化工学报 ›› 2022, Vol. 73 ›› Issue (11): 4859-4871.DOI: 10.11949/0438-1157.20221121
收稿日期:2022-08-08
修回日期:2022-10-13
出版日期:2022-11-05
发布日期:2022-12-06
通讯作者:
黄金保
作者简介:罗小松(1999—),男,硕士研究生,luoxiaosong1008@126.com
基金资助:
Xiaosong LUO( ), Jinbao HUANG(
), Jinbao HUANG( ), Mei ZHOU, Xin MU, Weiwei XU, Lei WU
), Mei ZHOU, Xin MU, Weiwei XU, Lei WU
Received:2022-08-08
Revised:2022-10-13
Online:2022-11-05
Published:2022-12-06
Contact:
Jinbao HUANG
摘要:
采用密度泛函理论M06-2X/6-311G(d)方法,对对苯二甲酸丁二醇酯二聚体的水/醇/氨解反应机理进行了量子化学理论研究。提出了各种可能的水/醇/氨解反应路径,对各反应的中间体、过渡态及产物进行了几何结构优化和频率计算以获得热力学与动力学参数值,分析了对苯二甲酸丁二醇酯二聚体主链酯键中的酰氧键位置水/醇/氨降解的反应机理。计算结果表明:水/醇/氨解条件下能够降低对苯二甲酸丁二醇酯二聚体主链酯键中的酰氧键裂解的反应活化能,使反应更易于进行,水/醇/氨解中主要基元反应步的反应能垒分别约为170.0、155.0和165.0 kJ/mol。对苯二甲酸丁二醇酯二聚体水解产物主要为对苯二甲酸和1,4-丁二醇,醇解产物主要为对苯二甲酸二甲酯和1,4-丁二醇,氨解产物主要为芳香腈和1,4-丁二醇等,其中1,4-丁二醇会进一步降解形成四氢呋喃。在对苯二甲酸丁二醇酯二聚体水/醇/氨解反应过程中,甲醇介质中的裂解反应优于氨气气氛中的反应、氨气气氛中的反应优于水分子环境中的反应,且反应温度的升高可以增加其自发性。
中图分类号:
罗小松, 黄金保, 周梅, 牟鑫, 徐伟伟, 吴雷. 对苯二甲酸丁二醇酯二聚体水/醇/氨解机理的理论研究[J]. 化工学报, 2022, 73(11): 4859-4871.
Xiaosong LUO, Jinbao HUANG, Mei ZHOU, Xin MU, Weiwei XU, Lei WU. Theoretical study on the mechanism of hydrolysis/alcoholysis/ammonolysis of butanediol terephthalate dimer[J]. CIESC Journal, 2022, 73(11): 4859-4871.
| Temperature/K | Hydrolysis[Path(1)]/ (kJ/mol) | Alcoholysis[Path(2)]/ (kJ/mol) | Ammonolysis[Path(3)]/ (kJ/mol) | Pure pyrolysis/ (kJ/mol) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1→TS1(1-a) | 1→TS4(1-b) | 1→TS5(1-c) | 1→TS7(2-a) | 1→TS10(2-b) | 1→TS12(2-c) | 1→TS15(3-a) | 1→TS22(3-b) | 1→TS24(3-c) | Six-membered ring | Four-membered ring | |||||
| 298 | 171.6 | 172.9 | 169.0 | 154.8 | 154.9 | 156.6 | 162.5 | 160.1 | 168.4 | 217.4 | 214.1 | 211.8 | 279.5 | 279.9 | 281.5 |
| 400 | 172.2 | 172.8 | 168.8 | 154.7 | 153.3 | 156.5 | 162.5 | 160.1 | 168.4 | 217.7 | 213.6 | 210.5 | 280.8 | 280.5 | 282.0 |
| 500 | 173.4 | 173.2 | 168.8 | 154.9 | 151.8 | 156.6 | 162.9 | 160.6 | 168.8 | 217.9 | 213.2 | 209.2 | 282.1 | 280.9 | 282.5 |
| 600 | 174.8 | 173.8 | 169.8 | 155.2 | 150.5 | 156.9 | 163.7 | 161.3 | 169.5 | 218.1 | 212.7 | 207.8 | 283.2 | 281.3 | 282.9 |
| 700 | 176.5 | 174.7 | 170.7 | 155.5 | 149.2 | 157.2 | 164.6 | 162.3 | 170.4 | 218.3 | 212.2 | 206.4 | 284.2 | 281.6 | 283.1 |
| 800 | 178.3 | 175.7 | 171.7 | 155.9 | 147.9 | 157.5 | 165.7 | 163.3 | 171.5 | 218.4 | 211.5 | 204.9 | 285.1 | 281.7 | 283.2 |
