化工学报 ›› 2024, Vol. 75 ›› Issue (1): 83-94.DOI: 10.11949/0438-1157.20230668
闫可欣1( ), 姜洪涛2, 高维群1, 郭晓晖2, 孙伟振1(
), 姜洪涛2, 高维群1, 郭晓晖2, 孙伟振1( ), 赵玲1
), 赵玲1
收稿日期:2023-07-03
修回日期:2023-09-04
出版日期:2024-01-25
发布日期:2024-03-11
通讯作者:
孙伟振
作者简介:闫可欣(2001—),女,硕士研究生,yankxin123@163.com
基金资助:
Kexin YAN1( ), Hongtao JIANG2, Weiqun GAO1, Xiaohui GUO2, Weizhen SUN1(
), Hongtao JIANG2, Weiqun GAO1, Xiaohui GUO2, Weizhen SUN1( ), Ling ZHAO1
), Ling ZHAO1
Received:2023-07-03
Revised:2023-09-04
Online:2024-01-25
Published:2024-03-11
Contact:
Weizhen SUN
摘要:
三氯氢硅和氢气中痕量硼磷杂质的含量是影响多晶硅品质的主要因素。提高硼磷杂质的脱除效率有利于电子级多晶硅的大规模生产,实现我国能源信息产业升级。综述了三氯氢硅和氢气中硼磷杂质脱除方法的研究进展,重点介绍了各类提纯方法的特点。其中,反应-吸附-精馏耦合技术集合了精馏法和化学提纯法的优点,是三氯氢硅精制过程中最具有发展前景的方法;吸附法凭借其负载的活性物质对硼磷杂质的高选择性,成为氢气精制最常使用的工艺。最后,系统探讨了吸附剂结构特点及与吸附性能的构效关系,在此基础上总结并展望了多晶硅原料中硼磷杂质脱除面临的挑战和发展方向。
中图分类号:
闫可欣, 姜洪涛, 高维群, 郭晓晖, 孙伟振, 赵玲. 电子级多晶硅原料中痕量硼磷杂质的脱除研究进展[J]. 化工学报, 2024, 75(1): 83-94.
Kexin YAN, Hongtao JIANG, Weiqun GAO, Xiaohui GUO, Weizhen SUN, Ling ZHAO. Recent advances in the removal of trace boron and phosphorus impurities from electronic grade silicon raw materials[J]. CIESC Journal, 2024, 75(1): 83-94.
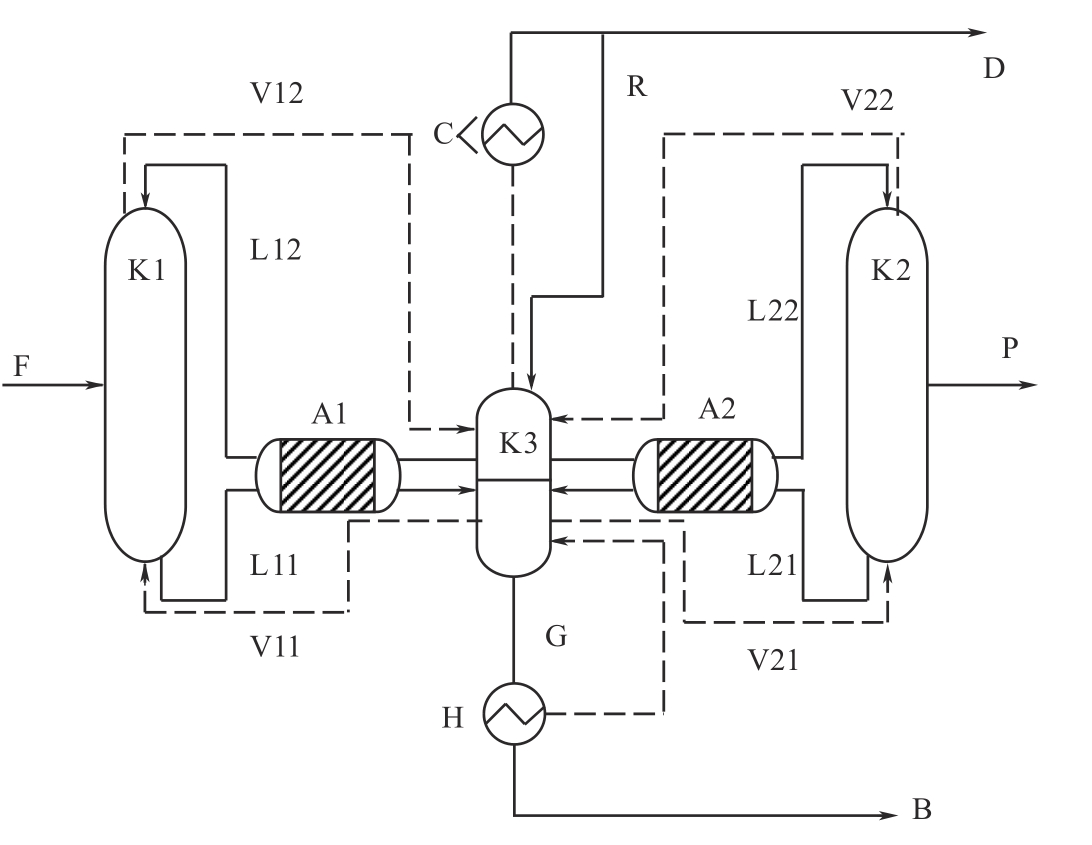
图3 使用水平隔板塔的精制流程[14]A1—吸附器1; A2—吸附器2; B—高沸点杂质; C—冷凝器; D—低沸点杂质; F—原料液; G—循环蒸汽; H—蒸发器; K1—精馏塔1; K2—精馏塔2; K3—水平隔板塔; L12—精馏塔K1塔顶的液相流股 (L代表液相流股, 1代表精馏塔K1, 2代表塔顶流股);V21—精馏塔塔底的气相流股 (V代表气相流股, 2代表精馏塔K2, 1代表塔底流股); P—产品流股; R—塔顶循环流股
Fig.3 The refining process with column using horizontal dividing wall[14]
| 1 | 江华. 我国电子级多晶硅发展情况分析[J]. 科技中国, 2021(4): 64-66. |
| Jiang H. Analysis on the development of electronic grade polysilicon in China[J]. Scitech in China, 2021(4): 64-66. | |
| 2 | 王晓英, 王宇光, 谷新春, 等. 多晶硅制备工艺及发展趋势[J]. 化工进展, 2013, 32(6): 1336-1340. |
| Wang X Y, Wang Y G, Gu X C, et al. Progress in preparation technology of polysilicon[J]. Chemical Industry and Engineering Progress, 2013, 32(6): 1336-1340. | |
| 3 | 周叶明. 电子级多晶硅循环氢中磷杂质的吸附脱除研究[D]. 天津: 天津大学, 2019. |
| Zhou Y M. Study on adsorption and removal of phosphorus impurities in circulating hydrogen of electronic grade polysilicon[D]. Tianjin: Tianjin University, 2019. | |
| 4 | Chen H, Morita K, Ma X D, et al. Boron removal for solar-grade silicon production by metallurgical route: a review[J]. Solar Energy Materials and Solar Cells, 2019, 203: 110169. |
| 5 | 何泰锋. 改良西门子法循环氢气中磷化氢的吸附脱除研究[D]. 天津: 天津大学, 2020. |
