化工学报 ›› 2023, Vol. 74 ›› Issue (6): 2427-2435.DOI: 10.11949/0438-1157.20230258
张希庆1( ), 王琰婷1(
), 王琰婷1( ), 徐彦红2, 常淑玲1, 孙婷婷1, 薛定3, 张立红1(
), 徐彦红2, 常淑玲1, 孙婷婷1, 薛定3, 张立红1( )
)
收稿日期:2023-03-20
修回日期:2023-05-28
出版日期:2023-06-05
发布日期:2023-07-27
通讯作者:
张立红
作者简介:张希庆(1997—),男,硕士研究生,zhangxiqing1997@163.com基金资助:
Xiqing ZHANG1( ), Yanting WANG1(
), Yanting WANG1( ), Yanhong XU2, Shuling CHANG1, Tingting SUN1, Ding XUE3, Lihong ZHANG1(
), Yanhong XU2, Shuling CHANG1, Tingting SUN1, Ding XUE3, Lihong ZHANG1( )
)
Received:2023-03-20
Revised:2023-05-28
Online:2023-06-05
Published:2023-07-27
Contact:
Lihong ZHANG
摘要:
采用一步法在Al2O3表面原位生长一系列Mg含量不同的超薄水滑石纳米片,并采用分步浸渍复原法逐步引入助剂In和活性组分Pt,进一步通过焙烧和还原处理制备了负载Pt-In双金属催化剂PtIn/HTR-x(x = 0.05,0.1,0.15,0.2 mol·L-1)。探究了催化剂及其前体的结构、物化性能与异丁烷直接脱氢性能之间的关系。结果表明,制备母液中Mg2+浓度会影响纳米片厚度,进而影响催化剂结构、还原能力、表面化学状态、表面酸性和脱氢性能。当Mg2+浓度为0.15 mol·L-1时,催化剂获得最佳脱氢性能,其中异丁烯产率高达58%。催化剂PtIn/HTR-0.15优异的活性、选择性、稳定性及良好的抗积炭性能与催化剂高的比表面积、低的强酸量和酸强度、强的金属载体相互作用以及高的表面In3+/In0原子比有关。
中图分类号:
张希庆, 王琰婷, 徐彦红, 常淑玲, 孙婷婷, 薛定, 张立红. Mg量影响的纳米片负载Pt-In催化异丁烷脱氢性能[J]. 化工学报, 2023, 74(6): 2427-2435.
Xiqing ZHANG, Yanting WANG, Yanhong XU, Shuling CHANG, Tingting SUN, Ding XUE, Lihong ZHANG. Effect of Mg content on isobutane dehydrogenation properties over nanosheets supported Pt-In catalysts[J]. CIESC Journal, 2023, 74(6): 2427-2435.
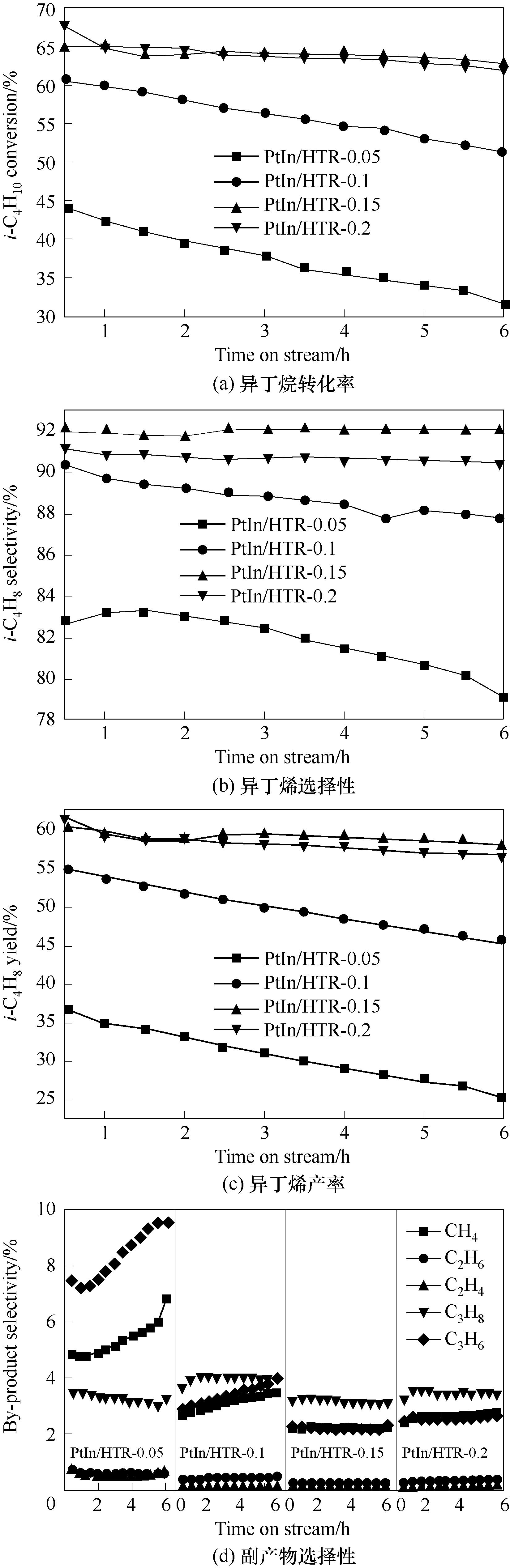
图1 不同Mg2+浓度制备的PtIn/HTR-x(x = 0.05, 0.1, 0.15, 0.2 mol·L-1)催化剂异丁烷直接脱氢性能
Fig.1 Isobutane direct dehydrogenation performance of PtIn/HTR-x (x = 0.05, 0.1, 0.15, 0.2 mol·L-1) with different Mg2+ concentration
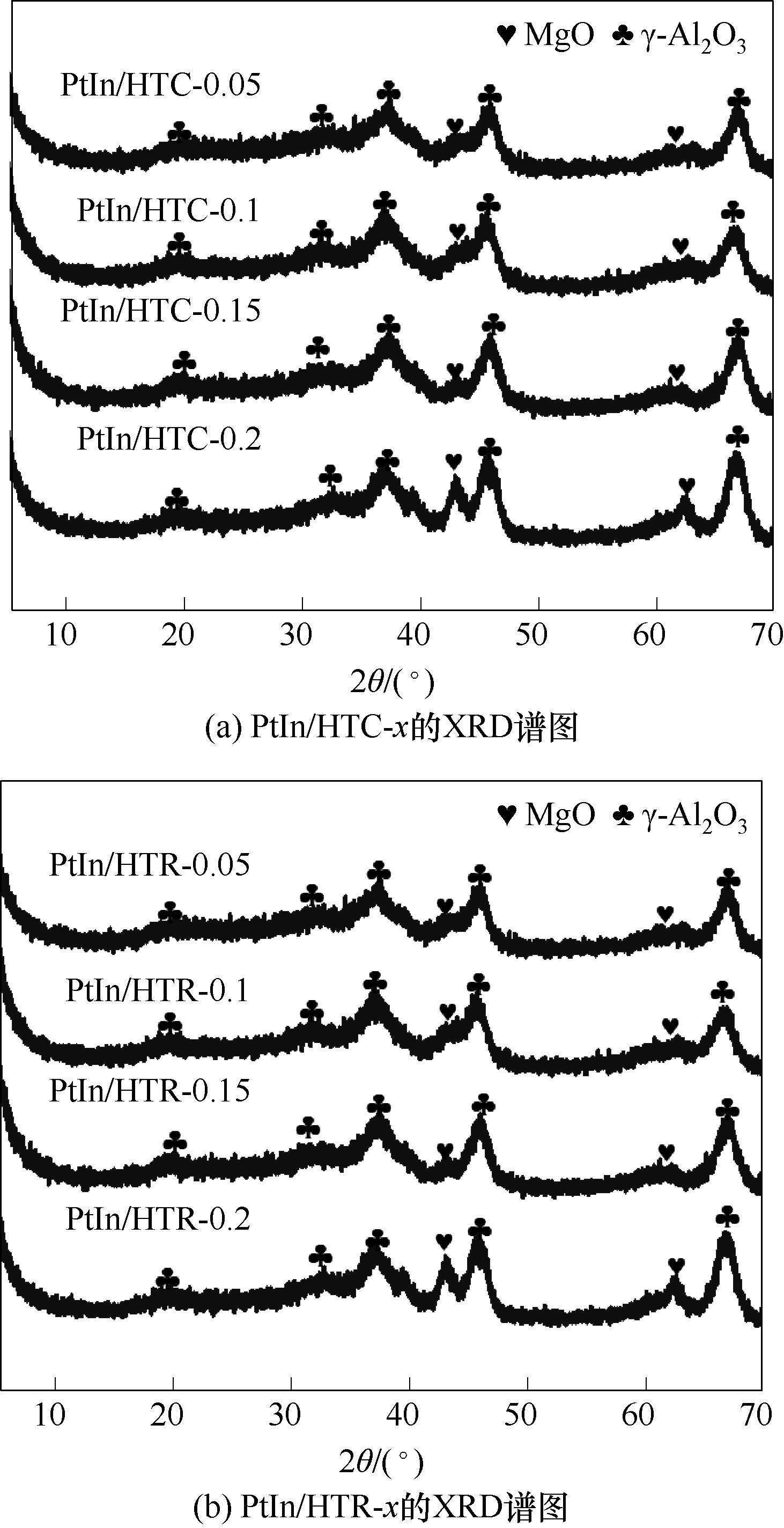
图3 焙烧催化剂PtIn/HTC-x和还原催化剂PtIn/HTR-x(x = 0.05, 0.1, 0.15, 0.2 mol·L-1)的XRD谱图
