化工学报 ›› 2021, Vol. 72 ›› Issue (12): 6340-6350.DOI: 10.11949/0438-1157.20210813
汪宇1( ),张禹2,童微雯1,叶光华1(
),张禹2,童微雯1,叶光华1( ),周兴贵1,袁渭康1
),周兴贵1,袁渭康1
收稿日期:2021-06-17
修回日期:2021-09-08
出版日期:2021-12-05
发布日期:2021-12-22
通讯作者:
叶光华
作者简介:汪宇 (1998—),男,硕士研究生,基金资助:
Yu WANG1( ),Yu ZHANG2,Weiwen TONG1,Guanghua YE1(
),Yu ZHANG2,Weiwen TONG1,Guanghua YE1( ),Xinggui ZHOU1,Weikang YUAN1
),Xinggui ZHOU1,Weikang YUAN1
Received:2021-06-17
Revised:2021-09-08
Online:2021-12-05
Published:2021-12-22
Contact:
Guanghua YE
摘要:
在较高充放电速率下,锂电池电极中Li+扩散受限严重,致使电池性能显著降低。为了减弱扩散限制,设计电极的孔结构是一种行之有效的方法。本工作以LiCoO2正极为模型电极,利用建立的二维模型,优化了电极中含低曲折因子孔道的多级孔道结构。这些低曲折因子孔道可作为Li+传输的“高速公路”,优化其孔隙率和孔径,可大幅度提升高放电倍率下的能量密度。低曲折因子孔道的最佳孔隙率高度依赖于其孔径,其直径小于10 μm较优。当电极厚度为200 μm且总孔隙率为0.36时,优化后的多级孔电极相比于传统电极,能量密度可提高45.9%~91.4%。此外,当Li+的扩散限制较弱时(例如,电极厚度≤50 μm和总孔隙率≥0.48),优化电极的多级孔结构并不会显著提升电极性能。本工作可为锂电池电极中多级孔道结构的设计提供一定的指导。
中图分类号:
汪宇, 张禹, 童微雯, 叶光华, 周兴贵, 袁渭康. 锂离子电池电极中多级孔道结构设计[J]. 化工学报, 2021, 72(12): 6340-6350.
Yu WANG, Yu ZHANG, Weiwen TONG, Guanghua YE, Xinggui ZHOU, Weikang YUAN. Engineering hierarchical pore network for Li-ion battery electrodes[J]. CIESC Journal, 2021, 72(12): 6340-6350.
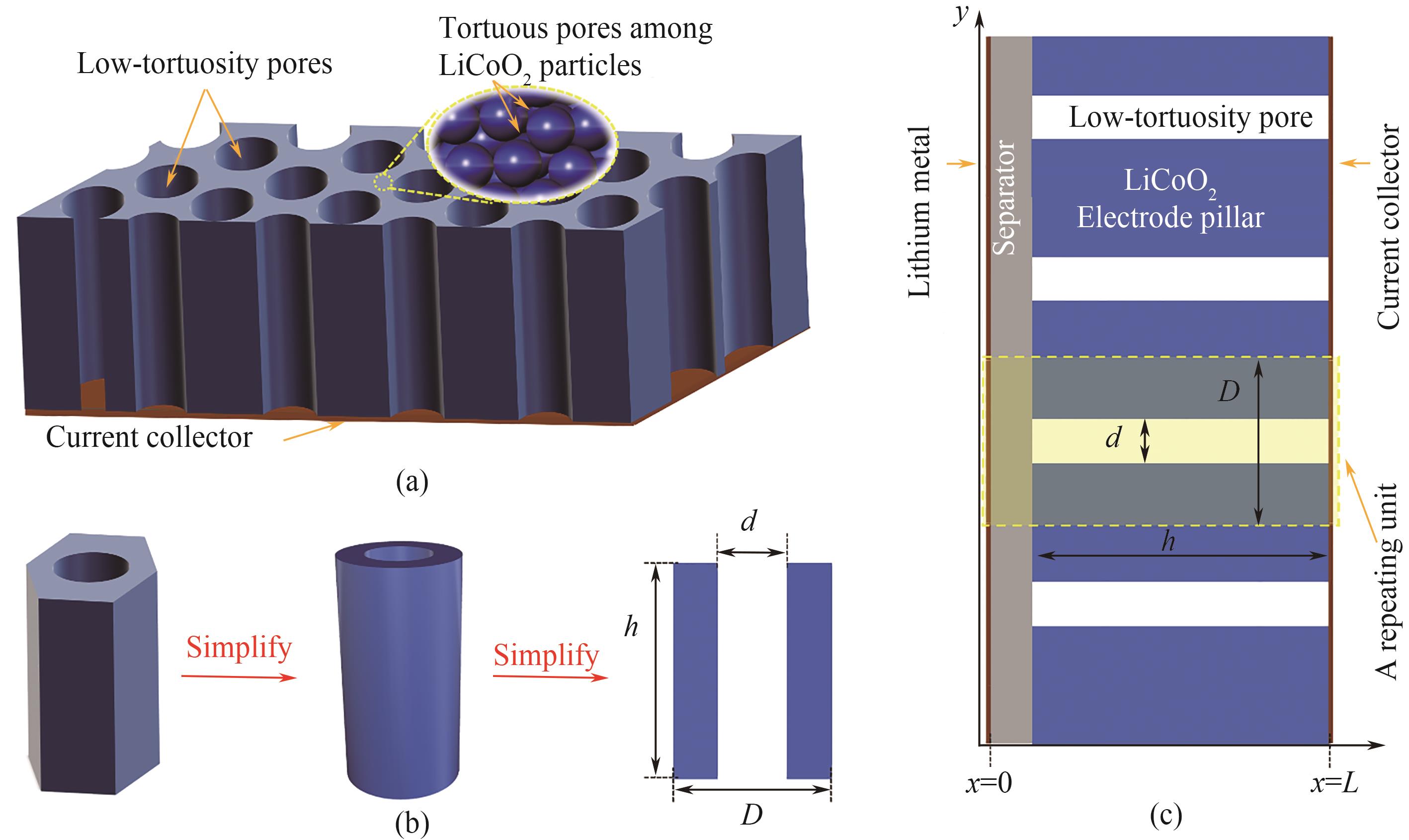
图1 多级孔电极的结构示意图(a);三维结构到二维重复单元的简化(b);以钴酸锂多级孔电极为正极和锂金属为负极的半电池的二维结构示意图(c)
Fig.1 A schematic of the electrode with the hierarchical pore network (a); Simplification of a three-dimensional repeating unit into a two-dimensional one (b); A two-dimensional schematic of the half-cell with the hierarchically structured electrode as LiCoO2 the cathode and the lithium metal as the anode (c)
| 参数 | 单位 | 数值 |
|---|---|---|
| 初始电解质盐浓度 (c0) | mol/m3 | 1200 ① |
| 最大嵌锂浓度 (cs,max) | mol/m3 | 20434 |
| 初始嵌锂浓度 (cs0) | mol/m3 | 9604 |
| 锂在电极材料内的扩散系数 (Ds) | m2/s | 10-11 ① |
| 锂离子在电解液中的扩散系数 (Dl) | m2/s | 2.6×10-10 ① |
| 电导率 (σ) | S/m | 10 ① |
| 膈膜厚度 (hs) | μm | 20 |
| 集流体的总厚度 (hc) | μm | 40 |
| 阳极锂金属厚度 (ha) | μm | 50 |
| 钴酸锂材料颗粒半径 (Rs) | μm | 4 ① |
