化工学报 ›› 2022, Vol. 73 ›› Issue (8): 3511-3517.DOI: 10.11949/0438-1157.20220262
俞夏琪1( ), 冯格1, 赵金燕1, 李嘉远1, 邓声威1, 郑靖楠1, 李雯雯1, 王亚秋1, 沈榄1, 刘旭1, 徐威威1, 王建国1, 王式彬1, 姚子豪1(
), 冯格1, 赵金燕1, 李嘉远1, 邓声威1, 郑靖楠1, 李雯雯1, 王亚秋1, 沈榄1, 刘旭1, 徐威威1, 王建国1, 王式彬1, 姚子豪1( ), 毛成立2(
), 毛成立2( )
)
收稿日期:2022-03-01
修回日期:2022-04-26
出版日期:2022-08-05
发布日期:2022-09-06
通讯作者:
姚子豪,毛成立
作者简介:俞夏琪(1997—),女,硕士研究生,yuxq4561@163.com
Xiaqi YU1( ), Ge FENG1, Jinyan ZHAO1, Jiayuan LI1, Shengwei DENG1, Jingnan ZHENG1, Wenwen LI1, Yaqiu WANG1, Lan SHEN1, Xu LIU1, Weiwei XU1, Jianguo WANG1, Shibin WANG1, Zihao YAO1(
), Ge FENG1, Jinyan ZHAO1, Jiayuan LI1, Shengwei DENG1, Jingnan ZHENG1, Wenwen LI1, Yaqiu WANG1, Lan SHEN1, Xu LIU1, Weiwei XU1, Jianguo WANG1, Shibin WANG1, Zihao YAO1( ), Chengli MAO2(
), Chengli MAO2( )
)
Received:2022-03-01
Revised:2022-04-26
Online:2022-08-05
Published:2022-09-06
Contact:
Zihao YAO, Chengli MAO
摘要:
采用密度泛函理论(DFT)研究了氧化剂高氯酸铵(001)、(210)、(011)、(201)四种晶面的表面能并对其进行从头算分子动力学(AIMD)模拟,测试其稳定性。基体组分甲苯二异氰酸酯(TDI)、三羟基甲基丙烷(TMP)、三氟化硼三乙醇胺络合物(T313)在氧化剂晶面的吸附能同样由DFT进行计算。针对基体和氧化剂之间的相互作用进行了化学理论分析,最后选取吸附物和晶面相互作用最强的T313-AP(201)体系,模拟其分子电子结构并观察原子间的电荷转移情况。通过多种理论方法在分子尺度上揭示键合剂(T313)与氧化剂(AP)的作用机制,证实老化过程中产生的关键产物来源。
中图分类号:
俞夏琪, 冯格, 赵金燕, 李嘉远, 邓声威, 郑靖楠, 李雯雯, 王亚秋, 沈榄, 刘旭, 徐威威, 王建国, 王式彬, 姚子豪, 毛成立. 基体(TDI-TMP-T313)与氧化剂(AP)相互作用的第一性原理研究[J]. 化工学报, 2022, 73(8): 3511-3517.
Xiaqi YU, Ge FENG, Jinyan ZHAO, Jiayuan LI, Shengwei DENG, Jingnan ZHENG, Wenwen LI, Yaqiu WANG, Lan SHEN, Xu LIU, Weiwei XU, Jianguo WANG, Shibin WANG, Zihao YAO, Chengli MAO. A first-principles study of the interaction between TDI-TMP-T313 and AP[J]. CIESC Journal, 2022, 73(8): 3511-3517.