Fig.3 XRD patterns of calcined catalysts PtIn/HTC-x and reduced catalysts PtIn/HTR-x (x = 0.05, 0.1, 0.15, 0.2 mol·L-1)
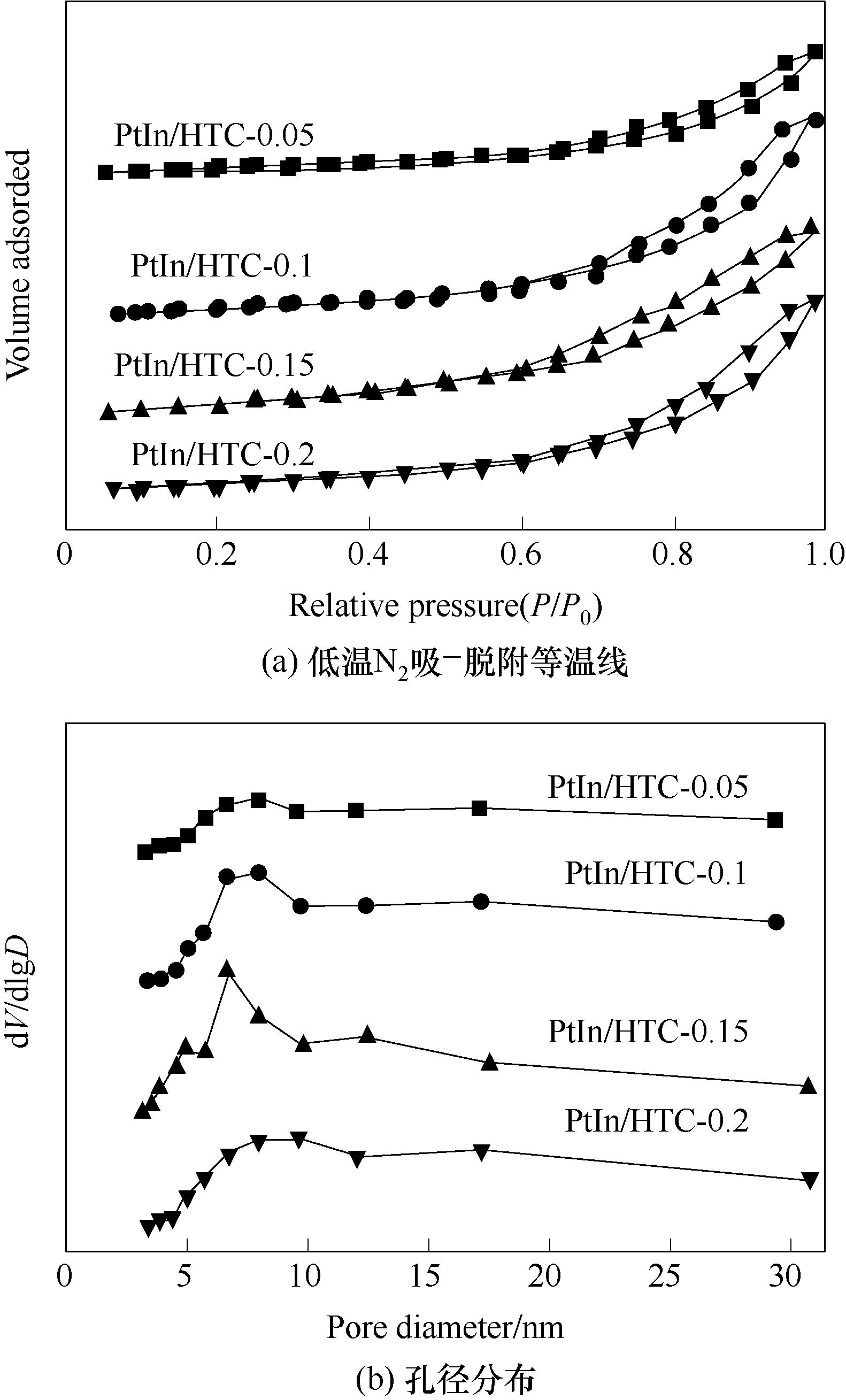
图4 焙烧催化剂PtIn/HTC-x(x = 0.05, 0.1, 0.15, 0.2 mol·L-1)的低温N2吸-脱附等温线和孔径分布曲线
Fig.4 Low temperature N2 adsorption-desorption isotherms and pore size distribution curves of calcined catalysts PtIn/HTC-x (x = 0.05, 0.1, 0.15, 0.2 mol·L-1)
| 催化剂 | 比表面积/(m2·g-1) | 孔体积/(cm3·g-1) | 平均孔径/nm |
|---|---|---|---|
| PtIn/HTC-0.05 | 136 | 0.34 | 6.6 |
| PtIn/HTC-0.1 | 173 | 0.58 | 6.6 |
| PtIn/HTC-0.15 | 247 | 0.57 | 6.6 |
| PtIn/HTC-0.2 | 203 | 0.56 | 7.8 |
表1 焙烧催化剂PtIn/HTC-x(x = 0.05, 0.1, 0.15, 0.2 mol·L-1)的织构数据
Table 1 Textural data of calcined catalysts PtIn/HTC-x (x = 0.05, 0.1, 0.15, 0.2 mol·L-1)
| 催化剂 | 比表面积/(m2·g-1) | 孔体积/(cm3·g-1) | 平均孔径/nm |
|---|---|---|---|
| PtIn/HTC-0.05 | 136 | 0.34 | 6.6 |
| PtIn/HTC-0.1 | 173 | 0.58 | 6.6 |
| PtIn/HTC-0.15 | 247 | 0.57 | 6.6 |
| PtIn/HTC-0.2 | 203 | 0.56 | 7.8 |
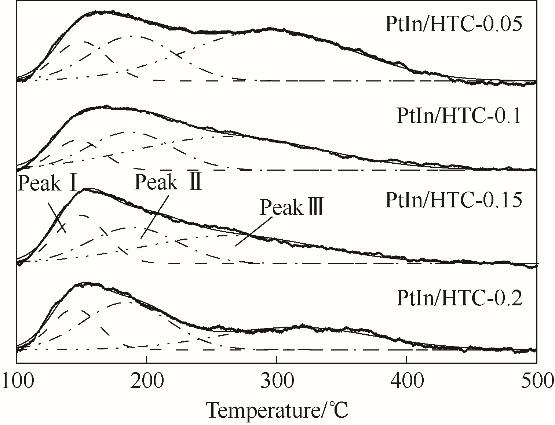
图5 焙烧催化剂PtIn/HTC-x(x = 0.05, 0.1, 0.15, 0.2 mol·L-1)的NH3-TPD谱图
Fig.5 NH3-TPD diagrams of calcined catalysts PtIn/HTC-x (x = 0.05, 0.1, 0.15, 0.2 mol·L-1)
| 催化剂 | TM /℃ | 总峰面积 | 峰面积占比/% | ||||
|---|---|---|---|---|---|---|---|
| Ⅰ | Ⅱ | Ⅲ | Ⅰ | Ⅱ | Ⅲ | ||
| PtIn/HTC-0.05 | 150 | 189 | 295 | 121 | 14.5 | 26.8 | 58.7 |
| PtIn/HTC-0.1 | 147 | 187 | 263 | 111 | 13.3 | 29.7 | 57.0 |
| PtIn/HTC-0.15 | 150 | 190 | 265 | 88 | 23.3 | 30.2 | 46.5 |
| PtIn/HTC-0.2 | 144 | 184 | 315 | 64 | 20.4 | 32.3 | 47.3 |
表2 焙烧催化剂PtIn/HTC-x(x = 0.05, 0.1, 0.15, 0.2 mol·L-1)的NH3-TPD结果
