化工学报 ›› 2022, Vol. 73 ›› Issue (1): 441-450.DOI: 10.11949/0438-1157.20211460
收稿日期:2021-10-13
修回日期:2021-11-25
出版日期:2022-01-05
发布日期:2022-01-18
通讯作者:
高翔
作者简介:郑哲楠(1991—),女,博士,讲师,基金资助:
Zhenan ZHENG1,2( ),Xiang GAO1(
),Xiang GAO1( ),Yingwu LUO1,Jie HUANG2
),Yingwu LUO1,Jie HUANG2
Received:2021-10-13
Revised:2021-11-25
Online:2022-01-05
Published:2022-01-18
Contact:
Xiang GAO
摘要:
制约全固态聚合物电解质开发应用的瓶颈在于如何同时实现高离子电导率与高机械强度。采用可逆加成断裂链转移(RAFT)溶液聚合技术,以3-环己烯-1-亚甲基丙烯酸酯(CEA)为后交联单体,聚乙二醇甲醚丙烯酸酯(PEGMA)为导离子单体,制备了不同链结构的全固态聚合物电解质,再通过硫醇-烯烃之间的“点击化学”反应形成化学交联网络结构。所制备的三嵌段共聚物电解质具有独立的导离子中间嵌段,且交联单体位于分子链两端,从而能够同时满足离子电导率与机械强度的要求。该三嵌段共聚物电解质在60℃下的离子电导率为6.13×10-5 S/cm,并应用于磷酸亚铁锂/锂(LiFePO4/Li)全固态电池。所得电池在0.5 C下循环130圈后,放电比容量为139.1 mAh/g,容量保持率为97.8%,库仑效率高于99.0%,显示出良好的电化学性能。
中图分类号:
郑哲楠, 高翔, 罗英武, 黄杰. 紫外光交联法制备全固态聚合物电解质[J]. 化工学报, 2022, 73(1): 441-450.
Zhenan ZHENG, Xiang GAO, Yingwu LUO, Jie HUANG. Preparation of all-solid-state polymer electrolyte by ultraviolet cross-linking method[J]. CIESC Journal, 2022, 73(1): 441-450.
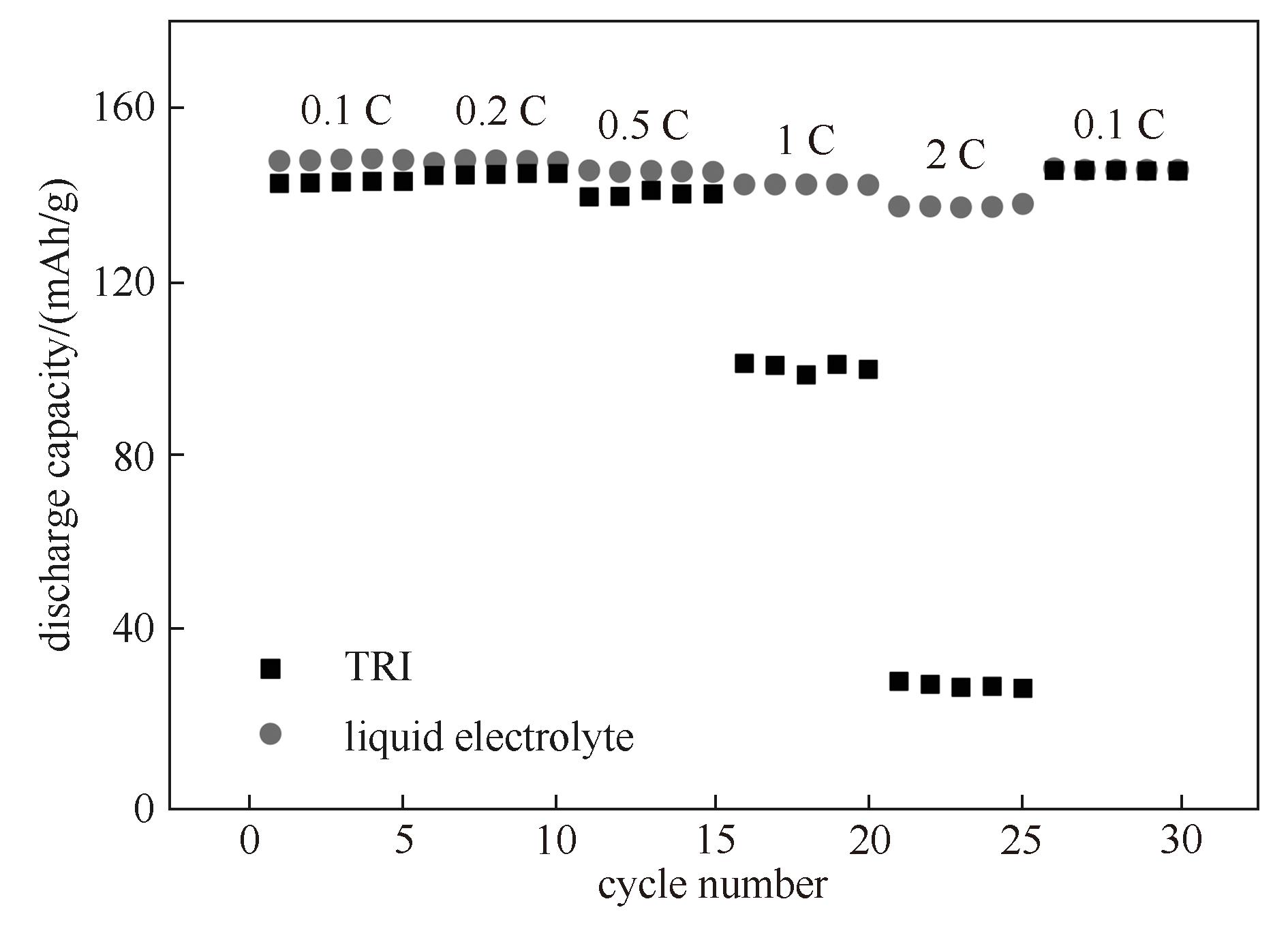
图8 采用三嵌段共聚物电解质与电解液的LiFePO4/Li电池的倍率性能(测试温度:三嵌段共聚物电解质为60℃,电解液为30℃)
Fig.8 Rate performance of LiFePO4/Li cell using TRI and commercial liquid electrolyte (The test temperature for TRI was 60℃, while that for liquid electrolyte was 30℃)