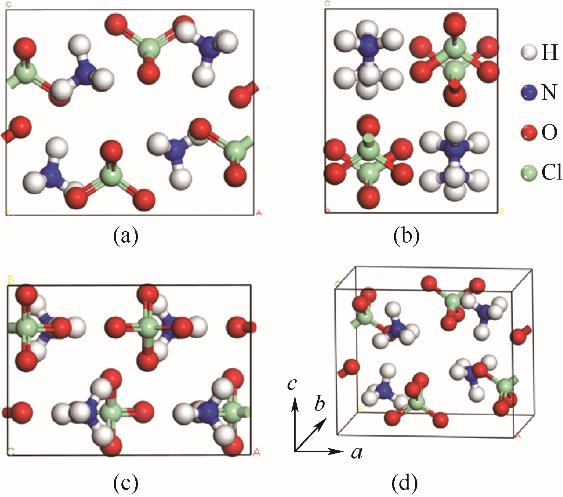
图1 高氯酸铵晶胞模型示意图(a) 主视图; (b) 侧视图; (c) 俯视图; (d) 立体图
Fig.1 Schematic representation of unit cell of AP(a) front view; (b) side view; (c) top view; (d) stereogram
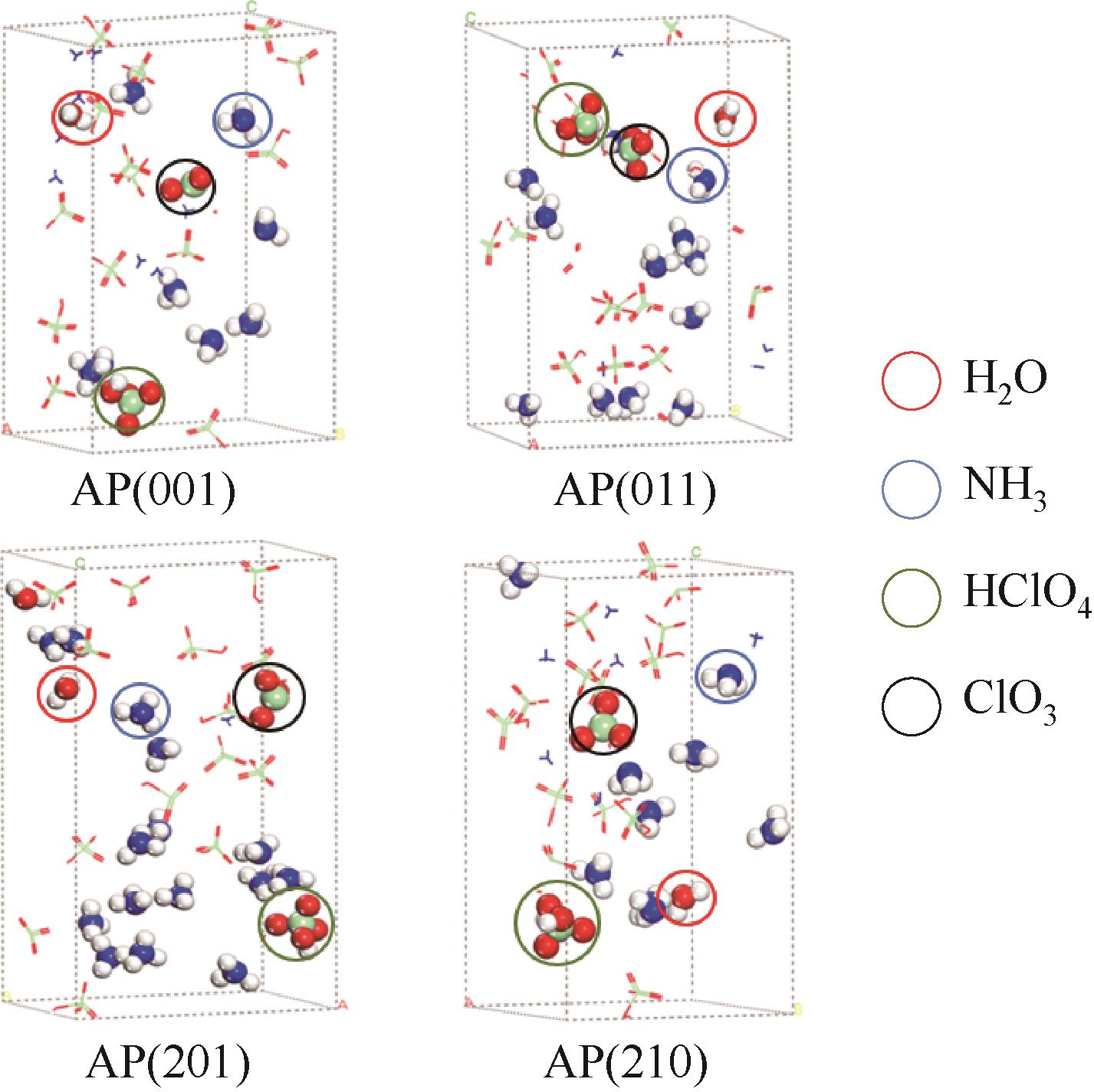
图3 基于AIMD计算的AP各晶面稳定性研究(红色圆圈内为H2O,蓝色为NH3,绿色为HClO4,黑色为ClO3)
Fig.3 Study on the stability of AP crystal planes based on AIMD(inside the red circle is H2O, blue is NH3, green is HClO4, black is ClO3)
| 物质-AP晶面 | 吸附能/eV | 物质-AP晶面 | 吸附能/eV |
|---|---|---|---|
| TDI-(001) | -1.47 | TMP- (201) | -1.68 |
| TMP-(001) | -1.63 | TDI- (201) | -0.66 |
| T313-(001) | -1.43 | T313-(201) | -2.20 |
| TDI- (011) | -0.59 | TMP-(210) | -1.08 |
| TMP- (011) | -1.32 | TDI-(210) | -0.58 |
| T313-(011) | -0.86 | T313-(210) | -0.27 |
表1 固化剂TDI、交联剂TMP、键合剂T313在氧化剂AP上的吸附能
Table 1 Adsorption energy of curing agent TDI, crosslinking agent TMP and bonding agent T313 on oxidizer AP
| 物质-AP晶面 | 吸附能/eV | 物质-AP晶面 | 吸附能/eV |
|---|---|---|---|
| TDI-(001) | -1.47 | TMP- (201) | -1.68 |
| TMP-(001) | -1.63 | TDI- (201) | -0.66 |
| T313-(001) | -1.43 | T313-(201) | -2.20 |
| TDI- (011) | -0.59 | TMP-(210) | -1.08 |
| TMP- (011) | -1.32 | TDI-(210) | -0.58 |
| T313-(011) | -0.86 | T313-(210) | -0.27 |
| 原子 | 数量 | 价态 | 电荷 | 得失电子情况 |
|---|---|---|---|---|
| B | 1 | 3 | 0.67 | 2.33 |
| C | 6 | 4 | 3.89 | 0.11 |
| Cl | 32 | 7 | 3.94 | 3.06 |
| F | 3 | 7 | 7.81 | -0.81 |
| H | 143 | 1 | 0.42 | 0.58 |
| N | 33 | 5 | 6.39 | -1.39 |
| O | 133 | 6 | 7.02 | -1.02 |
表2 T313-AP(201)的Bader电荷分析
Table 2 Bader charge analysis of T313-AP(201)
| 原子 | 数量 | 价态 | 电荷 | 得失电子情况 |
|---|---|---|---|---|
| B | 1 | 3 | 0.67 | 2.33 |
| C | 6 | 4 | 3.89 | 0.11 |
| Cl | 32 | 7 | 3.94 | 3.06 |
| F | 3 | 7 | 7.81 | -0.81 |
| H | 143 | 1 | 0.42 | 0.58 |
| N | 33 | 5 | 6.39 | -1.39 |
| O | 133 | 6 | 7.02 | -1.02 |
| 原子 | 数量 | 价态 | 电荷 | 得失电子情况 |
|---|---|---|---|---|
| B | 1 | 3 | 0.67 | 2.33 |
| C | 6 | 4 | 3.54 | 0.46 |
| F | 3 | 7 | 7.81 | -0.81 |
| H | 15 | 1 | 0.79 | 0.21 |
| N | 1 | 5 | 6.09 | -1.09 |
| O | 3 | 6 | 7.15 | -1.15 |
| 总体 | 29 | — | — | 1.21 |
表3 T313在T313-AP(201)的Bader电荷分析
