化工学报 ›› 2021, Vol. 72 ›› Issue (9): 4496-4503.DOI: 10.11949/0438-1157.20210215
马生贵1,2,3( ),田博文1,周雨薇1,陈琳1,江霞1,2,3(
),田博文1,周雨薇1,陈琳1,江霞1,2,3( ),高涛4
),高涛4
收稿日期:2021-02-04
修回日期:2021-05-11
出版日期:2021-09-05
发布日期:2021-09-05
通讯作者:
江霞
作者简介:马生贵(1990—),男,博士,助理研究员,基金资助:
Shenggui MA1,2,3( ),Bowen TIAN1,Yuwei ZHOU1,Lin CHEN1,Xia JIANG1,2,3(
),Bowen TIAN1,Yuwei ZHOU1,Lin CHEN1,Xia JIANG1,2,3( ),Tao GAO4
),Tao GAO4
Received:2021-02-04
Revised:2021-05-11
Online:2021-09-05
Published:2021-09-05
Contact:
Xia JIANG
摘要:
利用密度泛函理论研究H2S分子在氮掺杂Stone-Wales(SW)缺陷石墨烯上的吸附行为,通过吸附能、差分电荷密度、Bader电荷和电子态密度等分析了H2S分子在SW缺陷石墨烯及氮掺杂SW缺陷石墨烯上的吸附差异。计算结果表明氮原子掺杂可以有效提升H2S分子与石墨烯表面的相互作用,并加强二者之间的电荷转移。其中,氮原子主要作为电子传递的桥梁参与H2S与石墨烯表面之间的电荷转移。H2S分子被选择性吸附在SW缺陷及氮掺杂SW缺陷石墨烯的五元碳环中心处,这说明五元碳环的电荷分布促进H2S分子的吸附行为。
中图分类号:
马生贵, 田博文, 周雨薇, 陈琳, 江霞, 高涛. 氮掺杂Stone-Wales缺陷石墨烯吸附H2S的密度泛函理论研究[J]. 化工学报, 2021, 72(9): 4496-4503.
Shenggui MA, Bowen TIAN, Yuwei ZHOU, Lin CHEN, Xia JIANG, Tao GAO. DFT study of adsorption of H2S on N-doped Stone-Wales defected graphene[J]. CIESC Journal, 2021, 72(9): 4496-4503.
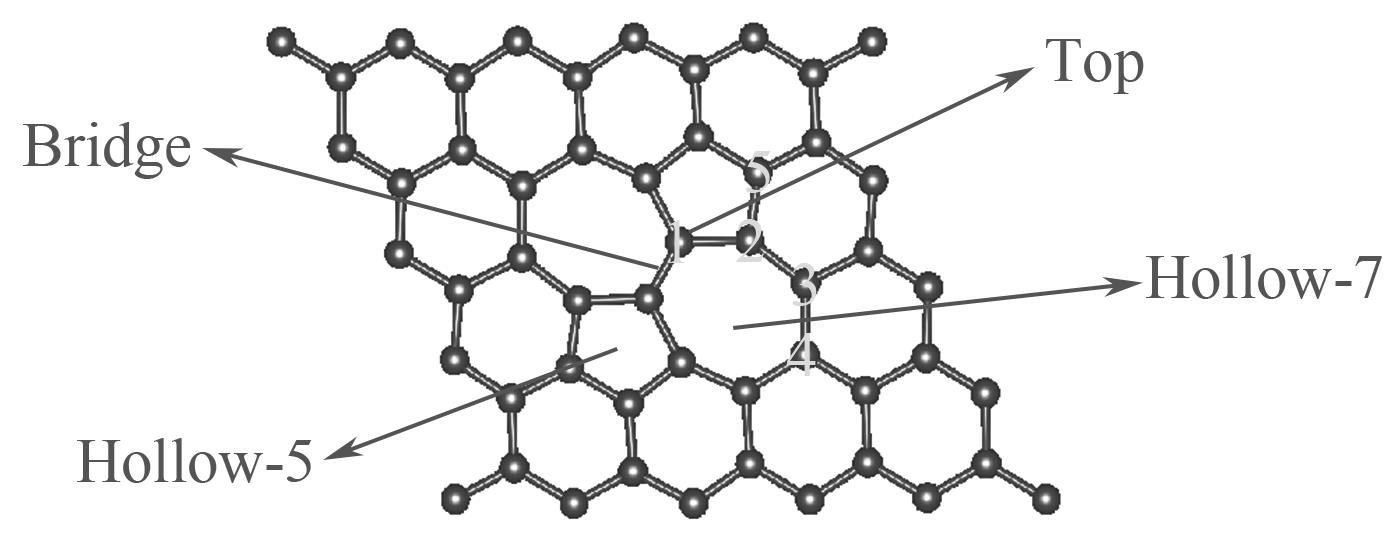
图2 SW缺陷石墨烯几何优化构型俯视图(Top、Bridge、Hollow-5和Hollow-7指H2S分子的吸附位点,数字1~5指氮原子的掺杂位点)
Fig.2 The top view optimized geometric structures of SW defected graphene (Top, Bridge, Hollow-5 and Hollow-7 are the adsorption sites of H2S, number 1—5 are the doping sites of N atom)
| H2S分子初始构型的几何方向 | 吸附位点 | 吸附能/eV |
|---|---|---|
| H-S键平行于石墨烯表面 | Top | -0.40 |
| Hollow-5 | -0.15 | |
| Hollow-7 | -0.14 | |
| Bridge | -0.17 | |
| 硫原子朝向石墨烯 | Top | -0.35 |
| Hollow-5 | -0.51 | |
| Hollow-7 | -0.13 | |
| Bridge | -0.35 | |
| 氢原子朝向石墨烯 | Top | -0.15 |
| Hollow-5 | -0.16 | |
| Hollow-7 | -0.15 | |
| Bridge | -0.14 |
表1 SW缺陷石墨烯吸附H2S分子的吸附能
Table 1 Adsorption energies of H2S molecules adsorbed on the SW defected graphene