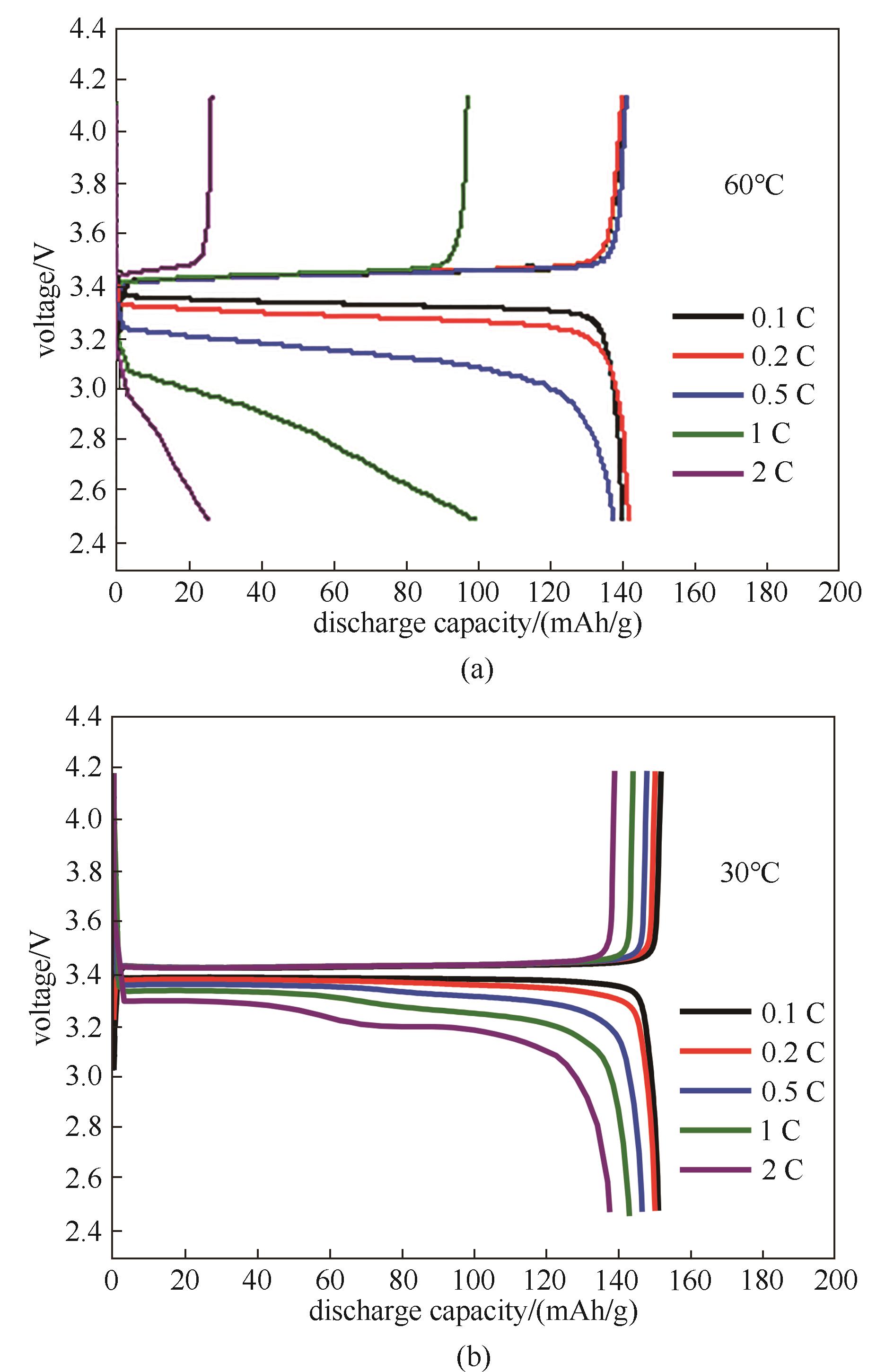
图9 采用三嵌段共聚物电解质(a)与电解液(b)的LiFePO4/Li电池在不同测试倍率下的充放电曲线
Fig.9 Charge/discharge profiles of LiFePO4/Li cells using TRI (a) and commercial liquid electrolyte (b) under different test rates
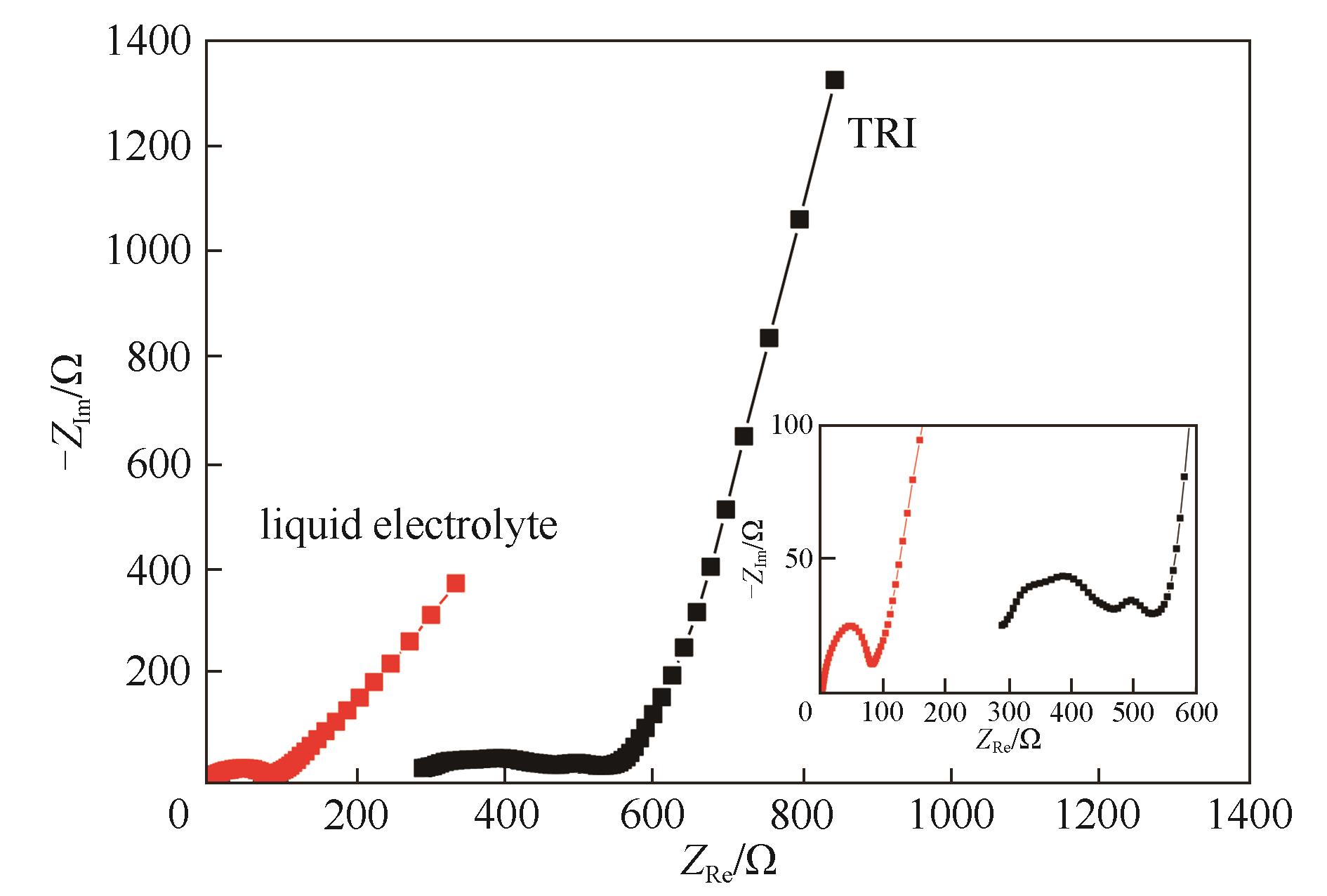
图10 采用三嵌段共聚物电解质与电解液的LiFePO4/Li电池的Nyquist图(测试温度:三嵌段共聚物电解质为60℃,电解液为30℃,插图为半圆区域的局部放大图)
Fig.10 Nyquist plots of LiFePO4/Li cells using TRI and commercial liquid electrolyte after rate performance tests and at discharge state (the test temperature for TRI was 60℃, while that for liquid electrolyte was 30℃; the inset image is a partial enlargement of the semicircular)
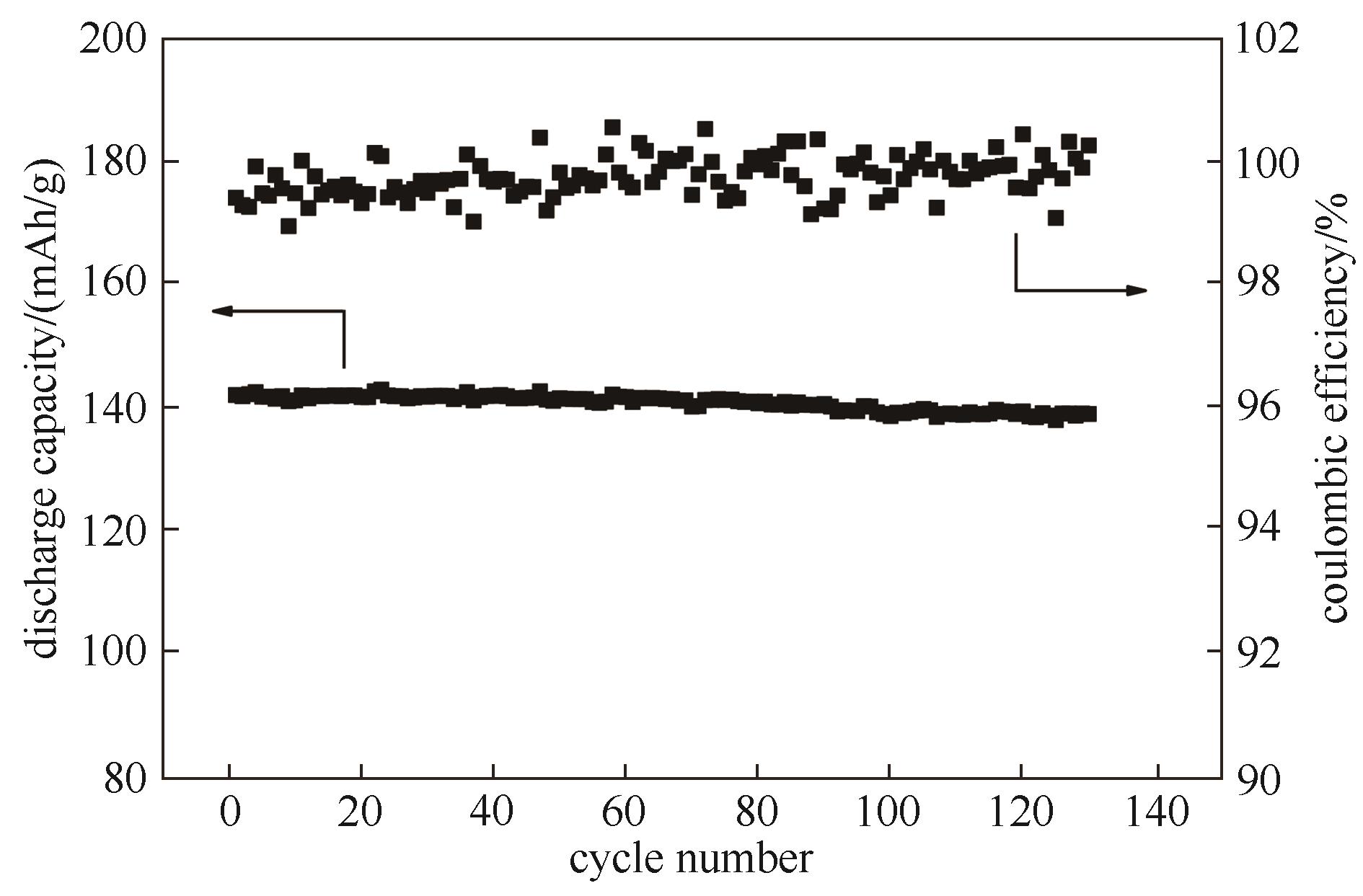
图11 采用三嵌段共聚物电解质的LiFePO4/Li电池的循环性能(测试温度为60℃,充/放电倍率均为0.5 C)
Fig.11 Cycling performance of LiFePO4/Li cell using TRI and its corresponding coulombic efficiencies (the test temperature was 60℃ and the test rate was 0.5 C for both charge and discharge procedures)
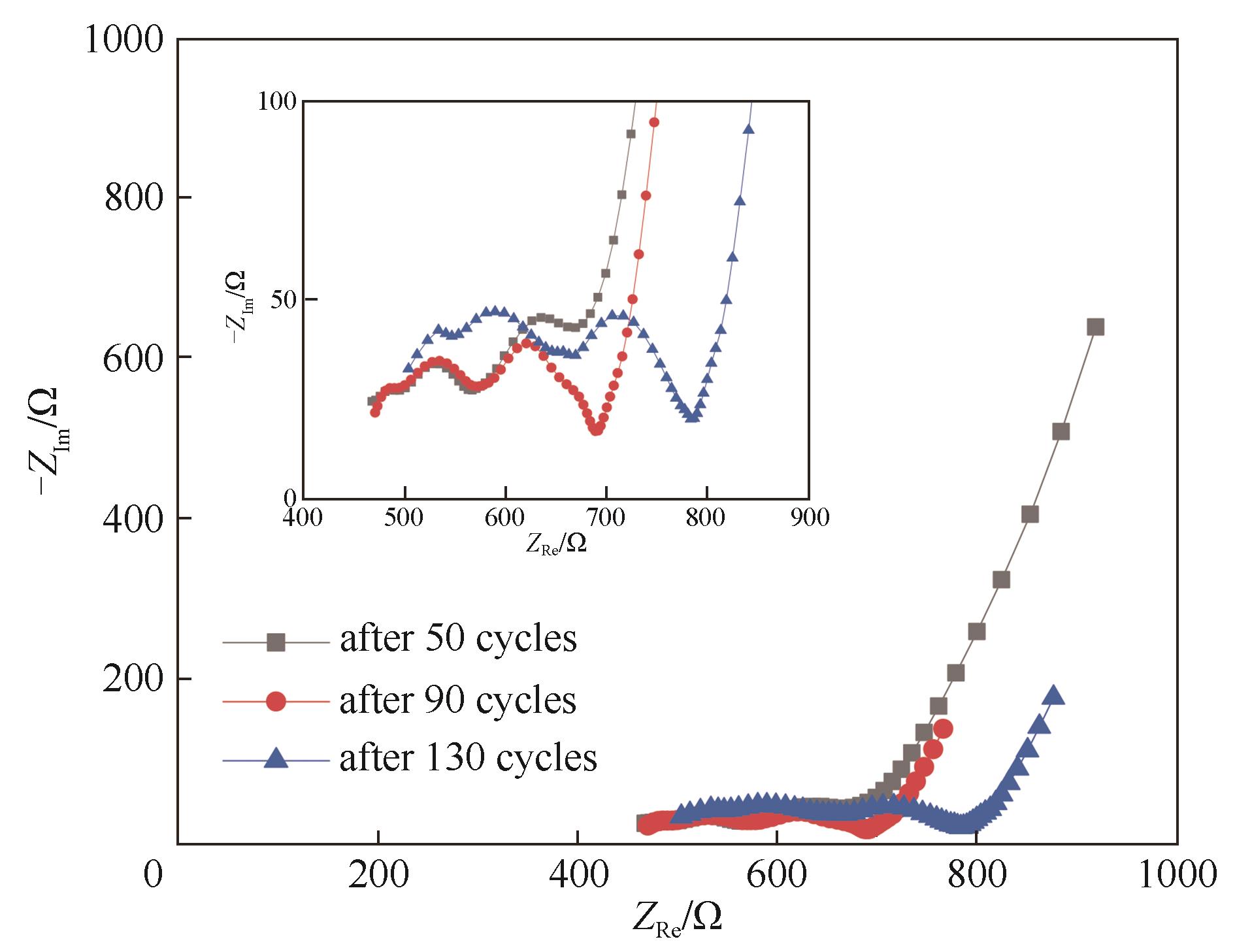
图12 采用三嵌段共聚物电解质的LiFePO4/Li电池经过不同循环次数后的Nyquist图(测试温度为60℃,内嵌图为半圆区域的局部放大图)
Fig.12 Nyquist plots of LiFePO4/Li cell using TRI after different amounts of cycles and at discharge state (the test temperature was 60℃, the inset image is a partial enlargement of the semicircular)
