid/5-hydroxy-ferulic acid O-methytransferase);CCoAOMT—咖啡酰辅酶A 3-O-甲基转移酶(caffeoyl CoA 3-O-methyltransferase);4CL—4-香豆酰辅酶A连接酶(4-hydroxycinnamate CoA ligase);CCR—肉桂酰辅酶A还原酶(cinnamoyl CoA reductase);CAD—肉桂醇脱氢酶(cinnamyl alcohol dehydrogenase);DP—同化蛋白(dirigent protein)
Fig.3 The synthesis pathway of pinoresinol in plants

图5 亚麻木酚素和鬼臼毒素的生物合成路径PLR—松脂素/落叶松脂素还原酶(pinoresinol/lariciresinol reductase);UGT74S1—尿苷糖基转移酶;SDH—开环异落叶松脂素脱氢酶(secoisolariciresinol dehydrogenase)
Fig.5 The biosynthetic pathway of secoisolariciresinol diglucoside and podophyllotoxin
| 1 | Lalaleo L, Alcazar R, Palazon J, et al. Comparing aryltetralin lignan accumulation patterns in four biotechnological systems of Linum album [J]. Journal of Plant Physiology, 2018, 228: 197-207. |
| 2 | Li Y, Xie S, Ying J, et al. Chemical structures of lignans and neolignans isolated from Lauraceae [J]. Molecules, 2018, 23(12): 3164. |
| 3 | Cravens A, Payne J, Smolke C D. Synthetic biology strategies for microbial biosynthesis of plant natural products [J]. Nature Communications, 2019, 10(1): 2142-2153. |
| 4 | Lv Y, Cheng X, Wu D, et al. Improving bioconversion of eugenol to coniferyl alcohol by in situ eliminating harmful H2O2 [J]. Bioresource Technology, 2018, 267: 578-583. |
| 5 | Chen Z, Sun X, Li Y, et al. Metabolic engineering of Escherichia coli for microbial synthesis of monolignols [J]. Metabolic Engineering, 2017, 39: 102-109. |
| 6 | Lv Y, Cheng X, Du G, et al. Engineering of an H2O2 auto-scavenging in vivo cascade for pinoresinol production [J]. Biotechnology and Bioengineering, 2017, 114(9): 2066-2074. |
| 7 | Yang J, Liang J, Shao L, et al. Green production of silybin and isosilybin by merging metabolic engineering approaches and enzymatic catalysis [J]. Metabolic Engineering, 2020, 59: 44-52. |
| 8 | Ono E, Nakai M, Fukui Y, et al. Formation of two methylenedioxy bridges by a sesamum CYP81Q protein yielding a furofuran lignan, (+)-sesamin [J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(26): 10116-10121. |
| 9 | Umezawa T. Diversity in lignan biosynthesis [J]. Phytochemistry Reviews, 2003, 2(3): 371-390. |
| 10 | Nakatsubo T, Mizutani M, Suzuki S, et al. Characterization of Arabidopsis thaliana pinoresinol reductase, a new type of enzyme involved in lignan biosynthesis [J]. Journal of Biological Chemistry, 2008, 283(23): 15550-15557. |
| 11 | Teponno R B, Kusari S, Spiteller M. Recent advances in research on lignans and neolignans [J]. Natural Product Reports, 2016, 33(9): 1044-1092. |
| 12 | Sellars J D, Steel P G. Advances in the synthesis of aryltetralin lignan lactones [J]. European Journal of Organic Chemistry, 2007, 2007(23): 3815-3828. |
| 13 | Shen W, Zou X, Chen M, et al. Effects of diphyllin as a novel V-ATPase inhibitor on gastric adenocarcinoma [J]. European Journal of Pharmacology, 2011, 667(1/2/3): 330-338. |
| 14 | Chen H, Liu P, Zhang T, et al. Effects of diphyllin as a novel V-ATPase inhibitor on TE-1 and ECA-109 cells [J]. Oncology Reports, 2018, 39(3): 921-928. |
| 15 | Thamburaj S, Ramaraj E, Sethupathy S, et al. Antibacterial and antibiofilm activities of diphyllin against fish pathogens [J]. Microbial Pathogenesis, 2020, 145: 104232. |
| 16 | Lin Y M, Kuo W W, Velmurugan B K, et al. Helioxanthin suppresses the cross talk of COX-2/PGE2 and EGFR/ERK pathway to inhibit Arecoline-induced Oral Cancer Cell (T28) proliferation and blocks tumor growth in xenografted nude mice [J]. Environmental Toxicology, 2016, 31(12): 2045-2056. |