| 阳极电荷转移系数 (αa) | — | 0.5 ① |
| 阴极电荷转移系数 (αc) | — | 0.5 ① |
| 添加剂所占孔隙率 (εadd) | — | 0.007 |
| 锂离子传递系数 ( | — | 0.363 ① |
| 温度 (T) | K | 298 |
| Bruggeman系数 (m) | — | 2.5 |
| 1C时电流密度 (I) | A/m2 | 38.2 |
| 钴酸锂阴极厚度 (h) | μm | 50~150 |
| 钴酸锂阴极总孔隙率 (εt) | — | 0.28~0.48 |
| 分层结构中孔的孔径 (d) | μm | 10~90 |
| 分层孔道结构的孔隙率 (d2/D2) | — | 0.04~0.25 |
表1 本工作数值模拟中所用到的参数
Table 1 Parameters for the numerical simulations in this work
| 参数 | 单位 | 数值 |
|---|---|---|
| 初始电解质盐浓度 (c0) | mol/m3 | 1200 ① |
| 最大嵌锂浓度 (cs,max) | mol/m3 | 20434 |
| 初始嵌锂浓度 (cs0) | mol/m3 | 9604 |
| 锂在电极材料内的扩散系数 (Ds) | m2/s | 10-11 ① |
| 锂离子在电解液中的扩散系数 (Dl) | m2/s | 2.6×10-10 ① |
| 电导率 (σ) | S/m | 10 ① |
| 膈膜厚度 (hs) | μm | 20 |
| 集流体的总厚度 (hc) | μm | 40 |
| 阳极锂金属厚度 (ha) | μm | 50 |
| 钴酸锂材料颗粒半径 (Rs) | μm | 4 ① |
| 阳极电荷转移系数 (αa) | — | 0.5 ① |
| 阴极电荷转移系数 (αc) | — | 0.5 ① |
| 添加剂所占孔隙率 (εadd) | — | 0.007 |
| 锂离子传递系数 ( | — | 0.363 ① |
| 温度 (T) | K | 298 |
| Bruggeman系数 (m) | — | 2.5 |
| 1C时电流密度 (I) | A/m2 | 38.2 |
| 钴酸锂阴极厚度 (h) | μm | 50~150 |
| 钴酸锂阴极总孔隙率 (εt) | — | 0.28~0.48 |
| 分层结构中孔的孔径 (d) | μm | 10~90 |
| 分层孔道结构的孔隙率 (d2/D2) | — | 0.04~0.25 |
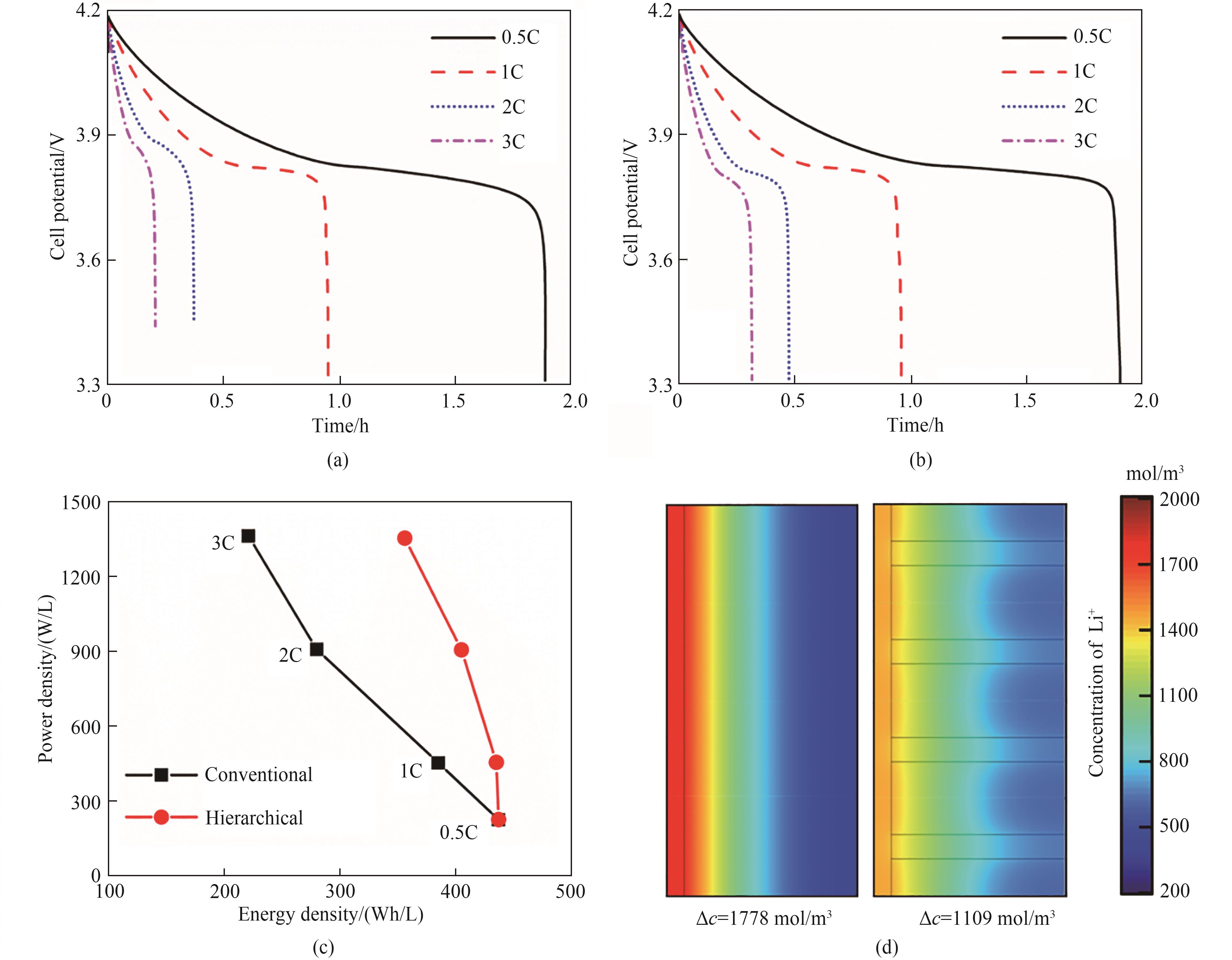
图2 普通电极(a)和多级孔电极(b)对应的半电池在0.5C、1C、2C和3C放电倍率下的放电特性曲线;由图2(a)、(b)计算得到的Ragone图(c);当放电倍率为2C时,普通电极和多级孔电极在放电120 s时的Li+浓度分布图(d)(模型参数:电池厚度h=200 μm,总孔隙率εt=0.36,低曲折因子孔道的直径d=20 μm,低曲折因子孔道所占的孔隙率d2/D2=0.0625,其他参数见表1)
Fig.2 Time-dependent cell potentials under the discharge rates of 0.5C, 1C, 2C and 3C for a conventional electrode (a) and a hierarchically structured electrode (b); The Ragone plots obtained from Figs. 2(a) and 2(b) (c); Typical concentration profiles of Li+ at t=120 s under the discharge rate of 2C for the conventional electrode and the hierarchically structured electrode (d) (Simulation parameters: h=200 μm, εt=0.36, d=20 μm, and d2/D2=0.0625. The other parameters are given in Table 1)
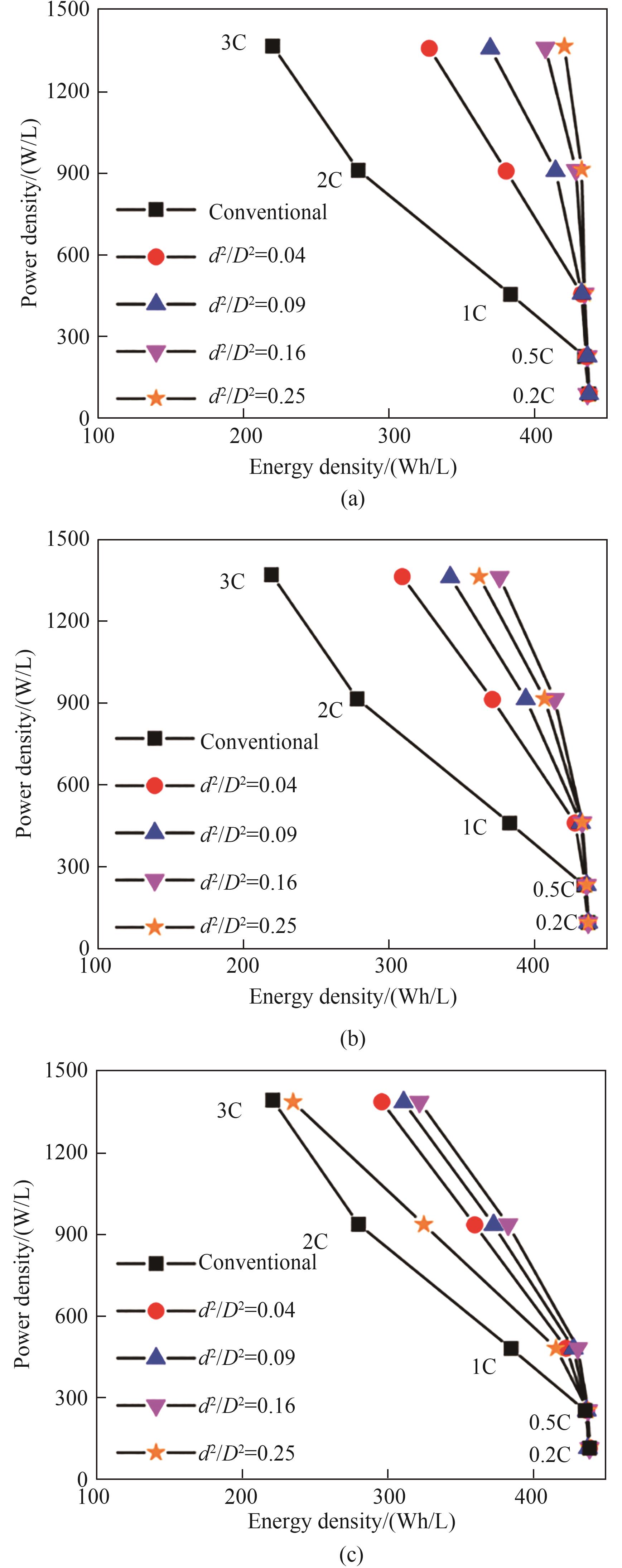