| 1 | Wang H C, Wang Q, Cao X, et al. Thiol-branched solid polymer electrolyte featuring high strength, toughness, and lithium ionic conductivity for lithium-metal batteries[J]. Advanced Materials, 2020, 32(37): 2001259. |
| 2 | Xu W, Wang J L, Ding F, et al. Lithium metal anodes for rechargeable batteries[J]. Energy Environ. Sci., 2014, 7(2): 513-537. |
| 3 | Lin D C, Liu Y Y, Cui Y. Reviving the lithium metal anode for high-energy batteries[J]. Nature Nanotechnology, 2017, 12(3): 194-206. |
| 4 | Cheng X B, Zhang R, Zhao C Z, et al. Toward safe lithium metal anode in rechargeable batteries: a review[J]. Chemical Reviews, 2017, 117(15): 10403-10473. |
| 5 | Young W S, Kuan W F, Epps I. Block copolymer electrolytes for rechargeable lithium batteries[J]. Journal of Polymer Science Part B: Polymer Physics, 2014, 52(1): 1-16. |
| 6 | Agrawal R C, Pandey G P. Solid polymer electrolytes: materials designing and all-solid-state battery applications: an overview[J]. Journal of Physics D: Applied Physics, 2008, 41(22): 223001. |
| 7 | Meyer W H. Polymer electrolytes for lithium-ion batteries[J]. Advanced Materials, 1998, 10(6): 439-448. |
| 8 | Young W S, Albert J N L, Schantz A B, et al. Mixed-salt effects on the ionic conductivity of lithium-doped PEO-containing block copolymers[J]. Macromolecules, 2011, 44(20): 8116-8123. |