| 17 | Kao T T, Lin C C, Shia K S. The total synthesis of Retrojusticidin B, Justicidin E, and Helioxanthin [J]. Journal of Organic Chemistry, 2015, 80(13): 6708-6714. |
| 18 | Guerram M, Jiang Z Z, Zhang L Y. Podophyllotoxin, a medicinal agent of plant origin: past, present and future [J]. Chinese Journal of Natural Medicines, 2012, 10(3): 161-169. |
| 19 | Canel C, Moraes R M, Dayan F E, et al. Podophyllotoxin [J]. Phytochemistry, 2000, 54(2): 115-120. |
| 20 | Bala M, Goel H C. Radioprotective effect of podophyllotoxin in Saccharomyces cerevisiae [J]. Journal of Environmental Pathology Toxicology and Oncology, 2004, 23(2): 139-144. |
| 21 | Casey M, Keaveney C M. A concise stereocontrolled formal total synthesis of (+/-)-podophyllotoxin using sulfoxide chemistry [J]. Chemical Communications, 2004, (2): 184-185. |
| 22 | Huang J X, Zhang J, Zhang X R, et al. Mucor fragilis as a novel source of the key pharmaceutical agents podophyllotoxin and kaempferol [J]. Pharmaceutical Biology, 2014, 52(10): 1237-1243. |
| 23 | Liu H, Sui X, Li X, et al. Lyoniresinol inhibits melanogenic activity through the induction of microphthalmia-associated transcription factor and extracellular receptor kinase activation [J]. Molecular and Cellular Biochemistry, 2013, 373(1/2): 211-216. |
| 24 | Rahman M A, Katayama T, Suzuki T, et al. Stereochemistry and biosynthesis of (+)-lyoniresinol, a syringyl tetrahydronaphthalene lignan in Lyonia ovalifolia var. elliptica (I): Isolation and stereochemistry of syringyl lignans and predicted precursors to (+)-lyoniresinol from wood [J]. Journal of Wood Science, 2007, 53(2): 161-167. |
| 25 | Takemoto M, Fukuyo A, Aoshima Y, et al. Synthesis of Lyoniresinol with combined utilization of synthetic chemistry and biotechnological methods [J]. Chemical & Pharmaceutical Bulletin, 2006, 54(2): 226-229. |
| 26 | Xu W H, Zhao P, Wang M, et al. Naturally occurring furofuran lignans: structural diversity and biological activities [J]. Natural Product Research, 2019, 33(9): 1357-1373. |
| 27 | Zhou H, Ren J, Li Z. Antibacterial activity and mechanism of pinoresinol from Cinnamomum Camphora leaves against food-related bacteria [J]. Food Control, 2017, 79: 192-199. |
| 28 | Hwang B, Lee J, Liu Q H, et al. Antifungal effect of (+)-pinoresinol isolated from Sambucus williamsii [J]. Molecules, 2010, 15(5): 3507-3516. |
| 29 | Yu J, Kwon H, Cho E, et al. The effects of pinoresinol on cholinergic dysfunction-induced memory impairments and synaptic plasticity in mice [J]. Food Chem. Toxicol., 2019, 125: 376-382. |
| 30 | Pathak N, Bhaduri A, Rai A K. Sesame: bioactive compounds and health benefits [M]//Mérillon J M, Ramawat K G. Bioactive Molecules in Food. Cham: Springer International Publishing, 2017: 1-20. |
| 31 | Katayama S, Sugiyama H, Kushimoto S, et al. Effects of sesaminol feeding on brain Aβ accumulation in a senescence-accelerated mouse-prone 8 [J]. Journal of Agricultural and Food Chemistry, 2016, 64(24): 4908-4913. |
| 32 | Watanabe M, Iizumi Y, Sukeno M, et al. The pleiotropic regulation of cyclin D1 by newly identified sesaminol-binding protein ANT2 [J]. Oncogenesis, 2017, 6(4): e311. |
| 33 | Schmitt J, Petersen M. Pinoresinol and matairesinol accumulation in a Forsythia x intermedia cell suspension culture [J]. Plant Cell Tissue and Organ Culture, 2002, 68(1): 91-98. |