Table 2 NH3-TPD results of calcined catalysts PtIn/HTC-x (x = 0.05, 0.1, 0.15, 0.2 mol·L-1)
| 催化剂 | TM /℃ | 总峰面积 | 峰面积占比/% | ||||
|---|---|---|---|---|---|---|---|
| Ⅰ | Ⅱ | Ⅲ | Ⅰ | Ⅱ | Ⅲ | ||
| PtIn/HTC-0.05 | 150 | 189 | 295 | 121 | 14.5 | 26.8 | 58.7 |
| PtIn/HTC-0.1 | 147 | 187 | 263 | 111 | 13.3 | 29.7 | 57.0 |
| PtIn/HTC-0.15 | 150 | 190 | 265 | 88 | 23.3 | 30.2 | 46.5 |
| PtIn/HTC-0.2 | 144 | 184 | 315 | 64 | 20.4 | 32.3 | 47.3 |

图6 焙烧催化剂PtIn/HTC-x(x = 0.05, 0.1, 0.15, 0.2 mol·L-1)的H2-TPR曲线
Fig. 6 H2-TPR curves of calcined catalysts PtIn/HTC-x (x = 0.05, 0.1, 0.15, 0.2 mol·L-1)
| 催化剂 | TM /℃ | 总峰面积 At | 各峰面积 | 峰面积比 | |||
|---|---|---|---|---|---|---|---|
| Ⅰ | Ⅱ | AⅠ | AⅡ | AII/AI | AII/At | ||
| PtIn/HTC-0.05 | 345 | 520 | 502 | 417 | 85 | 0.20 | 0.17 |
| PtIn/HTC-0.1 | 398 | 529 | 761 | 637 | 124 | 0.19 | 0.16 |
| PtIn/HTC-0.15 | 407 | 536 | 613 | 534 | 79 | 0.15 | 0.13 |
| PtIn/HTC-0.2 | 446 | 570 | 419 | 329 | 90 | 0.27 | 0.21 |
表3 焙烧催化剂PtIn/HTC-x(x = 0.05, 0.1, 0.15, 0.2 mol·L-1)的H2-TPR结果
Table 3 H2-TPR results of calcined catalysts PtIn/HTC-x (x = 0.05, 0.1, 0.15, 0.2 mol·L-1)
| 催化剂 | TM /℃ | 总峰面积 At | 各峰面积 | 峰面积比 | |||
|---|---|---|---|---|---|---|---|
| Ⅰ | Ⅱ | AⅠ | AⅡ | AII/AI | AII/At | ||
| PtIn/HTC-0.05 | 345 | 520 | 502 | 417 | 85 | 0.20 | 0.17 |
| PtIn/HTC-0.1 | 398 | 529 | 761 | 637 | 124 | 0.19 | 0.16 |
| PtIn/HTC-0.15 | 407 | 536 | 613 | 534 | 79 | 0.15 | 0.13 |
| PtIn/HTC-0.2 | 446 | 570 | 419 | 329 | 90 | 0.27 | 0.21 |
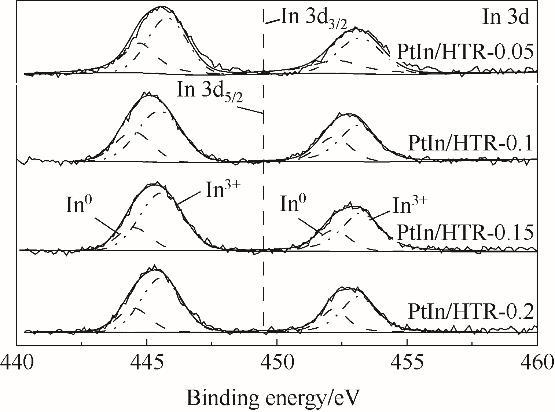
图7 还原催化剂PtIn/HTR-x(x = 0.05, 0.1, 0.15, 0.2 mol·L-1)的In 3d的XPS谱图
Fig.7 XPS spectra of In 3d region of reduced catalysts PtIn/HTR-x (x = 0.05, 0.1, 0.15, 0.2 mol·L-1)