| 9 | van Krevelen D W, te Nijenhuis K. Properties of Polymers[M]. Beijing: Science Press, 2010: 926-927. |
| 10 | 董甜甜, 张建军, 柴敬超, 等. 聚碳酸酯基固态聚合物电解质的研究进展[J]. 高分子学报, 2017(6): 906-921. |
| Dong T T, Zhang J J, Chai J C, et al. Research progress on polycarbonate-based solid-state polymer electrolytes[J]. Acta Polymerica Sinica, 2017(6): 906-921. | |
| 11 | 张建军, 杨金凤, 吴瀚, 等. 二次电池用原位生成聚合物电解质的研究进展[J]. 高分子学报, 2019, 50(9): 890-914. |
| Zhang J J, Yang J F, Wu H, et al. Research progress of in situ generated polymer electrolyte for rechargeable batteries[J]. Acta Polymerica Sinica, 2019, 50(9): 890-914. | |
| 12 | Takahashi Y, Tadokoro H. Structural studies of polyethers, (-(CH2)m-O-)n. Ⅹ. Crystal structure of poly(ethylene oxide)[J]. Macromolecules, 1973, 6(5): 672-675. |
| 13 | Long L Z, Wang S J, Xiao M, et al. Polymer electrolytes for lithium polymer batteries[J]. Journal of Materials Chemistry A, 2016, 4(26): 10038-10069. |
| 14 | Xue Z G, He D, Xie X L. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2015, 3(38): 19218-19253. |
| 15 | Zhang J X, Zhao N, Zhang M, et al. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: dispersion of garnet nanoparticles in insulating polyethylene oxide[J]. Nano Energy, 2016, 28: 447-454. |