| H2S分子初始构型的几何方向 | 吸附位点 | 吸附能/eV |
|---|---|---|
| H-S键平行于石墨烯表面 | Top | -0.40 |
| Hollow-5 | -0.15 | |
| Hollow-7 | -0.14 | |
| Bridge | -0.17 | |
| 硫原子朝向石墨烯 | Top | -0.35 |
| Hollow-5 | -0.51 | |
| Hollow-7 | -0.13 | |
| Bridge | -0.35 | |
| 氢原子朝向石墨烯 | Top | -0.15 |
| Hollow-5 | -0.16 | |
| Hollow-7 | -0.15 | |
| Bridge | -0.14 |

图3 H2S分子在SW缺陷石墨烯的三种吸附位点上的最稳定构型:(a) Top位点上吸附构型的侧视图和俯视图;(b) Hollow-5位点上吸附构型的侧视图和俯视图;(c) Bridge位点上吸附构型的侧视图和俯视图
Fig.3 The most stable structures of H2S molecules adsorbed on the SW defected graphene at three adsorption sites: (a) side and top view of the adsorption structure at Top site; (b) side and top view of the adsorption structure at Hollow-5 site; (c) side and top view of the adsorption structure at Bridge site

图4 S-Hollow-5吸附体系的电荷密度(a)和差分电荷密度图(b)(等值面值:0.0001)
Fig.4 Charge density (a) and deformation charge density (b) of the S-Hollow-5 adsorption system (values of absolute isosurface:0.0001)
| 原子 | 序号 | 吸附前电荷量/e | 吸附后电荷量/e | 转移电荷量/e |
|---|---|---|---|---|
| C | 1 | +0.005 | +0.022 | +0.017 |
| 2 | -0.079 | -0.012 | +0.067 | |
| 3 | -0.038 | -0.040 | -0.002 | |
| 4 | +0.180 | +0.046 | -0.134 | |
| 5 | +0.042 | -0.071 | -0.113 | |
| S | — | — | +0.049 | +0.049 |
| H | 1 | — | -0.010 | -0.010 |
| 2 | — | -0.021 | -0.021 |
表2 S-Hollow-5吸附体系的Bader电荷
Table 2 Bader atomic charges of the S-Hollow-5 adsorption system
| 原子 | 序号 | 吸附前电荷量/e | 吸附后电荷量/e | 转移电荷量/e |
|---|---|---|---|---|
| C | 1 | +0.005 | +0.022 | +0.017 |
| 2 | -0.079 | -0.012 | +0.067 | |
| 3 | -0.038 | -0.040 | -0.002 | |
| 4 | +0.180 | +0.046 | -0.134 | |
| 5 | +0.042 | -0.071 | -0.113 | |
| S | — | — | +0.049 | +0.049 |
| H | 1 | — | -0.010 | -0.010 |
| 2 | — | -0.021 | -0.021 |
| 氮掺杂位点 | Ef /eV |
|---|---|
| 1 | 2.94 |
| 2 | 3.14 |
| 3 | 3.93 |
| 4 | 3.75 |