| 34 | Szokol-Borsodi L, Sólyomváry A, Molnár-Perl I, et al. Optimum yields of dibenzylbutyrolactone-type lignans from cynareae fruits, during their ripening, germination and enzymatic hydrolysis processes, determined by on-line chromatographic methods [J]. Phytochemical Analysis, 2012, 23(6): 598-603. |
| 35 | Yamawaki M, Nishi K, Nishimoto S, et al. Immunomodulatory effect of (-)-matairesinol in vivo and ex vivo [J]. Bioscience Biotechnology and Biochemistry, 2011, 75(5): 859-863. |
| 36 | Choi S W, Park K I, Yeon J T, et al. Anti-osteoclastogenic activity of matairesinol via suppression of p38/ERK-NFATc1 signaling axis [J]. BMC Complementary and Alternative Medicine, 2014, 14: 1-8. |
| 37 | Su S, Cheng X, Wink M. Cytotoxicity of arctigenin and matairesinol against the T-cell lymphoma cell line CCRF-CEM [J]. Journal of Pharmacy and Pharmacology, 2015, 67(9): 1316-1323. |
| 38 | Okunishi T, Sakakibara N, Suzuki S, et al. Stereochemistry of matairesinol formation by Daphne secoisolariciresinol dehydrogenase [J]. Journal of Wood Science, 2004, 50(1): 77-81. |
| 39 | Zhu F, Li W, Wang Q, et al. Regioselective oxidative coupling approach to the synthesis of (+/-)-matairesinol and (+/-)-secoisolariciresinol [J]. Synlett, 2006, (11): 1780-1782. |
| 40 | Liu Y, Wang G, Yang M, et al. Arctigenin reduces blood pressure by modulation of nitric oxide synthase and NADPH oxidase expression in spontaneously hypertensive rats [J]. Biochemical and Biophysical Research Communications, 2015, 468(4): 837-842. |
| 41 | Yao X, Zhu F, Zhao Z, et al. Arctigenin enhances chemosensitivity of cancer cells to cisplatin through inhibition of the STAT3 signaling pathway [J]. Journal of Cellular Biochemistry, 2011, 112(10): 2837-2849. |
| 42 | Borbely S, Jocsak G, Moldovan K, et al. Arctigenin reduces neuronal responses in the somatosensory cortex via the inhibition of non-NMDA glutamate receptors [J]. Neurochemistry International, 2016, 97: 83-90. |
| 43 | Joncour A, Decor A, Liu J M, et al. Asymmetric synthesis of antimicrotubule biaryl hybrids of allocolchicine and steganacin [J]. Chemistry-a European Journal, 2007, 13(19): 5450-5465. |
| 44 | Augros D, Yalcouye B, Choppin S, et al. Transition-metal-free synthesis of a known intermediate in the formal synthesis of (-)-steganacin [J]. European Journal of Organic Chemistry, 2017, 2017(3): 497-503. |
| 45 | Ooi K L, Loh S I, Sattar M A, et al. Cytotoxic, caspase-3 induction and in vivo hepatoprotective effects of phyllanthin, a major constituent of Phyllanthus niruri [J]. Journal of Functional Foods, 2015, 14: 236-243. |
| 46 | Lam P L, Gambari R, Yip J, et al. Development of phyllanthin containing microcapsules and their improved biological activity towards skin cells and Staphylococcus aureus [J]. Bioorganic & Medicinal Chemistry Letters, 2012, 22(1): 468-471. |
| 47 | Dunkoksung W, Vardhanabhuti N, Jianmongkol S. Potential p-glycoprotein-mediated herb-drug interaction of phyllanthin at the intestinal absorptive barrier [J]. Journal of Pharmacy and Pharmacology, 2019, 71(2): 213-219. |
| 48 | Bruno-Colmenarez J, Usubillaga A, Khouri N, et al. Cubebin, a lignan isolated from Aristolochia odoratissima L [J]. Acta Crystallographica Section E-Crystallographic Communications, 2007, 63: O2046-O2047. |
| 49 | Rajalekshmi D S, Kabeer F A, Madhusoodhanan A R, et al. Anticancer activity studies of cubebin isolated from Piper cubeba and its synthetic derivatives [J]. Bioorganic & Medicinal Chemistry Letters, 2016, 26(7): 1767-1771. |