| 16 | Chen L, Li Y T, Li S P, et al. PEO/garnet composite electrolytes for solid-state lithium batteries: from “ceramic-in-polymer” to “polymer-in-ceramic”[J]. Nano Energy, 2018, 46: 176-184. |
| 17 | Abetz V, Simon P F W. Phase Behaviour and Morphologies of Block Copolymers[M]//Abetz V. Block Copolymers I. Berlin: Springer, 2005: 125-212. |
| 18 | Bouchet R, Phan T N T, Beaudoin E, et al. Charge transport in nanostructured PS-PEO-PS triblock copolymer electrolytes[J]. Macromolecules, 2014, 47(8): 2659-2665. |
| 19 | Wang C X, Sakai T, Watanabe O, et al. All solid-state lithium-polymer battery using a self-cross-linking polymer electrolyte[J]. Journal of the Electrochemical Society, 2003, 150(9): A1166. |
| 20 | Panday A, Mullin S, Gomez E D, et al. Effect of molecular weight and salt concentration on conductivity of block copolymer electrolytes[J]. Macromolecules, 2009, 42(13): 4632-4637. |
| 21 | Wanakule N S, Panday A, Mullin S A, et al. Ionic conductivity of block copolymer electrolytes in the vicinity of order-disorder and order-order transitions[J]. Macromolecules, 2009, 42(15): 5642-5651. |