| 催化剂 | In 3d5/2 结合能 /eV | In 3d3/2 结合能 /eV | In3+/In0① | ||
|---|---|---|---|---|---|
| In0 | In3+ | In0 | In3+ | ||
| PtIn/HTR-0.05 | 444.5 | 445.8 | 452.2 | 453.2 | 1.57 |
| PtIn/HTR-0.1 | 444.5 | 445.6 | 452.2 | 453.1 | 2.00 |
| PtIn/HTR-0.15 | 444.7 | 445.6 | 452.3 | 453.3 | 2.87 |
| PtIn/HTR-0.2 | 444.5 | 445.5 | 452.2 | 453.1 | 2.27 |
表4 还原催化剂PtIn/HTR-x(x = 0.05,0.1,0.15,0.2 mol·L-1)的In 3d数据
Table 4 In 3d region data of reduced catalysts PtIn/HTR-x (x = 0.05,0.1,0.15,0.2 mol·L-1)
| 催化剂 | In 3d5/2 结合能 /eV | In 3d3/2 结合能 /eV | In3+/In0① | ||
|---|---|---|---|---|---|
| In0 | In3+ | In0 | In3+ | ||
| PtIn/HTR-0.05 | 444.5 | 445.8 | 452.2 | 453.2 | 1.57 |
| PtIn/HTR-0.1 | 444.5 | 445.6 | 452.2 | 453.1 | 2.00 |
| PtIn/HTR-0.15 | 444.7 | 445.6 | 452.3 | 453.3 | 2.87 |
| PtIn/HTR-0.2 | 444.5 | 445.5 | 452.2 | 453.1 | 2.27 |
| 1 | Venugopalan A T, Kandasamy P, Gupta N N, et al. Promoted mesoporous Fe-alumina catalysts for the non-oxidative dehydrogenation of isobutane[J]. Catalysis Communications, 2021, 150: 106263. |
| 2 | Im J, Choi M. Physicochemical stabilization of Pt against sintering for a dehydrogenation catalyst with high activity, selectivity, and durability[J]. ACS Catalysis, 2016, 6(5): 2819-2826. |
| 3 | 郭丹, 方雨洁, 许一寒, 等. 乙烷和二氧化碳催化转化的研究进展[J]. 化工学报, 2022, 73(8): 3406-3416. |
| Guo D, Fang Y J, Xu Y H, et al. Research progress of the catalytic conversion of ethane and carbon dioxide[J]. CIESC Journal, 2022, 73(8): 3406-3416. | |
| 4 | Gao X Q, Song W, Li W C, et al. Anti-coke behavior of an alumina nanosheet supported Pt-Sn catalyst for isobutane dehydrogenation[J]. Catalysis Science & Technology, 2021, 11(7): 2597-2603. |