| 900 | 180.2 | 176.8 | 172.7 | 156.3 | 146.7 | 157.9 | 166.8 | 164.5 | 172.6 | 218.4 | 210.8 | 203.3 | 285.9 | 281.7 | 283.2 |
表1 对苯二甲酸丁二醇酯二聚体在不同温度下纯热解和水/醇/氨解过程初始反应步骤的活化能
Table 1 Activation energy of initial reaction steps in pure pyrolysis and hydrolysis/alcoholysis/ammonolysis processes of butanediol terephthalate dimer at different temperatures
| Temperature/K | Hydrolysis[Path(1)]/ (kJ/mol) | Alcoholysis[Path(2)]/ (kJ/mol) | Ammonolysis[Path(3)]/ (kJ/mol) | Pure pyrolysis/ (kJ/mol) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1→TS1(1-a) | 1→TS4(1-b) | 1→TS5(1-c) | 1→TS7(2-a) | 1→TS10(2-b) | 1→TS12(2-c) | 1→TS15(3-a) | 1→TS22(3-b) | 1→TS24(3-c) | Six-membered ring | Four-membered ring | |||||
| 298 | 171.6 | 172.9 | 169.0 | 154.8 | 154.9 | 156.6 | 162.5 | 160.1 | 168.4 | 217.4 | 214.1 | 211.8 | 279.5 | 279.9 | 281.5 |
| 400 | 172.2 | 172.8 | 168.8 | 154.7 | 153.3 | 156.5 | 162.5 | 160.1 | 168.4 | 217.7 | 213.6 | 210.5 | 280.8 | 280.5 | 282.0 |
| 500 | 173.4 | 173.2 | 168.8 | 154.9 | 151.8 | 156.6 | 162.9 | 160.6 | 168.8 | 217.9 | 213.2 | 209.2 | 282.1 | 280.9 | 282.5 |
| 600 | 174.8 | 173.8 | 169.8 | 155.2 | 150.5 | 156.9 | 163.7 | 161.3 | 169.5 | 218.1 | 212.7 | 207.8 | 283.2 | 281.3 | 282.9 |
| 700 | 176.5 | 174.7 | 170.7 | 155.5 | 149.2 | 157.2 | 164.6 | 162.3 | 170.4 | 218.3 | 212.2 | 206.4 | 284.2 | 281.6 | 283.1 |
| 800 | 178.3 | 175.7 | 171.7 | 155.9 | 147.9 | 157.5 | 165.7 | 163.3 | 171.5 | 218.4 | 211.5 | 204.9 | 285.1 | 281.7 | 283.2 |
| 900 | 180.2 | 176.8 | 172.7 | 156.3 | 146.7 | 157.9 | 166.8 | 164.5 | 172.6 | 218.4 | 210.8 | 203.3 | 285.9 | 281.7 | 283.2 |

图12 对苯二甲酸丁二醇酯二聚体水/醇/氨解的初始反应步骤活化能与温度的相关性
Fig.12 Relationship between activation energy of initial reaction steps in hydrolysis/alcoholysis/ammonolysis processes of butanediol terephthalate dimer and temperature
| Temperature/K | Hydrolysis[Path(1)] | Alcoholysis[Path(2)] | Ammonolysis[Path(3)] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ΔH/ (kJ/mol) | ΔG/ (kJ/mol) | ΔS/ (J/(mol·K)) | ΔH/ (kJ/mol) | ΔG/ (kJ/mol) | ΔS/ (J/(mol·K)) | ΔH/ (kJ/mol) | ΔG/ (kJ/mol) | ΔS/ (J/(mol·K)) | |
| 298 | 19.4 | -6.1 | 85.6 | -11.7 | -27.0 | 51.1 | 83.0 | 55.8 | 91.3 |
| 400 | 17.3 | -14.4 | 79.4 | -10.8 | -32.3 | 53.7 | 82.9 | 46.5 | 91.0 |