| 22 | Schulze M W, McIntosh L D, Hillmyer M A, et al. High-modulus, high-conductivity nanostructured polymer electrolyte membranes via polymerization-induced phase separation[J]. Nano Letters, 2014, 14(1): 122-126. |
| 23 | Hamersky M W, Hillmyer M A, Tirrell M, et al. Block copolymer self-diffusion in the gyroid and cylinder morphologies[J]. Macromolecules, 1998, 31(16): 5363-5370. |
| 24 | Young W S, Epps T H. Ionic conductivities of block copolymer electrolytes with various conducting pathways: sample preparation and processing considerations[J]. Macromolecules, 2012, 45(11): 4689-4697. |
| 25 | Niitani T, Shimada M, Kawamura K, et al. Characteristics of new-type solid polymer electrolyte controlling nano-structure[J]. Journal of Power Sources, 2005, 146(1/2): 386-390. |
| 26 | Ferguson C J, Hughes R J, Nguyen D, et al. Ab initio emulsion polymerization by RAFT-controlled self-assembly[J]. Macromolecules, 2005, 38(6): 2191-2204. |
| 27 | Yang L, Xu J Q, Sun P, et al. Ab initio emulsion and miniemulsion polymerization of styrene mediated by a cyclohexenyl-functionalized amphiphilic RAFT agent[J]. Industrial & Engineering Chemistry Research, 2014, 53(28): 11259-11268. |
| 28 | Ma J, Cheng C, Wooley K L. Cycloalkenyl-functionalized polymers and block copolymers: syntheses via selective RAFT polymerizations and demonstration of their versatile reactivity[J]. Macromolecules, 2009, 42(5): 1565-1573. |