| 5 | Zhu Y R, An Z, Song H Y, et al. Lattice-confined Sn (Ⅳ/Ⅱ) stabilizing raft-like Pt clusters: high selectivity and durability in propane dehydrogenation[J]. ACS Catalysis, 2017, 7(10): 6973-6978. |
| 6 | Liu J F, Zhou W, Jiang D Y, et al. Insights into the doping effect of rare-earth metal on ZnAl2O4 supported PtSn catalyzed isobutane dehydrogenation[J]. Catalysis Today, 2021, 368: 58-65. |
| 7 | Xia K, Lang W Z, Li P P, et al. The properties and catalytic performance of PtIn/Mg(Al)O catalysts for the propane dehydrogenation reaction: effects of pH value in preparing Mg(Al)O supports by the co-precipitation method[J]. Journal of Catalysis, 2016, 338: 104-114. |
| 8 | Xia K, Lang W Z, Li P P, et al. The influences of Mg/Al molar ratio on the properties of PtIn/Mg(Al)O-x catalysts for propane dehydrogenation reaction[J]. Chemical Engineering Journal, 2016, 284: 1068-1079. |
| 9 | Tolek W, Suriye K, Praserthdam P, et al. Effect of preparation method on the Pt-In modified Mg(Al)O catalysts over dehydrogenation of propane[J]. Catalysis Today, 2020, 358: 100-108. |
| 10 | Bauer T, Maisel S, Blaumeiser D, et al. Operando DRIFTS and DFT study of propane dehydrogenation over solid- and liquid-supported Ga x Pt y catalysts[J]. ACS Catalysis, 2019, 9(4): 2842-2853. |
| 11 | Rochlitz L, Searles K, Alfke J, et al. Silica-supported, narrowly distributed, subnanometric Pt-Zn particles from single sites with high propane dehydrogenation performance[J]. Chemical Science, 2020, 11(6): 1549-1555. |