| 5 | 2.72 |
表3 氮掺杂SW缺陷石墨烯的形成能
Table 3 Formation energies of N doped SW defected graphene
| 氮掺杂位点 | Ef /eV |
|---|---|
| 1 | 2.94 |
| 2 | 3.14 |
| 3 | 3.93 |
| 4 | 3.75 |
| 5 | 2.72 |
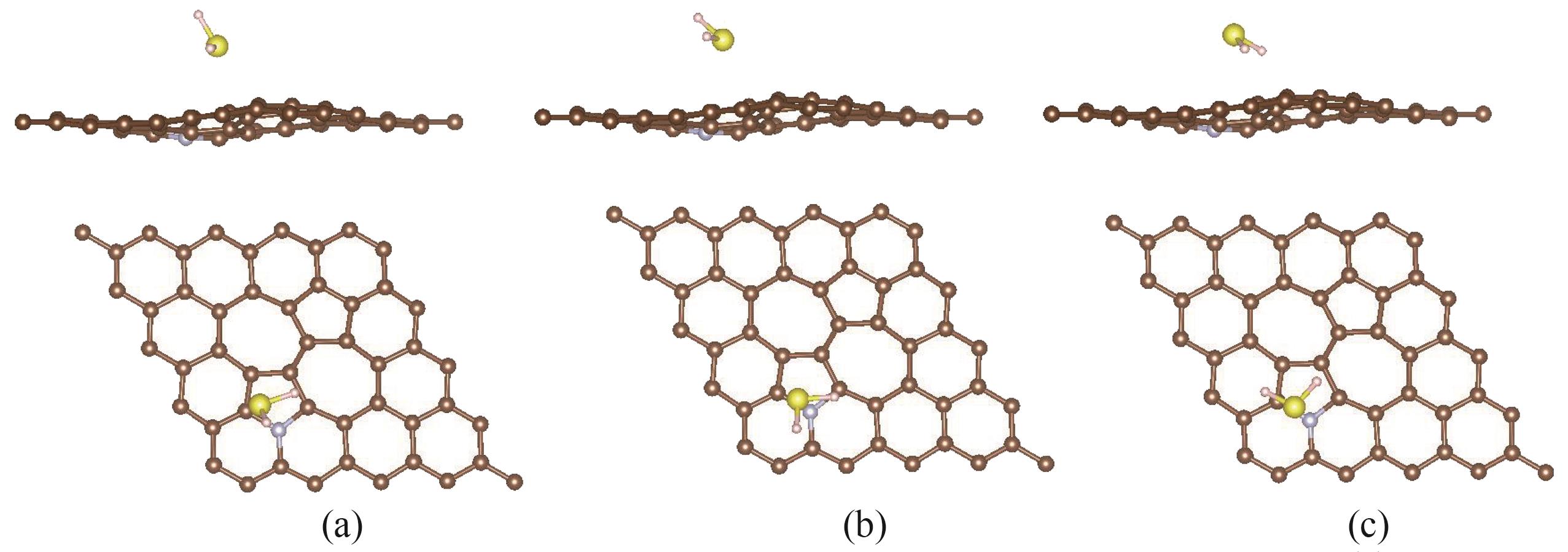
图6 H2S分子在氮掺杂SW缺陷石墨烯上的三种吸附体系的稳定构型:(a) S-H体系吸附构型的侧视图和俯视图;(b) S-T体系吸附构型的侧视图和俯视图;(c) H-H体系吸附构型的侧视图和俯视图
Fig.6 The stable structures of three adsorption systems of H2S molecules adsorbed on the N doped SW defected graphene: (a) side and top view of the adsorption structure of S-H system; (b) side and top view of the adsorption structure of S-T system; (c) side and top view of the adsorption structure of H-H system
| 吸附构型 | Eads /eV |
|---|---|
| S-T | -0.60 |
| S-H | -0.56 |
| H-H | -0.65 |
表4 氮掺杂SW缺陷石墨烯吸附H2S分子的吸附能
Table 4 Adsorption energies of H2S molecules adsorbed on the N doped SW defected graphene
| 吸附构型 | Eads /eV |
|---|---|
| S-T | -0.60 |
| S-H | -0.56 |
| H-H | -0.65 |
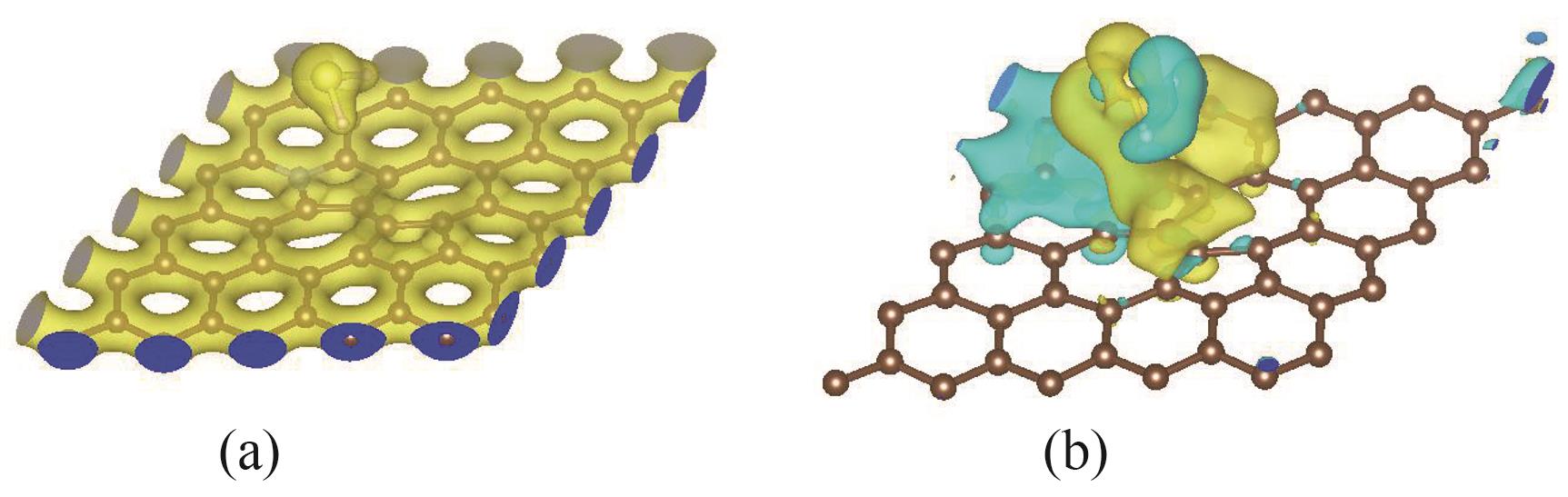
图7 H-H吸附体系的电荷密度(a)和差分电荷密度图(b) (等值面值:0.0001)
Fig.7 Charge density (a) and deformation charge density (b) of the H-H adsorption system (values of absolute isosurface:0.0001)
| 原子 | 序号 | 吸附前电荷量/e | 吸附后电荷量/e | 转移电荷量/e |
|---|---|---|---|---|
| C | 1 | +0.512 | +0.512 | 0 |
| 2 | +0.339 | +0.339 | 0 | |
| 3 | -0.101 | -0.105 | -0.004 | |
| 4 | +0.015 | +0.006 | -0.009 | |
| N | — | -1.283 | -1.283 | 0 |
| S | — | — | -0.076 | -0.076 |
| H | 1 | — | +0.053 | +0.053 |
| 2 | — | +0.051 | +0.051 |
表5 H-H吸附体系的Bader电荷
Table 5 Bader atomic charges of the H-H adsorption system
| 原子 | 序号 | 吸附前电荷量/e | 吸附后电荷量/e | 转移电荷量/e |
|---|---|---|---|---|
| C | 1 | +0.512 | +0.512 | 0 |
| 2 | +0.339 | +0.339 | 0 | |
| 3 | -0.101 | -0.105 | -0.004 | |
| 4 | +0.015 | +0.006 | -0.009 | |
| N | — | -1.283 | -1.283 | 0 |
| S | — | — | -0.076 | -0.076 |