| 50 | Niwa A M, Marcarini J C, Sartori D, et al. Effects of (-)-cubebin (Piper cubeba) on cytotoxicity, mutagenicity and expression of p38 MAP kinase and GSTa2 in a hepatoma cell line [J]. Journal of Food Composition and Analysis, 2013, 30(1): 1-5. |
| 51 | Xiao Y, Ji Q, Gao S, et al. Combined transcriptome and metabolite profiling reveals that IiPLR1 plays an important role in lariciresinol accumulation in Isatis indigotica [J]. Journal of Experimental Botany, 2015, 66(20): 6259-6271. |
| 52 | Bajpai V K, Shukla S, Paek W K, et al. Efficacy of (+)-lariciresinol to control bacterial growth of Staphylococcus aureus and Escherichia coli O157:H7 [J]. Frontiers in Microbiology, 2017, 8: 8. |
| 53 | Hwang B, Cho J, Hwang I S, et al. Antifungal activity of lariciresinol derived from Sambucus williamsii and their membrane-active mechanisms in Candida albicans [J]. Biochemical and Biophysical Research Communications, 2011, 410(3): 489-493. |
| 54 | Ma Z J, Lu L, Yang J J, et al. Lariciresinol induces apoptosis in HepG2 cells via mitochondrial-mediated apoptosis pathway [J]. European Journal of Pharmacology, 2018, 821: 1-10. |
| 55 | Arruda C, Eugenio D D, Moreira M R, et al. Biotransformation of (-)-cubebin by Aspergillus spp. into (-)-hinokinin and (-)-parabenzlactone, and their evaluation against oral pathogenic bacteria [J]. Natural Product Research, 2018, 32(23): 2803-2816. |
| 56 | Xie L H, Akao T, Hamasaki K, et al. Biotransformation of pinoresinol diglucoside to mammalian lignans by human intestinal microflora, and isolation of Enterococcus faecalis strain PDG-1 responsible for the transformation of (+)-pinoresinol to (+)-lariciresinol [J]. Chemical & Pharmaceutical Bulletin, 2003, 51(5): 508-515. |
| 57 | Ono E, Murata J, Toyonaga H, et al. Formation of a methylenedioxy bridge in (+)-epipinoresinol by CYP81Q3 corroborates with diastereomeric specialization in sesame lignans [J]. Plant and Cell Physiology, 2018, 59(11): 2278-2287. |
| 58 | Murata J, Ono E, Yoroizuka S, et al. Oxidative rearrangement of (+)-sesamin by CYP92B14 co-generates twin dietary lignans in sesame [J]. Nature Communications, 2018, 9: 2140-2149. |
| 59 | Ghosal S, Banerjee S, Srivastava R S. Simplexolin, a new lignan from Justicia simplex [J]. Phytochemistry, 1979, 18(3): 503-505. |
| 60 | Chen C, Zhu H, Zhao D, et al. Two new lignans from Phryma leptostachya L [J]. Helvetica Chimica Acta, 2013, 96(7): 1392-1396. |
| 61 | Tsai H Y, Lee W J, Chu I H, et al. Formation of samin diastereomers by acid-catalyzed transformation of sesamolin with hydrogen peroxide [J]. J. Agric. Food Chem., 2020, 68(23): 6430-6438. |
| 62 | Ono E, Waki T, Oikawa D, et al. Glycoside-specific glycosyltransferases catalyze regio-selective sequential glucosylations for a sesame lignan, sesaminol triglucoside [J]. Plant J., 2020, 101(5): 1221-1233. |
| 63 | Kezimana P, Dmitriev A A, Kudryavtseva A V, et al. Secoisolariciresinol diglucoside of flaxseed and its metabolites: biosynthesis and potential for nutraceuticals [J]. Frontiers in Genetics, 2018, 9: 641. |
| 64 | Cardoso Carraro J C, de Souza Dantas M I, Rocha Espeschit A C, et al. Flaxseed and human health: reviewing benefits and adverse effects [J]. Food Reviews International, 2012, 28(2): 203-230. |
| 65 | von Heimendahl C B I, Schafer K M, Eklund P, et al. Pinoresinol-lariciresinol reductases with different stereospecificity from Linum album and Linum usitatissimum [J]. Phytochemistry, 2005, 66(11): 1254-1263. |