| 12 | Xu M, Wei M. Layered double hydroxide-based catalysts: recent advances in preparation, structure, and applications[J]. Advanced Functional Materials, 2018, 28(47): 1802943. |
| 13 | Zhang M, Song Z, Guo M Q, et al. Effect of reduction atmosphere on structure and catalytic performance of PtIn/Mg(Al)O/ZnO for propane dehydrogenation[J]. Catalysts, 2020, 10(5): 485. |
| 14 | Li J X, Zhang M, Song Z, et al. Hierarchical PtIn/Mg(Al)O derived from reconstructed PtIn-hydrotalcite-like compounds for highly efficient propane dehydrogenation[J]. Catalysts, 2019, 9(9): 767. |
| 15 | Li Z, Sun Y, Liu X E, et al. Bottom-up fabrication of ultrathin CoFe layered double hydroxide nanosheets on oxidized carbon nanotube as a water oxidation electrocatalyst[J]. Journal of Alloys and Compounds, 2021, 857: 157570. |
| 16 | Wang Z L, Xu S M, Xu Y Q, et al. Single Ru atoms with precise coordination on a monolayer layered double hydroxide for efficient electrooxidation catalysis[J]. Chemical Science, 2019, 10(2): 378-384. |
| 17 | Chi H Y, Dong J W, Li T, et al. Scaled-up synthesis of defect-rich layered double hydroxide monolayers without organic species for efficient oxygen evolution reaction[J]. Green Energy & Environment, 2022, 7(5): 975-982. |
| 18 | Guo T T, Chen L Y, Li Y W, et al. Controllable synthesis of ultrathin defect-rich LDH nanoarrays coupled with MOF-derived Co-NC microarrays for efficient overall water splitting[J]. Small, 2022, 18(29): 2107739. |
| 19 | Zhao Y F, Wang Q, Bian T, et al. Ni3+ doped monolayer layered double hydroxide nanosheets as efficient electrodes for supercapacitors[J]. Nanoscale, 2015, 7(16): 7168-7173. |