图3 普通电极与具有不同低曲折因子孔道孔隙率(d2/D2=0.04、0.09、0.16和0.25)多级孔电极对应的Ragone图:(a) d = 30 μm; (b) d=60 μm;(c) d=90 μm(模型参数:h=200 μm,εt=0.36,其他参数列于表1)
Fig.3 Ragone plots for the conventional electrode and the hierarchically structured electrodes with d2/D2=0.04, 0.09, 0.16, and 0.25. (a) d=30 μm; (b) d=60 μm; (c) d=90 μm (Simulation parameters: h=200 μm, εt=0.36. The other parameters are given in Table 1)
| d2/D2 | 能量密度/(Wh/L) | |||
|---|---|---|---|---|
| 0 μm | 30 μm | 60 μm | 90 μm | |
| 0 | 220① | — | — | — |
| 0.04 | — | 328 | 310 | 295 |
| 0.09 | — | 370 | 343 | 308 |
| 0.16 | — | 408 | 377 | 321 |
| 0.25 | — | 421 | 363 | 234 |
表2 3C放电倍率下普通电极与具有不同低曲折因子孔道孔隙率多级孔电极对应的能量密度
Table 2 Energy densities at the 3C discharge rate for the conventional electrode and the hierarchically structured electrodes with different porosities
| d2/D2 | 能量密度/(Wh/L) | |||
|---|---|---|---|---|
| 0 μm | 30 μm | 60 μm | 90 μm | |
| 0 | 220① | — | — | — |
| 0.04 | — | 328 | 310 | 295 |
| 0.09 | — | 370 | 343 | 308 |
| 0.16 | — | 408 | 377 | 321 |
| 0.25 | — | 421 | 363 | 234 |

图4 多级孔电极以2C的放电倍率下放电120 s时的浓度分布:(a) d=30 μm,d2/D2=0.04;(b) d=30 μm,d2/D2=0.09;(c) d=30 μm,d2/D2=0.16;(d) d=30 μm,d2/D2=0.25;(e) d=90 μm,d2/D2=0.04;(f) d=90 μm,d2/D2=0.09;(g) d=90 μm,d2/D2=0.16;(h) d=90 μm,d2/D2=0.25(模型参数:h=200 μm,εt=0.36,其他参数列于表1)
Fig.4 Concentration profiles of Li+ in the hierarchically structured electrodes at t=120 s under the discharge rate of 2C. (a) d=30 μm, d2/D2=0.04; (b) d=30 μm, d2/D2=0.09; (c) d=30 μm, d2/D2=0.16; (d) d=30 μm, d2/D2=0.25; (e) d=90 μm, d2/D2=0.04; (f) d=90 μm, d2/D2=0.09; (g) d=90 μm, d2/D2=0.16; (h) d=90 μm, d2/D2=0.25 (Simulation parameters: h=200 μm, εt=0.36. The other parameters are given in Table 1)
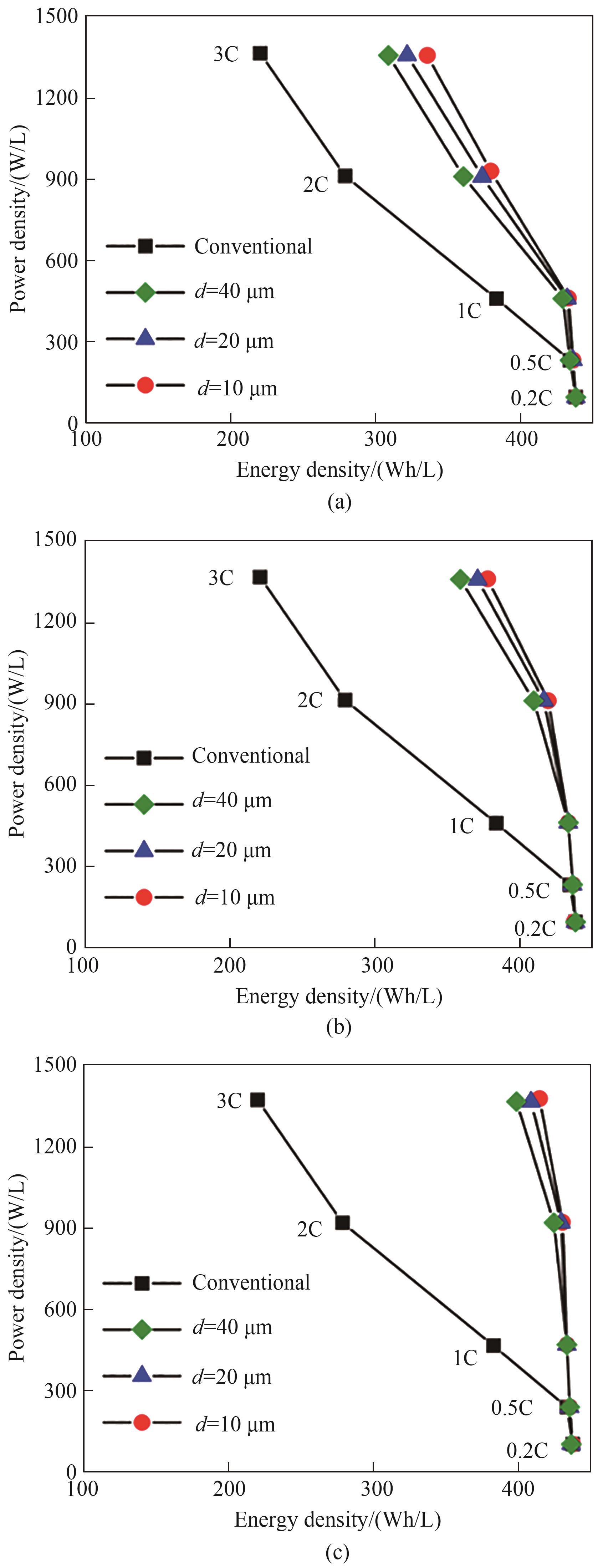
图5 普通电极与具有不同低曲折因子孔道直径(d=10、20和40 μm)多级孔电极对应的Ragone图:(a) d2/D2=0.04;(b) d2/D2=0.09;(c) d2/D2=0.16(模型参数:h=200 μm,εt=0.36,其他参数列于表1)