Table 3 Bader charge analysis of T313 in T313-AP(201)
| 原子 | 数量 | 价态 | 电荷 | 得失电子情况 |
|---|---|---|---|---|
| B | 1 | 3 | 0.67 | 2.33 |
| C | 6 | 4 | 3.54 | 0.46 |
| F | 3 | 7 | 7.81 | -0.81 |
| H | 15 | 1 | 0.79 | 0.21 |
| N | 1 | 5 | 6.09 | -1.09 |
| O | 3 | 6 | 7.15 | -1.15 |
| 总体 | 29 | — | — | 1.21 |
| 1 | Chaturvedi S, Dave P N. Solid propellants: AP/HTPB composite propellants[J]. Arabian Journal of Chemistry, 2019, 12(8): 2061-2068. |
| 2 | Hunter S, Davidson A J, Morrison C A, et al. Combined experimental and computational hydrostatic compression study of crystalline ammonium perchlorate[J]. The Journal of Physical Chemistry C, 2011, 115(38): 18782-18788. |
| 3 | Kellogg R, Lapidus S, Hedman T, et al. Synchrotron based measurement of the temperature dependent thermal expansion coefficient of ammonium perchlorate[J]. Propellants, Explosives, Pyrotechnics, 2020, 45(3): 480-485. |
| 4 | Kroonblawd M P, Koroglu B, Zaug J M, et al. Effects of pressure on the structure and lattice dynamics of ammonium perchlorate: a combined experimental and theoretical study[J]. The Journal of Chemical Physics, 2018, 149(3): 034501. |
| 5 | Zhu W H, Wei T, Zhu W, et al. Comparative DFT study of crystalline ammonium perchlorate and ammonium dinitramide[J]. The Journal of Physical Chemistry. A, 2008, 112(20): 4688-4693. |
| 6 | Liu Y B, Zhang S H, Gou R J, et al. Theoretical study on the intermolecular interactions between energetic oxidizer and pyrazine - 1, 4 - dioxide[J]. Materials Today Communications, 2020, 24: 101020. |
| 7 | Wu Q, Li C L, Tan L H, et al. Comparative DFT and DFT-D studies on structural, electronic, vibrational and absorption properties of crystalline ammonium perchlorate[J]. RSC Advances, 2016, 6(54): 48489-48497. |
| 8 | Yedukondalu N, Vaitheeswaran G. Polymorphism, phase transition, and lattice dynamics of energetic oxidizer ammonium perchlorate under high pressure[J]. The Journal of Physical Chemistry C, 2019, 123(4): 2114-2126. |
| 9 | Yeh I C, Andzelm J W. Computational study of structural and energetic properties of ammonium perchlorate at interfaces[J]. The Journal of Physical Chemistry C, 2021, 125(22): 12297-12304. |