| 500 | 16.1 | -22.2 | 76.6 | -9.9 | -37.8 | 55.7 | 83.5 | 37.4 | 92.3 |
| 600 | 15.5 | -29.8 | 75.5 | -9.0 | -43.4 | 57.4 | 84.5 | 28.0 | 94.1 |
| 700 | 15.2 | -37.3 | 75.1 | -8.1 | -49.2 | 58.8 | 85.6 | 18.6 | 95.7 |
| 800 | 15.3 | -44.9 | 75.2 | -7.2 | -55.2 | 60.0 | 86.7 | 8.9 | 97.3 |
| 900 | 15.5 | -52.4 | 75.4 | -6.3 | -61.2 | 61.0 | 87.8 | -0.9 | 98.6 |
表2 对苯二甲酸丁二醇酯二聚体在不同温度下水/醇/氨解过程初始反应步骤的ΔH/ΔG/ΔS
Table 2 ΔH/ΔG/ΔS of initial reaction steps in hydrolysis/alcoholysis/ammonolysis processes of butanediol terephthalate dimer at different temperatures
| Temperature/K | Hydrolysis[Path(1)] | Alcoholysis[Path(2)] | Ammonolysis[Path(3)] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ΔH/ (kJ/mol) | ΔG/ (kJ/mol) | ΔS/ (J/(mol·K)) | ΔH/ (kJ/mol) | ΔG/ (kJ/mol) | ΔS/ (J/(mol·K)) | ΔH/ (kJ/mol) | ΔG/ (kJ/mol) | ΔS/ (J/(mol·K)) | |
| 298 | 19.4 | -6.1 | 85.6 | -11.7 | -27.0 | 51.1 | 83.0 | 55.8 | 91.3 |
| 400 | 17.3 | -14.4 | 79.4 | -10.8 | -32.3 | 53.7 | 82.9 | 46.5 | 91.0 |
| 500 | 16.1 | -22.2 | 76.6 | -9.9 | -37.8 | 55.7 | 83.5 | 37.4 | 92.3 |
| 600 | 15.5 | -29.8 | 75.5 | -9.0 | -43.4 | 57.4 | 84.5 | 28.0 | 94.1 |
| 700 | 15.2 | -37.3 | 75.1 | -8.1 | -49.2 | 58.8 | 85.6 | 18.6 | 95.7 |
| 800 | 15.3 | -44.9 | 75.2 | -7.2 | -55.2 | 60.0 | 86.7 | 8.9 | 97.3 |
| 900 | 15.5 | -52.4 | 75.4 | -6.3 | -61.2 | 61.0 | 87.8 | -0.9 | 98.6 |
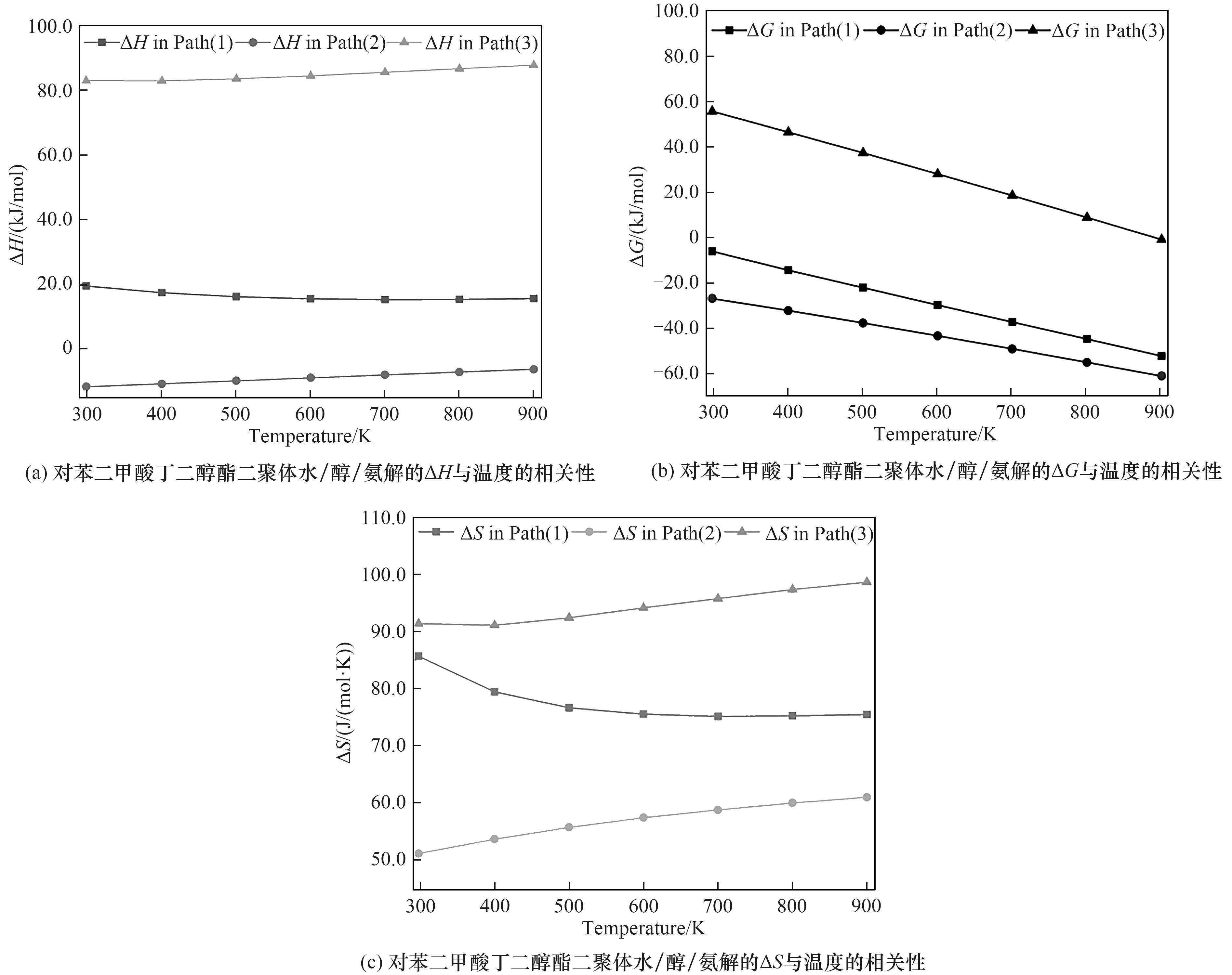
图13 对苯二甲酸丁二醇酯二聚体水/醇/氨解的ΔH/ΔG/ΔS与温度的相关性
Fig.13 Relationship between ΔH/ΔG/ΔS in hydrolysis/alcoholysis/ammonolysis processes of butanediol terephthalate dimer and temperature
| 1 | Sablong R, Duchateau R, Koning C E, et al. Incorporation of a flame retardancy enhancing phosphorus-containing diol into poly(butylene terephthalate) via solid state polycondensation: a comparative study[J]. Polymer Degradation and Stability, 2011, 96(3): 334-341. |
| 2 | Nogales A, Sanz A, Ezquerra T A, et al. Molecular dynamics of poly(butylene tert-butyl isophthalate) and its copolymers with poly(butylene terephthalate) as revealed by broadband dielectric spectroscopy[J]. Polymer, 2006, 47(20): 7078-7084. |
| 3 | Cho M, Yang J, Noh S, et al. Production of PBT(polybutylene terephthalate) oligomer from recycled PET(polyethylene terephthalate)[J]. Korean Chemical Engineering Research, 2016, 54(4): 437-442. |