| H | 1 | — | +0.053 | +0.053 |
| 2 | — | +0.051 | +0.051 |
| 1 | 谢乐, 蒋崇文. 生物滴滤塔去除高浓度H2S废气的模拟研究[J]. 化工学报, 2021, 72(8): 4346-4353. |
| Xie L, Jiang C W. Simulation study on the removal of high concentration H2S waste gas by biotrickling filter[J]. CIESC Journal, 2021, 72(8): 4346-4353. | |
| 2 | 张敏, 李涛, 陈曙旸, 等. 我国硫化氢中毒的特点与控制对策[J]. 工业卫生与职业病, 2005, 31(1): 12-14. |
| Zhang M, Li T, Chen S Y, et al. Characteristics and control measures of hydrogen sulfide poisoning in China [J]. Industrial Health and Occupational Diseases, 2005, 31(1): 12-14. | |
| 3 | 杨嫱, 董小刚, 贺雪红, 等. 油田硫化氢腐蚀原因及防护措施[J]. 化工设计通讯, 2019, 45(8): 45-46. |
| Yang Q, Dong X G, He X H, et al. Causes and protective measures of hydrogen sulfide in oil fields[J]. Chemical Engineering Design Communications, 2019, 45(8): 45-46. | |
| 4 | 杨振宇. 关于硫化氢废气处理新方法研究[J]. 节能与环保, 2019 (7): 75-77. |
| Yang Z Y. Study on new treatment method of hydrogen sulfide waste gas[J]. Energy Conservation & Environmental Protection,2019 (7): 75-77. | |
| 5 | Bagreev A, Bandosz T J. H2S adsorption/oxidation on unmodified activated carbons: importance of prehumidification[J]. Carbon, 2001, 39(15): 2303-2311. |
| 6 | Adib F, Bagreev A, Bandosz T J. Adsorption/oxidation of hydrogen sulfide on nitrogen-containing activated carbons[J]. Langmuir, 2000, 16(4): 1980-1986. |
| 7 | Yang W J, Gao Z Y, Liu X S, et al. Single-atom iron catalyst with single-vacancy graphene-based substrate as a novel catalyst for NO oxidation: a theoretical study[J]. Catalysis Science & Technology, 2018, 8(16): 4159-4168. |
| 8 | Tetlow H, Posthuma de Boer J, Ford I J, et al. Growth of epitaxial graphene: theory and experiment[J]. Physics Reports, 2014, 542(3): 195-295. |
| 9 | 张慧娟. SO2和NO在石墨烯氧化物上吸附氧化的第一性原理研究[D]. 昆明: 昆明理工大学, 2015. |