Fig.5 Ragone plots for the conventional electrode and the hierarchically structured electrodes with d=10, 20, and 40 μm. (a) d2/D2=0.04; (b) d2/D2=0.09; (c) d2/D2=0.16 (Simulation parameters: h=200 μm, εt=0.36. The other parameters are given in Table 1)
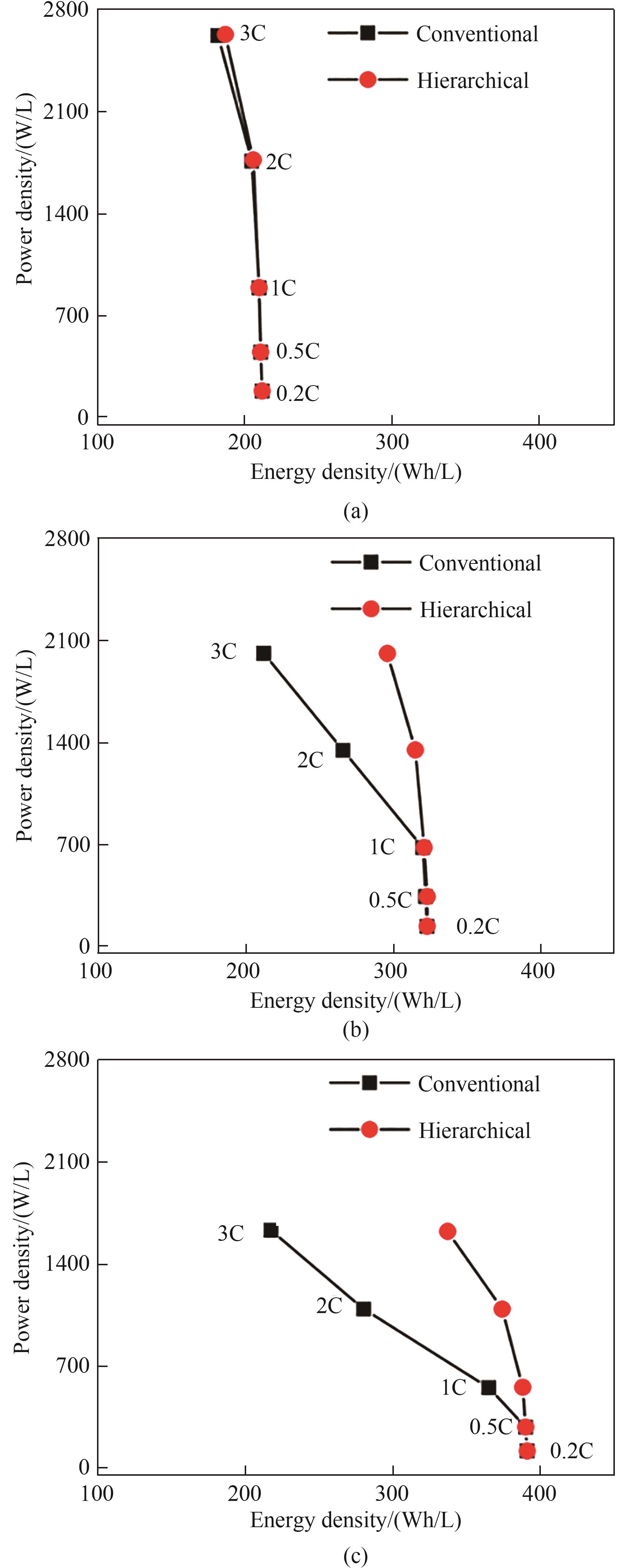
图6 普通电极与多级孔电极在不同的电极厚度下的Ragone图:(a) h=50 μm;(b) h=100 μm;(c) h=150 μm(模型参数:d=25 μm,d2/D2=0.0625,εt=0.36,其他参数列于表1)
Fig.6 Ragone plots for the conventional electrode and the hierarchically structured electrode under different electrode thicknesses. (a) h=50 μm; (b) h=100 μm; (c) h=150 μm (simulation parameters: d=25 μm, d2/D2=0.0625, εt=0.36. The other parameters are given in Table 1)

图7 普通电极与多级孔电极在不同电极总孔隙率下的Ragone图:(a) εt=0.28;(b) εt=0.38;(c) εt=0.48(模型参数:d=30 μm,D=100 μm,h=200 μm,其他参数列于表1)
Fig.7 Ragone plots for the conventional electrode and the hierarchically structured electrode under different total porosities. (a) εt=0.28; (b) εt=0.38; (c) εt=0.48 (simulation parameters: d=30 μm, D=100 μm, h=200 μm. The other parameters are given in Table 1)
| d/μm | 能量密度/(Wh/L) | |||
|---|---|---|---|---|
| d2/D2=0 | d2/D2=0.04 | d2/D2=0.09 | d2/D2=0.16 | |
| 0 | 220① | — | — | — |
| 10 | — | 336 | 378 | 416 |
| 20 | — | 322 | 371 | 410 |
| 40 | — | 309 | 359 | 400 |
表3 3C放电倍率下普通电极与具有不同低曲折因子孔道直径多级孔电极对应的能量密度
Table 3 Energy densities at the 3C discharge rate for the conventional electrode and the hierarchically structured electrodes with different pore diameters
| d/μm | 能量密度/(Wh/L) | |||
|---|---|---|---|---|
| d2/D2=0 | d2/D2=0.04 | d2/D2=0.09 | d2/D2=0.16 | |
| 0 | 220① | — | — | — |
| 10 | — | 336 | 378 | 416 |
| 20 | — | 322 | 371 | 410 |
| 40 | — | 309 | 359 | 400 |
| 电极 | 能量密度/(Wh/L) | ||
|---|---|---|---|
| 50 μm | 100 μm | 150 μm | |
| 传统电极 | 182 | 212 | 217 |
| 多级孔电极 | 187 | 296 | 337 |
表4 3C放电倍率下普通电极与多级孔电极在不同电极厚度下的能量密度
Table 4 Energy densities at the 3C discharge rate for the conventional electrode and the hierarchically structured electrodes with different electrode thicknesses