| 20 | Yu J F, Martin B R, Clearfield A, et al. One-step direct synthesis of layered double hydroxide single-layer nanosheets[J]. Nanoscale, 2015, 7(21): 9448-9451. |
| 21 | Zhao Y F, Zhao Y X, Waterhouse G I N, et al. Layered-double-hydroxide nanosheets as efficient visible-light-driven photocatalysts for dinitrogen fixation[J]. Advanced Materials, 2017, 29(42): 1703828. |
| 22 | Wang Y, Zhao F L, Feng Y Y, et al. Ultrathin layered double hydroxide nanosheets prepared by original precursor method for photoelectrochemical photodetectors[J]. Nano Research, 2022, 15(10): 9392-9401. |
| 23 | Huo J M, Ma Z L, Wang Y, et al. Monodispersed Pt sites supported on NiFe-LDH from synchronous anchoring and reduction for high efficiency overall water splitting[J]. Small, 2023, 19(11): 2207044. |
| 24 | Yu J F, Liu J J, Clearfield A, et al. Synthesis of layered double hydroxide single-layer nanosheets in formamide[J]. Inorganic Chemistry, 2016, 55(22): 12036-12041. |
| 25 | Yue Y Z, Liu F, Zhao L, et al. Loading oxide nano sheet supported Ni-Co alloy nanoparticles on the macroporous walls of monolithic alumina and their catalytic performance for ethanol steam reforming[J]. International Journal of Hydrogen Energy, 2015, 40(22): 7052-7063. |
| 26 | Chen Z K, Huang M H, Cölfen H. Synthesis of ultrathin metal oxide and hydroxide nanosheets using formamide in water at room temperature[J]. CrystEngComm, 2021, 23(21): 3794-3801. |
| 27 | Wang Q, Chen L F, Guan S L, et al. Ultrathin and vacancy-rich CoAl-layered double hydroxide/graphite oxide catalysts: promotional effect of cobalt vacancies and oxygen vacancies in alcohol oxidation[J]. ACS Catalysis, 2018, 8(4): 3104-3115. |