| 66 | Markulin L, Corbin C, Renouard S, et al. Characterization of LuWRKY36, a flax transcription factor promoting secoisolariciresinol biosynthesis in response to Fusarium oxysporum elicitors in Linum usitatissimum L. hairy roots [J]. Planta, 2019, 250(1): 347-366. |
| 67 | Ford J D, Huang K S, Wang H B, et al. Biosynthetic pathway to the cancer chemopreventive secoisolariciresinol diglucoside-hydroxymethyl glutaryl ester-linked lignan oligomers in flax (Linum usitatissimum) seed [J]. Journal of Natural Products, 2002, 65(5): 800. |
| 68 | Caputi L, Malnoy M, Goremykin V, et al. A genome-wide phylogenetic reconstruction of family 1 UDP-glycosyltransferases revealed the expansion of the family during the adaptation of plants to life on land [J]. Plant J., 2012, 69(6): 1030-1042. |
| 69 | Barvkar V T, Pardeshi V C, Kale S M, et al. Phylogenomic analysis of UDP glycosyltransferase 1 multigene family in Linum usitatissimum identified genes with varied expression patterns [J]. BMC Genomics, 2012, 13: 175. |
| 70 | Ghose K, Selvaraj K, Mccallum J, et al. Identification and functional characterization of a flax UDP-glycosyltransferase glucosylating secoisolariciresinol (SECO) into secoisolariciresinol monoglucoside (SMG) and diglucoside (SDG) [J]. BMC Plant Biology, 2014, 14: 82. |
| 71 | Li M X, Zhu H Y, Yang D H, et al. Production of secoisolariciresinol from defatted flaxseed by bacterial biotransformation [J]. Journal of Applied Microbiology, 2012, 113(6): 1352-1361. |
| 72 | Luo X, Reiter M A, D'espaux L, et al. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast [J]. Nature, 2020, 580(7802): 123-126. |
| 73 | Ajikumar P K, Xiao W H, Tyo K E J, et al. Isoprenoid pathway optimization for taxol precursor overproduction in Escherichia coli [J]. Science, 2010, 330(6000): 70-74. |
| 74 | Biggs B W, Lim C G, Sagliani K, et al. Overcoming heterologous protein interdependency to optimize P450-mediated Taxol precursor synthesis in Escherichia coli [J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(12): 3209-3214. |
| 75 | Aschenbrenner J, Marx P, Pietruszka J, et al. Microbial production of natural and unnatural monolignols with Escherichia coli [J]. Chembiochem, 2019, 20(7): 949-954. |
| 76 | Ricklefs E, Girhard M, Koschorreck K, et al. Two-step one-pot synthesis of pinoresinol from eugenol in an enzymatic cascade [J]. ChemCatChem, 2015, 7(12): 1857-1864. |
| 77 | Ricklefs E, Girhard M, Urlacher V B. Three-steps in one-pot: whole-cell biocatalytic synthesis of enantiopure (+)- and (-)-pinoresinol via kinetic resolution [J]. Microbial Cell Factories, 2016, 15: 78. |
| 78 | Dalton D A, Diaz Del Castillo L, Kahn M L, et al. Heterologous expression and characterization of soybean cytosolic ascorbate peroxidase [J]. Arch. Biochem. Biophys., 1996, 328(1): 1-8. |
| 79 | Zhao X R, Choi K R, Lee S Y. Metabolic engineering of Escherichia coli for secretory production of free haem [J]. Nature Catalysis, 2018, 1(9): 720-728. |
| 80 | Chandra K, Sinha A, Arumugam N. Gene isolation, heterologous expression, purification and functional confirmation of sesamin synthase from Sesamum indicum L [J]. Biotechnology Reports, 2019, 22: e00336. |
| 81 | Chemler J A, Fowler Z L, Mchugh K P, et al. Improving NADPH availability for natural product biosynthesis in Escherichia coli by metabolic engineering [J]. Metabolic Engineering, 2010, 12(2): 96-104. |
| 82 | Koenig L, Brixius-Anderko S, Milhim M, et al. Identification and circumvention of bottlenecks in CYP21A2-mediated premedrol production using recombinant Escherichia coli [J]. Biotechnology and Bioengineering, 2020, 117(4): 901-911. |