| 电极 | 能量密度/(Wh/L) | ||
|---|---|---|---|
| 50 μm | 100 μm | 150 μm | |
| 传统电极 | 182 | 212 | 217 |
| 多级孔电极 | 187 | 296 | 337 |
| 电极 | 能量密度/(Wh/L) | ||
|---|---|---|---|
| 0.28 | 0.38 | 0.48 | |
| 传统电极 | 252 | 239 | 295 |
| 多级孔电极 | 368 | 302 | 302 |
表5 3C放电倍率下普通电极与多级孔电极在不同电极总孔隙率下的能量密度
Table 5 Energy densities at the 3C discharge rate for the conventional electrode and the hierarchically structured electrodes with different total porosities
| 电极 | 能量密度/(Wh/L) | ||
|---|---|---|---|
| 0.28 | 0.38 | 0.48 | |
| 传统电极 | 252 | 239 | 295 |
| 多级孔电极 | 368 | 302 | 302 |
| 1 | Etacheri V, Marom R, Elazari R, et al. Challenges in the development of advanced Li-ion batteries: a review[J]. Energy & Environmental Science, 2011, 4(9):3243-3262. |
| 2 | Manthiram A. A reflection on lithium-ion battery cathode chemistry[J]. Nature Communications, 2020, 11: 1550. |
| 3 | Nitta N, Wu F X, Lee J T, et al. Li-ion battery materials: present and future[J]. Materials Today, 2015, 18(5): 252-264. |
| 4 | Lu L G, Han X B, Li J Q, et al. A review on the key issues for lithium-ion battery management in electric vehicles[J]. Journal of Power Sources, 2013, 226:272-288. |
| 5 | Kang B, Ceder G. Battery materials for ultrafast charging and discharging[J]. Nature, 2009, 458(7235):190-193. |
| 6 | Palacín M R. Recent advances in rechargeable battery materials: a chemist's perspective [J]. Chemical Society Reviews, 2009, 38(9):2565-2575. |
| 7 | Bae C J, Erdonmez C K, Halloran J W, et al. Design of battery electrodes with dual-scale porosity to minimize tortuosity and maximize performance[J]. Advanced Materials, 2013, 25(9): 1254-1258. |
| 8 | Billaud J, Bouville F, Magrini T, et al. Magnetically aligned graphite electrodes for high-rate performance Li-ion batteries[J]. Nature Energy, 2016, 1: 16097. |