| Zhang H J. A first principles study of adsorption and oxidation of SO2 and NO by graphene oxides[D]. Kunming: Kunming University of Science and Technology, 2015. | |
| 10 | Zhou Q X, Fu Z B, Tang Y J, et al. First-principle study of the transition-metal adatoms on B-doped vacancy-defected graphene[J]. Physica E: Low-Dimensional Systems and Nanostructures, 2014, 60: 133-138. |
| 11 | Ye X, Ma S G, Jiang X, et al. The adsorption of acidic gaseous pollutants on metal and nonmetallic surface studied by first-principles calculation: a review[J]. Chinese Chemical Letters, 2019, 30(12): 2123-2131. |
| 12 | Gao Z Y, Yang W J, Ding X L, et al. Support effects on adsorption and catalytic activation of O2 in single atom iron catalysts with graphene-based substrates[J]. Physical Chemistry Chemical Physics: PCCP, 2018, 20(10): 7333-7341. |
| 13 | Hohenberg P, Kohn W. Inhomogeneous electron gas[J]. Physical Review, 1964, 136(3B): b864. |
| 14 | Mineva T, Krishnamurty S, Salahub D R, et al. Temperature dependence of the molecular conformations of dilauroyl phosphatidylcholine: a density functional study[J]. International Journal of Quantum Chemistry, 2013, 113(5): 631-636. |
| 15 | Sham L J, Kohn W. One-particle properties of an inhomogeneous interacting electron gas[J]. Physical Review, 1966, 145(2): 561. |
| 16 | Leenaerts O, Partoens B, Peeters F M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: a first-principles study[J]. Physical Review B, 2008, 77(12): 125416. |
| 17 | Lazar P, Karlický F, Jurečka P, et al. Adsorption of small organic molecules on graphene[J]. Journal of the American Chemical Society, 2013, 135(16): 6372-6377. |
| 18 | Ambrusi R E, Luna C R, Juan A, et al. DFT study of Rh-decorated pristine, B-doped and vacancy defected graphene for hydrogen adsorption[J]. RSC Advances, 2016, 6(87): 83926-83941. |