| 83 | Liang B, Sun G, Wang Z, et al. Production of 3-hydroxypropionate using a novel malonyl-CoA-mediated biosynthetic pathway in genetically engineered E. coli strain [J]. Green Chemistry, 2019, 21(22): 6103-6115. |
| 84 | Xue H, Liu H, Lv B, et al. Controlling chemo- and regioselectivity of a plant P450 in yeast cell toward rare licorice triterpenoid biosynthesis [J]. ACS Catalysis, 2020, 10(7): 4253-4260. |
| 85 | Kuo H J, Wei Z Y, Lu P C, et al. Bioconversion of pinoresinol into matairesinol by use of recombinant Escherichia coli [J]. Applied and Environmental Microbiology, 2014, 80(9): 2687-2692. |
| 86 | Arneaud S L B, Porter J R. Investigation and expression of the secoisolariciresinol dehydrogenase gene involved in podophyllotoxin biosynthesis [J]. Molecular Biotechnology, 2015, 57(11): 961-973. |
| 87 | Marques J V, Kim K W, Lee C, et al. Next generation sequencing in predicting gene function in podophyllotoxin biosynthesis [J]. Journal of Biological Chemistry, 2013, 288(1): 466-479. |
| 88 | Moon J H, Lee K, Lee J H, et al. Redesign and reconstruction of a steviol-biosynthetic pathway for enhanced production of steviol in Escherichia coli [J]. Microbial Cell Factories, 2020, 19(1): 20. |
| 89 | Gibson D G, Young L, Chuang R Y, et al. Enzymatic assembly of DNA molecules up to several hundred kilobases [J]. Nature Methods, 2009, 6(5): 343-345. |
| 90 | Zhou S, Ding R, Chen J, et al. Obtaining a panel of cascade promoter-5'-UTR complexes in Escherichia coli [J]. ACS Synthetic Biology, 2017, 6(6): 1065-1075. |
| 91 | Gao S, Zhou H, Zhou J, et al. Promoter-library-based pathway optimization for efficient (2S)-naringenin production from p-coumaric acid in Saccharomyces cerevisiae [J]. Journal of Agricultural and Food Chemistry, 2020, 68(25): 6884-6891. |
| 92 | Li N, Zeng W, Xu S, et al. Obtaining a series of native gradient promoter-5'-UTR sequences in Corynebacterium glutamicum ATCC 13032 [J]. Microbial Cell Factories, 2020, 19(1): 120. |
| 93 | Jiang Y, Chen B, Duan C, et al. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system [J]. Applied and Environmental Microbiology, 2016, 82(12): 3693. |
| [1] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [2] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [3] | 陈雅鑫, 袁航, 刘冠章, 毛磊, 杨纯, 张瑞芳, 张光亚. 蛋白质纳米笼介导的酶自固定化研究进展[J]. 化工学报, 2023, 74(7): 2773-2782. |
| [4] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [5] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [6] | 张兰河, 赖青燚, 王铁铮, 关潇卓, 张明爽, 程欣, 徐小惠, 贾艳萍. H2O2对SBR脱氮效率和污泥性能的影响[J]. 化工学报, 2023, 74(5): 2186-2196. |
| [7] | 贾露凡, 王艺颖, 董钰漫, 李沁园, 谢鑫, 苑昊, 孟涛. 微流控双水相贴壁液滴流动强化酶促反应研究[J]. 化工学报, 2023, 74(3): 1239-1246. |
| [8] | 刘瑞琪, 周栖桐, 张悦, 贺莹, 高静, 马丽. 基于金纳米颗粒修饰二氧化硅纳米花的生物传感器构建及应用[J]. 化工学报, 2023, 74(3): 1247-1259. |
| [9] | 苏伟怡, 丁佳慧, 李春利, 王洪海, 姜艳军. 酶促反应结晶研究进展[J]. 化工学报, 2023, 74(2): 617-629. |
| [10] | 刘昕, 戈钧, 李春. 光驱动微生物杂合系统提高生物制造水平[J]. 化工学报, 2023, 74(1): 330-341. |
| [11] | 谭卓涛, 齐思雨, 许梦蛟, 戴杰, 朱晨杰, 应汉杰. 辅酶自循环的氧化还原级联体系在生物催化过程中的应用:机遇与挑战[J]. 化工学报, 2023, 74(1): 45-59. |
| [12] | 胡阳, 孙彦. 酶分子的自驱动及其介导的微纳马达[J]. 化工学报, 2023, 74(1): 116-132. |
| [13] | 刘雪, 张莉娟, 赵广荣. 大肠杆菌偏利共培养系统合成大豆苷元[J]. 化工学报, 2022, 73(9): 4015-4024. |
| [14] | 安绍杰, 许洪峰, 李思, 许远航, 李佳锡. 利用分子机器的组装与分解构建pH敏感性谷胱甘肽过氧化物人工酶[J]. 化工学报, 2022, 73(8): 3669-3678. |
| [15] | 张劢, 田瑶, 郭之旗, 王叶, 窦广进, 宋浩. 光催化-生物杂合系统设计优化用于燃料和化学品绿色合成[J]. 化工学报, 2022, 73(7): 2774-2789. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号