| 29 | Dondoni A. The emergence of thiol-ene coupling as a click process for materials and bioorganic chemistry[J]. Angewandte Chemie International Edition, 2008, 47(47): 8995-8997. |
| 30 | Lowe A B. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis[J]. Polym. Chem., 2010, 1(1): 17-36. |
| 31 | Yang L, Xu J Q, Han J L, et al. A novel method for preparing click-ready latex and latex with stability against high electrolyte concentrations[J]. Industrial & Engineering Chemistry Reaction, 2015, 54(20): 5536-5542. |
| 32 | Xie P L, Yang X X, Li T F, et al. Highly stretchable, transparent, and colorless electrodes from a diblock copolymer electrolyte[J]. Journal of Materials Chemistry C, 2017, 38(5): 9865-9872. |
| 33 | Timachova K, Watanabe H, Balsara N P. Effect of molecular weight and salt concentration on ion transport and the transference number in polymer electrolytes[J]. Macromolecules, 2015, 48(21): 7882-7888. |
| 34 | Petrucci R H, Herring F G, Bissonnette C, et al. General Chemistry: Principles and Modern Applications[M]. 11th ed. Toronto: Pearson Canada Inc., 2017. |
| 35 | Dehmelt H. A single atomic particle forever floating at rest in free space: new value for electron radius[J]. Physica Scripta, 1988, T22: 102. |