| 10 | Forquet V, Miró Sabaté C, Chermette H, et al. Energetic properties of rocket propellants evaluated through the computational determination of heats of formation of nitrogen-rich compounds[J]. Chemistry-An Asian Journal, 2016, 11(5): 730-744. |
| 11 | Tang P F, Yang B, Li R, et al. Ti3C2 MXene: a reactive combustion catalyst for efficient burning rate control of ammonium perchlorate based solid propellant[J]. Carbon, 2022, 186: 678-687. |
| 12 | 吴芳, 熊中年, 燕为光, 等. Bu-NENA/PBT推进剂安全性能[J]. 固体火箭技术, 2019, 42(4): 483-487. |
| Wu F, Xiong Z N, Yan W G, et al. Safety properties of Bu-NENA/PBT propellants[J]. Journal of Solid Rocket Technology, 2019, 42(4): 483-487. | |
| 13 | 周水平, 王艳萍, 唐根, 等. 黏合剂固化网络参数对叠氮聚醚推进剂低温性能的影响[J]. 化学推进剂与高分子材料, 2017, 15(5): 49-54. |
| Zhou S P, Wang Y P, Tang G, et al. Influence of binder curing network parameters on cryogenic performance of azido polyether propellant[J]. Chemical Propellants & Polymeric Materials, 2017, 15(5): 49-54. | |
| 14 | 周水平, 吴芳, 唐根, 等. 含能交联剂对PBT高能推进剂力学性能的影响[J]. 化学推进剂与高分子材料, 2016, 14(4): 54-59. |
| Zhou S P, Wu F, Tang G, et al. Influence of energetic crosslinking agents on mechanical performance of PBT-based high energy propellants[J]. Chemical Propellants & Polymeric Materials, 2016, 14(4): 54-59. | |
| 15 | 姚维尚, 李倩, 谭惠民. NEPE推进剂黏合剂性能的分子模拟研究[J]. 含能材料, 2007, 15(6): 650-655. |
| Yao W S, Li Q, Tan H M. Molecular simulation on properties of NEPE propellant binders[J]. Chinese Journal of Energetic Materials, 2007, 15(6): 650-655. | |
| 16 | 焦东明, 杨月诚, 强洪夫, 等. 键合剂对HTPB与Al/Al2O3之间界面作用的分子模拟[J]. 火炸药学报, 2009, 32(4): 60-63. |
| Jiao D M, Yang Y C, Qiang H F, et al. Molecular simulation of effect of bonding agents on interface interaction for HTPB and Al/Al2O3 [J]. Chinese Journal of Explosives & Propellants, 2009, 32(4): 60-63. | |
| 17 | Choi C S, Prask H J, Prince E. Crystal structure of NH4ClO4 at 298, 78, and 10 by neutron diffraction[J]. The Journal of Chemical Physics, 1974, 61(9): 3523-3529. |