| 19 | Yang W J, Gao Z Y, Liu X S, et al. Directly catalytic reduction of NO without NH3 by single atom iron catalyst: a DFT calculation[J]. Fuel, 2019, 243: 262-270. |
| 20 | Zhou Q X, Wang C Y, Fu Z B, et al. Adsorption of formaldehyde molecule on Stone-Wales defected graphene doped with Cr, Mn, and Co: a theoretical study[J]. Computational Materials Science, 2014, 83: 398-402. |
| 21 | 刘笑涵. 石墨烯气体传感器吸附CO和CO2性能研究[D]. 西安: 西安电子科技大学, 2019. |
| Liu X H. Study for adsorption of CO and CO2 on graphene gas sensor[D]. Xi'an: Xidian University, 2019. | |
| 22 | Dai J Y, Yuan J M, Giannozzi P. Gas adsorption on graphene doped with B, N, Al, and S: a theoretical study[J]. Applied Physics Letters, 2009, 95(23): 232105. |
| 23 | Jia X T, Zhang H, Zhang Z M, et al. Effect of doping and vacancy defects on the adsorption of CO on graphene[J]. Materials Chemistry and Physics, 2020, 249: 123114. |
| 24 | Kresse G, Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Computational Materials Science, 1996, 6(1): 15-50. |
| 25 | Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set[J]. Physical Review. B, Condensed Matter, 1996, 54(16): 11169-11186. |
| 26 | Kresse G, Hafner J. Ab initio molecular dynamics for open-shell transition metals[J]. Physical Review. B, Condensed Matter, 1993, 48(17): 13115-13118. |
| 27 | Perdew J P, Burke K, Ernzerhof M. Generalized gradient approximation made simple[J]. Physical Review Letters, 1996, 77(18): 3865-3868. |
| 28 | Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method[J]. Physical Review B, 1999, 59(3): 1758. |
| 29 | Goerigk L, Grimme S. A thorough benchmark of density functional methods for general main group thermochemistry, kinetics, and noncovalent interactions[J]. Physical Chemistry Chemical Physics, 2011, 13(14): 6670-6688. |