| 9 | Park M, Zhang X C, Chung M, et al. A review of conduction phenomena in Li-ion batteries[J]. Journal of Power Sources, 2010, 195(24):7904-7929. |
| 10 | Zheng H H, Li J, Song X Y, et al. A comprehensive understanding of electrode thickness effects on the electrochemical performances of Li-ion battery cathodes[J]. Electrochimica Acta, 2012, 71: 258-265. |
| 11 | Dai Y L, Srinivasan V. On graded electrode porosity as a design tool for improving the energy density of batteries[J]. Journal of the Electrochemical Society, 2015, 163(3): A406-A416. |
| 12 | De S, Northrop P W C, Ramadesigan V, et al. Model-based simultaneous optimization of multiple design parameters for lithium-ion batteries for maximization of energy density[J]. Journal of Power Sources, 2013, 227:161-170. |
| 13 | Golmon S, Maute K, Dunn M L. A design optimization methodology for Li+ batteries[J]. Journal of Power Sources, 2014, 253:239-250. |
| 14 | Fuller T F, Doyle M, Newman J. Simulation and optimization of the dual lithium ion insertion cell[J]. Journal of the Electrochemical Society, 1994, 141(1): 1-10. |
| 15 | Henquín E R, Aguirre P A. Model based analysis of lithium batteries considering particle-size distribution[J]. AIChE Journal, 2018, 64(3): 904-915. |
| 16 | Lueth S, Sauter U S, Bessler W G. An agglomerate model of lithium-ion battery cathodes[J]. Journal of the Electrochemical Society, 2015, 163(2): A210-A222. |
| 17 | Newman J. Optimization of porosity and thickness of a battery electrode by means of a reaction-zone model[J]. Journal of the Electrochemical Society, 1995, 142(1): 97-101. |
| 18 | Srinivasan V, Newman J. Design and optimization of a natural graphite/iron phosphate lithium-ion cell[J]. Journal of the Electrochemical Society, 2004, 151(10): A1530. |
| 19 | Ramadesigan V, Methekar R N, Latinwo F, et al. Optimal porosity distribution for minimized ohmic drop across a porous electrode[J]. Journal of the Electrochemical Society, 2010, 157(12): A1328. |