| [1] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [2] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [3] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [4] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [5] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [6] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [7] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [8] | 刘杰, 吴立盛, 李锦锦, 罗正鸿, 周寅宁. 含乙烯基胺酯键聚醚类可逆交联聚合物的制备及性能研究[J]. 化工学报, 2023, 74(7): 3051-3057. |
| [9] | 张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| [10] | 龙臻, 王谨航, 任俊杰, 何勇, 周雪冰, 梁德青. 离子液体协同PVCap抑制天然气水合物生成实验研究[J]. 化工学报, 2023, 74(6): 2639-2646. |
| [11] | 杨琴, 秦传鉴, 李明梓, 杨文晶, 赵卫杰, 刘虎. 用于柔性传感的双形状记忆MXene基水凝胶的制备及性能研究[J]. 化工学报, 2023, 74(6): 2699-2707. |
| [12] | 李瑞康, 何盈盈, 卢维鹏, 王园园, 丁皓东, 骆勇名. 电化学强化钴基阴极活化过一硫酸盐的研究[J]. 化工学报, 2023, 74(5): 2207-2216. |
| [13] | 王承泽, 顾凯丽, 张晋华, 石建轩, 刘艺娓, 李锦祥. 硫化协同老化零价铁增效去除水中Cr(Ⅵ)的作用机制[J]. 化工学报, 2023, 74(5): 2197-2206. |
| [14] | 张建华, 陈萌萌, 孙雅雯, 彭永臻. 部分短程硝化同步除磷耦合Anammox实现生活污水高效脱氮除磷[J]. 化工学报, 2023, 74(5): 2147-2156. |
| [15] | 郭旭, 张永政, 夏厚兵, 杨娜, 朱真珍, 齐晶瑶. 碳基材料电氧化去除水体污染物的研究进展[J]. 化工学报, 2023, 74(5): 1862-1874. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号