| 30 | Zhang H P, Luo X G, Song H T, et al. DFT study of adsorption and dissociation behavior of H2S on Fe-doped graphene[J]. Applied Surface Science, 2014, 317: 511-516. |
| [1] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [2] | 顾浩, 张福建, 刘珍, 周文轩, 张鹏, 张忠强. 力电耦合作用下多孔石墨烯膜时间维度的脱盐性能及机理研究[J]. 化工学报, 2023, 74(5): 2067-2074. |
| [3] | 党迎喜, 谈朋, 刘晓勤, 孙林兵. 辐射冷却和太阳能加热驱动的CO2变温捕获[J]. 化工学报, 2023, 74(1): 469-478. |
| [4] | 余后川, 任腾, 张宁, 姜晓滨, 代岩, 张晓鹏, 鲍军江, 贺高红. 二维氧化石墨烯膜离子选择性传递调控的研究进展[J]. 化工学报, 2023, 74(1): 303-312. |
| [5] | 杜峰, 尹思琦, 罗辉, 邓文安, 李传, 黄振薇, 王文静. H2在Mo x S y 团簇上吸附解离的尺寸效应研究[J]. 化工学报, 2022, 73(9): 3895-3903. |
| [6] | 俞夏琪, 冯格, 赵金燕, 李嘉远, 邓声威, 郑靖楠, 李雯雯, 王亚秋, 沈榄, 刘旭, 徐威威, 王建国, 王式彬, 姚子豪, 毛成立. 基体(TDI-TMP-T313)与氧化剂(AP)相互作用的第一性原理研究[J]. 化工学报, 2022, 73(8): 3511-3517. |
| [7] | 黄凯, 王思洁, 苏海萍, 练成, 刘洪来. 石墨烯层间距调控抑制锂枝晶生长的第一性原理研究[J]. 化工学报, 2022, 73(8): 3501-3510. |
| [8] | 韩双, 张楠, 王慧, 张璇, 杨金栾, 张蔓琳, 张志超. 金霉素分子印迹电化学传感器的制备与应用[J]. 化工学报, 2022, 73(8): 3758-3767. |
| [9] | 赵继昊, 唐伟强, 徐小飞, 赵双良, 贺炅皓. 高分子复合材料中键合剂在不同纳米填料表面的吸附能计算[J]. 化工学报, 2022, 73(7): 3174-3181. |
| [10] | 刘洪超, 陈苏航, 段先力, 吴凡, 徐小飞, 宋先雨, 赵双良, 刘洪来. Janus石墨烯量子点在生物膜中的输运行为:分子动力学模拟[J]. 化工学报, 2022, 73(7): 2835-2843. |
| [11] | 李智超, 郑瑜, 张润楠, 姜忠义. 高通量抗污染氧化石墨烯膜研究进展[J]. 化工学报, 2022, 73(6): 2370-2380. |
| [12] | 张苗, 杨洪海, 尹勇, 徐悦, 沈俊杰, 卢心诚, 施伟刚, 王军. 氧化石墨烯/水脉动热管的启动及传热特性[J]. 化工学报, 2022, 73(3): 1136-1146. |
| [13] | 陈雪梅, 王彤, 高玉箔, 彭鼎程, 罗雨婷. 利用激光诱导石墨烯实现高效太阳能界面蒸发[J]. 化工学报, 2022, 73(12): 5648-5659. |
| [14] | 罗小松, 黄金保, 周梅, 牟鑫, 徐伟伟, 吴雷. 对苯二甲酸丁二醇酯二聚体水/醇/氨解机理的理论研究[J]. 化工学报, 2022, 73(11): 4859-4871. |
| [15] | 徐欢, 柯律, 张生辉, 张子林, 韩广东, 崔金声, 唐道远, 黄东辉, 高杰峰, 何新建. GO表面原位生长CNTs改善聚丙烯导热复合材料分散与界面形态[J]. 化工学报, 2022, 73(11): 5150-5157. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号