| 28 | Gao W, Zhao Y F, Chen H R, et al. Core shell Cu@(CuCo-alloy)/Al2O3 catalysts for the synthesis of higher alcohols from syngas[J]. Green Chemistry, 2015, 17(3): 1525-1534. |
| 29 | Shi J J, Zhou Y M, Zhang Y W, et al. Synthesis of magnesium-modified mesoporous Al2O3 with enhanced catalytic performance for propane dehydrogenation[J]. Journal of Materials Science, 2014, 49(16): 5772-5781. |
| 30 | Jiang H X, Yao C X, Wang Y D, et al. Synthesis and catalytic performance of highly dispersed platinum nanoparticles supported on alumina via supercritical fluid deposition[J]. The Journal of Supercritical Fluids, 2020, 166: 105014. |
| [1] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [2] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [5] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [6] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [7] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [8] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [9] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [10] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [11] | 张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| [12] | 王辰, 史秀锋, 武鲜凤, 魏方佳, 张昊虹, 车寅, 吴旭. 氧化还原法制备Mn3O4催化剂及其甲苯催化氧化性能与机理研究[J]. 化工学报, 2023, 74(6): 2447-2457. |
| [13] | 李勇, 高佳琦, 杜超, 赵亚丽, 李伯琼, 申倩倩, 贾虎生, 薛晋波. Ni@C@TiO2核壳双重异质结的构筑及光热催化分解水产氢[J]. 化工学报, 2023, 74(6): 2458-2467. |
| [14] | 周继鹏, 何文军, 李涛. 异形催化剂上乙烯催化氧化失活动力学反应工程计算[J]. 化工学报, 2023, 74(6): 2416-2426. |
| [15] | 郭旭, 张永政, 夏厚兵, 杨娜, 朱真珍, 齐晶瑶. 碳基材料电氧化去除水体污染物的研究进展[J]. 化工学报, 2023, 74(5): 1862-1874. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号