| 20 | Pérez-Ramírez J, Christensen C H, Egeblad K, et al. Hierarchical zeolites: enhanced utilisation of microporous crystals in catalysis by advances in materials design[J]. Chemical Society Reviews, 2008, 37(11): 2530-2542. |
| 21 | Schwieger W, Machoke A G, Weissenberger T, et al. Hierarchy concepts: classification and preparation strategies for zeolite containing materials with hierarchical porosity[J]. Chemical Society Reviews, 2016, 45(12): 3353-3376. |
| 22 | Yang X Y, Chen L H, Li Y, et al. Hierarchically porous materials: synthesis strategies and structure design[J]. Chemical Society Reviews, 2017, 46(2): 481-558. |
| 23 | Sander J S, Erb R M, Li L, et al. High-performance battery electrodes via magnetic templating[J]. Nature Energy, 2016, 1: 16099. |
| 24 | Delattre B, Amin R, Sander J, et al. Impact of pore tortuosity on electrode kinetics in lithium battery electrodes: study in directionally freeze-cast LiNi0.8Co0.15Al0.05O2(NCA)[J]. Journal of the Electrochemical Society, 2018, 165(2): A388-A395. |
| 25 | Ye G H, Tong W W, Liu X L, et al. An analytical method for the optimization of pore network in lithium-ion battery electrodes[J]. Chemical Engineering Research and Design, 2019, 149: 226-234. |
| 26 | Newman J, Tiedemann W. Porous-electrode theory with battery applications[J]. AIChE Journal, 1975, 21(1): 25-41. |
| 27 | Doyle M, Fuller T F, Newman J. Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell[J]. Journal of the Electrochemical Society, 1993, 140(6): 1526-1533. |
| 28 | Antolini E. LiCoO2: formation, structure, lithium and oxygen nonstoichiometry, electrochemical behaviour and transport properties[J]. Solid State Ionics, 2004, 170(3/4): 159-171. |
| 29 | Marom R, Amalraj S F, Leifer N, et al. A review of advanced and practical lithium battery materials[J]. Journal of Materials Chemistry, 2011, 21(27): 9938. |