| 4 | 刘丽. 聚对苯二甲酸丁二醇酯在亚临界酸性水溶液和超/亚临界乙醇中的解聚研究[D]. 杭州: 浙江工业大学, 2011. |
| Liu L. Depolymerization of polybutylene terephthalate in subcritical aqueous acid solution and sub/superctitical ethanol[D]. Hangzhou: Zhejiang University of Technology, 2011. | |
| 5 | 孙锴. 废塑料催化热解制备芳香烃的研究[D]. 杭州: 浙江大学, 2021. |
| Sun K. Study on aromatics production from catalytic pyrolysis of waste plastics[D]. Hangzhou: Zhejiang University, 2021. | |
| 6 | Chiu S J, Wu Y S. A comparative study on thermal and catalytic degradation of polybutylene terephthalate[J]. Journal of Analytical and Applied Pyrolysis, 2009, 86(1): 22-27. |
| 7 | 王媚娴, 潘志彦, 戴娟娟, 等. 超/亚临界水中聚对苯二甲酸乙二醇酯的解聚[J]. 高校化学工程学报, 2011, 25(5): 904-910. |
| Wang M X, Pan Z Y, Dai J J, et al. Depolymerization of polyethylene terephthalate in sub- and supercritical water[J]. Journal of Chemical Engineering of Chinese Universities, 2011, 25(5): 904-910. | |
| 8 | 戴娟娟. 亚临界水中聚对苯二甲酸丁二醇酯的(催化)解聚研究[D]. 杭州: 浙江工业大学, 2010. |
| Dai J J. Depolymerization of polybutylene terephthalate in subcritical water with and without catalyst[D]. Hangzhou: Zhejiang University of Technology, 2010. | |
| 9 | 杨伟. 聚酯复合材料无卤协效阻燃研究及机理的研究[D]. 合肥: 中国科学技术大学, 2012. |
| Yang W. Investigations on synergistic flame retardancy and mechanisms of halogen-free flame retardant polyester composites[D]. Hefei: University of Science and Technology of China, 2012. | |
| 10 | Balabanovich A I. The effect of ammonium polyphosphate on the combustion and thermal decomposition behavior of poly(butylene terephthalate)[J]. Journal of Fire Sciences, 2003, 21(4): 285-298. |
| 11 | 黄婕, 齐文杰, 吴勇强, 等. 超临界甲醇降解对苯二甲酸丁二醇酯的研究[J]. 高分子学报, 2005(2): 309-312. |
| Huang J, Qi W J, Wu Y Q, et al. Depolymerization of polybutylene terephthalate in supercritical methanol[J]. Acta Polymerica Sinica, 2005(2): 309-312. | |
| 12 | Levchik S V, Weil E D. A review on thermal decomposition and combustion of thermoplastic polyesters[J]. Polymers for Advanced Technologies, 2004, 15(12): 691-700. |
| 13 | 刘丽, 戴娟娟, 黄园园, 等. 聚对苯二甲酸丁二醇酯在亚临界水中的催化解聚[J]. 高校化学工程学报, 2012, 26(3): 524-530. |
| Liu L, Dai J J, Huang Y Y, et al. Catalytic depolymerization of polybutylene terephthalate in subcritical water[J]. Journal of Chemical Engineering of Chinese Universities, 2012, 26(3): 524-530. | |