| 18 | Mao Y, Hu P. Identification of the active sites and mechanism for partial methane oxidation to methanol over copper-exchanged CHA zeolites[J]. Science China Chemistry, 2020, 63(6): 850-859. |
| 19 | Wei Z Z, Yao Z H, Zhou Q, et al. Optimizing alkyne hydrogenation performance of Pd on carbon in situ decorated with oxygen-deficient TiO2 by integrating the reaction and diffusion[J]. ACS Catalysis, 2019, 9(12): 10656-10667. |
| 20 | Yao Z H, Guo C X, Mao Y, et al. Quantitative determination of C—C coupling mechanisms and detailed analyses on the activity and selectivity for Fischer-Tropsch synthesis on Co(0001): microkinetic modeling with coverage effects[J]. ACS Catalysis, 2019, 9(7): 5957-5973. |
| 21 | Yao Z H, Zhao J Y, Bunting R J, et al. Quantitative insights into the reaction mechanism for the direct synthesis of H2O2 over transition metals: coverage-dependent microkinetic modeling[J]. ACS Catalysis, 2021, 11(3): 1202-1221. |
| 22 | Yao Z H, Zhao J Y, Zhao C X, et al. A first-principles study of reaction mechanism over carbon decorated oxygen-deficient TiO2 supported Pd catalyst in direct synthesis of H2O2 [J]. Chinese Journal of Chemical Engineering, 2021, 31: 126-134. |
| 23 | Khan M A S, Vijayalakshmi R, Singh A, et al. Morphology of ammonium perchlorate in the presence of ethylene glycol as an additive: a first principle study[J]. CrystEngComm, 2019, 21(48): 7519-7527. |
| 24 | Kang L, Li S R, Wang B, et al. Exploration of the energetic material ammonium perchlorate at high pressures: combined Raman spectroscopy and X-ray diffraction study[J]. The Journal of Physical Chemistry C, 2018, 122(28): 15937-15944. |
| 25 | Ma Z Y, Pang A M, Li W, et al. Preparation and characterization of ultra-fine ammonium perchlorate crystals using a microfluidic system combined with ultrasonication[J]. Chemical Engineering Journal, 2021, 405: 126516. |
| 26 | Xie P F, Ding J, Yao Z H, et al. Oxo dicopper anchored on carbon nitride for selective oxidation of methane[J]. Nature Communications, 2022, 13(1): 1375. |
| 27 | Reuter K, Scheffler M. Composition, structure, and stability of RuO2(110) as a function of oxygen pressure[J]. Physical Review B, 2001, 65(3): 035406. |