| 30 | Hutzenlaub T, Asthana A, Becker J, et al. FIB/SEM-based calculation of tortuosity in a porous LiCoO2 cathode for a Li-ion battery[J]. Electrochemistry Communications, 2013, 27: 77-80. |
| 31 | Cobb C L., Blanco M. Modeling mass and density distribution effects on the performance of co-extruded electrodes for high energy density lithium-ion batteries[J]. Journal of Power Sources, 2014, 249:357-366. |
| 32 | Doyle M, Fuentes Y. Computer simulations of a lithium-ion polymer battery and implications for higher capacity next-generation battery designs[J]. Journal of the Electrochemical Society, 2003, 150(6): A706. |
| [1] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [2] | 康飞, 吕伟光, 巨锋, 孙峙. 废锂离子电池放电路径与评价研究[J]. 化工学报, 2023, 74(9): 3903-3911. |
| [3] | 何松, 刘乔迈, 谢广烁, 王斯民, 肖娟. 高浓度水煤浆管道气膜减阻两相流模拟及代理辅助优化[J]. 化工学报, 2023, 74(9): 3766-3774. |
| [4] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [5] | 齐聪, 丁子, 余杰, 汤茂清, 梁林. 基于选择吸收纳米薄膜的太阳能温差发电特性研究[J]. 化工学报, 2023, 74(9): 3921-3930. |
| [6] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [7] | 张曼铮, 肖猛, 闫沛伟, 苗政, 徐进良, 纪献兵. 危废焚烧处理耦合有机朗肯循环系统工质筛选与热力学优化[J]. 化工学报, 2023, 74(8): 3502-3512. |
| [8] | 诸程瑛, 王振雷. 基于改进深度强化学习的乙烯裂解炉操作优化[J]. 化工学报, 2023, 74(8): 3429-3437. |
| [9] | 陈国泽, 卫东, 郭倩, 向志平. 负载跟踪状态下的铝空气电池堆最优功率点优化方法[J]. 化工学报, 2023, 74(8): 3533-3542. |
| [10] | 刘文竹, 云和明, 王宝雪, 胡明哲, 仲崇龙. 基于场协同和 耗散的微通道拓扑优化研究[J]. 化工学报, 2023, 74(8): 3329-3341. 耗散的微通道拓扑优化研究[J]. 化工学报, 2023, 74(8): 3329-3341. |
| [11] | 吴文涛, 褚良永, 张玲洁, 谭伟民, 沈丽明, 暴宁钟. 腰果酚生物基自愈合微胶囊的高效制备工艺研究[J]. 化工学报, 2023, 74(7): 3103-3115. |
| [12] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [13] | 王志龙, 杨烨, 赵真真, 田涛, 赵桐, 崔亚辉. 搅拌时间和混合顺序对锂离子电池正极浆料分散特性的影响[J]. 化工学报, 2023, 74(7): 3127-3138. |
| [14] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [15] | 文兆伦, 李沛睿, 张忠林, 杜晓, 侯起旺, 刘叶刚, 郝晓刚, 官国清. 基于自热再生的隔壁塔深冷空分工艺设计及优化[J]. 化工学报, 2023, 74(7): 2988-2998. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号