| 14 | Goje A S. Auto-catalyzed hydrolytic depolymerization of poly(butylene terephthalate) waste at high temperature[J]. Polymer-Plastics Technology and Engineering, 2006, 45(2): 171-181. |
| 15 | 戴娟娟, 潘志彦. 聚对苯二甲酸丁二醇酯的解聚研究进展[J]. 环境科学与技术, 2010, 33(S1): 184-187. |
| Dai J J, Pan Z Y. The research progress of depolymerization of polybutylene terephthalate[J]. Environmental Science & Technology, 2010, 33(S1): 184-187. | |
| 16 | Huang J, Yang J H, Chyu M K, et al. Continuous-distribution kinetics for degradation of polybutylene terephthalate (PBT) in supercritical methanol[J]. Polymer Degradation and Stability, 2009, 94(12): 2142-2148. |
| 17 | Balabanovich A I. The effect of melamine on the combustion and thermal decomposition behaviour of poly(butylene terephthalate)[J]. Polymer Degradation and Stability, 2004, 84(3): 451-458. |
| 18 | Huang J B, He C, Pan G Y, et al. A theoretical research on pyrolysis reactions mechanism of coumarone-contained lignin model compound[J]. Computational and Theoretical Chemistry, 2016, 1091: 92-98. |
| 19 | Long B, Xia Y, Bao J L, et al. Reaction of SO3 with HONO2 and implications for sulfur partitioning in the atmosphere[J]. Journal of the American Chemical Society, 2022, 144(20): 9172-9177. |
| 20 | Huang J B, Mu X, Luo X S, et al. DFT studies on pyrolysis mechanisms of tetrabromobisphenol A (TBBPA)[J]. Environmental Science and Pollution Research International, 2021, 28(48): 68817-68833. |
| 21 | Yang J H, Huang J, Chyu M K, et al. Degradation of poly(butylene terephthalate) in different supercritical alcohol solvents[J]. Journal of Applied Polymer Science, 2010, 116(4): 2269-2274. |
| 22 | Frisch M J, Trucks G W, Schlegel J, et al. Gaussian 09, revision C.01[CP]. 2010. |
| 23 | Huang J B, Li X S, Meng H X, et al. Studies on pyrolysis mechanisms of syndiotactic polystyrene using DFT method[J]. Chemical Physics Letters, 2020, 747: 137334. |
| 24 | Cheng H, Wu S B, Huang J B, et al. Direct evidence from in situ FTIR spectroscopy that o-quinonemethide is a key intermediate during the pyrolysis of guaiacol[J]. Analytical and Bioanalytical Chemistry, 2017, 409(10): 2531-2537. |
| 25 | Huang J B, Meng H X, Luo X S, et al. Insights into the thermal degradation mechanisms of polyethylene terephthalate dimer using DFT method[J]. Chemosphere, 2022, 291: 133112. |