| 28 | Reuter K, Scheffler M. Composition and structure of the RuO2(110) surface in an O2 and CO environment: implications for the catalytic formation of CO2 [J]. Physical Review B, 2003, 68(4): 045407. |
| 29 | Reuter K, Scheffler M. Oxide formation at the surface of late 4d transition metals: insights from first-principles atomistic thermodynamics[J]. Applied Physics A, 2004, 78(6): 793-798. |
| 30 | Rogal J, Reuter K, Scheffler M. Thermodynamic stability of PdO surfaces[J]. Physical Review B, 2004, 69(7): 075421. |
| 31 | Therrien A J, Zhang R Q, Lucci F R, et al. Structurally accurate model for the “29”-structure of Cu x O/Cu(111): a DFT and STM study[J]. The Journal of Physical Chemistry C, 2016, 120(20): 10879-10886. |
| [1] | 吴馨, 龚建英, 靳龙, 王宇涛, 黄睿宁. 超声波激励下铝板表面液滴群输运特性的研究[J]. 化工学报, 2023, 74(S1): 104-112. |
| [2] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [3] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [4] | 杨越, 张丹, 郑巨淦, 涂茂萍, 杨庆忠. NaCl水溶液喷射闪蒸-掺混蒸发的实验研究[J]. 化工学报, 2023, 74(8): 3279-3291. |
| [5] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [6] | 陈天华, 刘兆轩, 韩群, 张程宾, 李文明. 喷雾冷却换热强化研究进展及影响因素[J]. 化工学报, 2023, 74(8): 3149-3170. |
| [7] | 何晓崐, 刘锐, 薛园, 左然. MOCVD生长AlN单晶薄膜的气相和表面化学反应综述[J]. 化工学报, 2023, 74(7): 2800-2813. |
| [8] | 王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588. |
| [9] | 李晨曦, 刘永峰, 张璐, 刘海峰, 宋金瓯, 何旭. O2/CO2氛围下正庚烷的燃烧机理研究[J]. 化工学报, 2023, 74(5): 2157-2169. |
| [10] | 徐文超, 孙志高, 李翠敏, 李娟, 黄海峰. 静态条件下表面活性剂E-1310对HCFC-141b水合物生成的影响[J]. 化工学报, 2023, 74(5): 2179-2185. |
| [11] | 葛运通, 王玮, 李楷, 肖帆, 于志鹏, 宫敬. 多相分散体系中微油滴与改性二氧化硅表面间作用力的AFM研究[J]. 化工学报, 2023, 74(4): 1651-1659. |
| [12] | 罗来明, 张劲, 郭志斌, 王海宁, 卢善富, 相艳. 1~5 kW高温聚合物电解质膜燃料电池堆的理论模拟与组装测试[J]. 化工学报, 2023, 74(4): 1724-1734. |
| [13] | 钱志广, 樊越, 王世学, 岳利可, 王金山, 朱禹. 吹扫条件对PEMFC阻抗弛豫现象和低温启动的影响[J]. 化工学报, 2023, 74(3): 1286-1293. |
| [14] | 张家庆, 蒋榕培, 史伟康, 武博翔, 杨超, 刘朝晖. 煤基/石油基火箭煤油高参数黏温特性与组分特性研究[J]. 化工学报, 2023, 74(2): 653-665. |
| [15] | 王峰, 张顺鑫, 余方博, 刘亚, 郭烈锦. 光催化CO2还原制碳氢燃料系统优化策略研究[J]. 化工学报, 2023, 74(1): 29-44. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号