| 26 | Kelleher P G, Wentz R P, Falcone D R. Hydrolysis of poly(butylene terephthalate)[J]. Polymer Engineering & Science, 1982, 22(4): 260-264. |
| 27 | Loyer C, Régnier G, Duval V, et al. PBT plasticity loss induced by oxidative and hydrolysis ageing[J]. Polymer Degradation and Stability, 2020, 181: 109368. |
| 28 | Goje A S, Chauhan Y P, Mishra S. Chemical recycling and kinetics of aqueous alkaline depolymerization of poly(butylene terephthalate) waste[J]. Chemical Engineering & Technology, 2004, 27(7): 790-799. |
| 29 | 黄婕, 齐文杰, 黄科, 等. 聚对苯二甲酸丁二醇酯在超临界甲醇中降解机理的研究[J]. 高校化学工程学报, 2007, 21(1): 48-53. |
| Huang J, Qi W J, Huang K, et al. The depolymerization mechanism of polybutylene terephthalate in supercritical methanol[J]. Journal of Chemical Engineering of Chinese Universities, 2007, 21(1): 48-53. | |
| 30 | Huang J, Huang K, Qi W J, et al. Process analysis of depolymerization polybutylene terephthalate in supercritical methanol[J]. Polymer Degradation and Stability, 2006, 91(10): 2527-2531. |
| 31 | Shibata M, Masuda T, Yosomiya R, et al. Depolymerization of poly(butylene terephthalate) using high-temperature and high-pressure methanol[J]. Journal of Applied Polymer Science, 2000, 77(14): 3228-3233. |
| 32 | Pan Z Y, Shi Y H, Liu L, et al. Depolymerization of poly(butylene terephthalate) in sub- and supercritical ethanol in a fused silica capillary reactor or autoclave reactor[J]. Polymer Degradation and Stability, 2013, 98(7): 1287-1292. |
| 33 | Balabanovich A I, Balabanovich A M, Engelmann J. Intumescence in poly(butylene terephthalate): the effect of 2-methyl-1, 2-oxaphospholan-5-one 2-oxide and ammonium polyphosphate[J]. Polymer International, 2003, 52(8): 1309-1314. |
| 34 | Xu B, Wu X, Qian L J, et al. Intumescent flame-retardant poly(1, 4-butylene terephthalate) with ammonium polyphosphate and a hyperbranched triazine charring-foaming agent: flame retardancy performance and mechanisms[J]. Journal of Fire Sciences, 2017, 35(4): 317-340. |
| 35 | Zhang W Z, Ren J W, Wei T, et al. Synergistic effect between ammonium polyphosphate and expandable graphite on flame-retarded poly(butylene terephthalate)[J]. Materials Research Express, 2018, 5(2): 025310. |
| 36 | 罗小松, 黄金保, 吴雷, 等. 聚对苯二甲酸丁二醇酯二聚体热降解机理的理论研究[J]. 燃料化学学报, DOI: 10.1016/S1872-5813(22)60043-4 . |
| Luo X S, Huang J B, Wu L, et al. Theoretical study on thermal degradation mechanism of polybutylene terephthalate dimer[J]. Journal of Fuel Chemistry and Technology, DOI: 10.1016/S1872-5813(22)60043-4 . | |
| 37 | Huang J B, Liu C, Wu D, et al. Density functional theory studies on pyrolysis mechanism of β-O-4 type lignin dimer model compound[J]. Journal of Analytical and Applied Pyrolysis, 2014, 109: 98-108. |
| 38 | 黄金保, 刘朝, 魏顺安, 等. 丙三醇脱水反应机理的密度泛函理论研究[J]. 化学学报, 2010, 68(11): 1043-1049. |
| Huang J B, Liu C, Wei S A, et al. Density functional theory study on the dehydration mechanism of glycerine[J]. Acta Chimica Sinica, 2010, 68(11): 1043-1049. | |
| 39 | 程小彩, 黄金保, 潘贵英, 等. 聚苯乙烯热降解机理的理论研究[J]. 燃料化学学报, 2019, 47(7): 884-896. |
| Cheng X C, Huang J B, Pan G Y, et al. Theoretical study on thermal degradation mechanism of polystyrene[J]. Journal of Fuel Chemistry and Technology, 2019, 47(7): 884-896. | |
| 40 | 黄金保, 童红, 曾桂生, 等. 丁醇醛和丁醇酸热解形成CO和CO2机理的密度泛函理论研究[J]. 燃料化学学报, 2012, 40(8): 979-984. |
| Huang J B, Tong H, Zeng G S, et al. Density functional theory studies on the formation mechanism of CO and CO2 in pyrolysis of hydroxyl butyraldehyde and butyric acid[J]. Journal of Fuel Chemistry and Technology, 2012, 40(8): 979-984. |
| [1] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [2] | 常明慧, 王林, 苑佳佳, 曹艺飞. 盐溶液蓄能型热泵循环特性研究[J]. 化工学报, 2023, 74(S1): 329-337. |
| [3] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [4] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [5] | 张化福, 童莉葛, 张振涛, 杨俊玲, 王立, 张俊浩. 机械蒸汽压缩蒸发技术研究现状与发展趋势[J]. 化工学报, 2023, 74(S1): 8-24. |
| [6] | 肖明堃, 杨光, 黄永华, 吴静怡. 浸没孔液氧气泡动力学数值研究[J]. 化工学报, 2023, 74(S1): 87-95. |
| [7] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [8] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [9] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [10] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [11] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [12] | 张曼铮, 肖猛, 闫沛伟, 苗政, 徐进良, 纪献兵. 危废焚烧处理耦合有机朗肯循环系统工质筛选与热力学优化[J]. 化工学报, 2023, 74(8): 3502-3512. |
| [13] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [14] | 曾如宾, 沈中杰, 梁钦锋, 许建良, 代正华, 刘海峰. 基于分子动力学模拟的Fe2O3纳米颗粒烧结机制研究[J]. 化工学报, 2023, 74(8): 3353-3365. |
| [15] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号