| He T F. Study on adsorption and removal of phosphine from circulating hydrogen by improved Siemens method[D]. Tianjin: Tianjin University, 2020. | |
| 6 | 钱浩. 液体氯硅烷中硼磷杂质高效分离工艺[D]. 天津: 天津大学, 2016. |
| Qian H. Efficient separation process of boron and phosphorus impurities in liquid chlorosilane[D]. Tianjin: Tianjin University, 2016. | |
| 7 | Díez E, Rodríguez A, Gómez J M, et al. Distillation assisted heat pump in a trichlorosilane purification process[J]. Chemical Engineering and Processing: Process Intensification, 2013, 69: 70-76. |
| 8 | Zhang H, Lu P, Ding Z, et al. Design optimization and control of dividing wall column for purification of trichlorosilane[J]. Chemical Engineering Science, 2022, 257: 117716. |
| 9 | Yin M, Hua C, Lu P, et al. Design and control of pressure-swing heat integration distillation for the trichlorosilane purification process[J]. ACS Omega, 2022, 7(11): 9254-9266. |
| 10 | Sturm A G, Karaca U S, Heinz M, et al. Siemens reloaded: chloride-assisted selective hydrodechlorination of SiCl4 to HSiCl3 [J]. ChemSusChem, 2023, 16(5): e202201953. |
| 11 | Szabó L, Balaton M, Németh S, et al. Analysing divided wall columns[J]. Clean Technologies and Environmental Policy, 2011, 13(4): 633-636. |
| 12 | 裴艳红, 李强, 马国栋, 等. 一种三氯氢硅精制方法: 102807223B[P]. 2014-03-26. |
| Pei Y H, Li Q, Ma G D, et al. Method for refining trichlorosilane: 102807223B[P]. 2014-03-26. | |
| 13 | Lee S K, Shin J H, Lee J K, et al. Trihalosilane refining device: US20140076711[P]. 2014-03-20. |
| 14 | Aigner M, Paetzold U, Prochaska J. Purification of chlorosilanes by means of distillation and adsorption: US10632398[P]. 2020-04-28. |
| 15 | 沈祖祥, 严大洲, 汤传斌, 等. 三氯氢硅加压提纯方法及其装置: 1693192A[P]. 2005-11-09. |
| Shen Z X, Yan D Z, Tang C B, et al. Process for pressure purification of silicon trichlorohydrgen and apparatus thereof: 1693192A[P]. 2005-11-09. | |
| 16 | 华超, 黄国强, 王红星, 等. 高精密精馏提纯三氯氢硅的分离装置和方法: 101249312A[P]. 2008-08-27. |
| Hua C, Huang G Q, Wang H X, et al. Separation apparatus and method of high precision rectification purify trichlorosilane: 101246912A[P]. 2008-08-27. | |
| 17 | Choi C H, Lee J S, Choi G U, et al. Method for purifying trichlorosilane: KR1292545B1[P]. 2013-08-12. |
| 18 | Zhu J X, Hao L, Wei H Y. Sustainable concept design including economic, environment and inherent safety criteria: process intensification-reactive pressure swing distillation[J]. Journal of Cleaner Production, 2021, 314: 127852. |
| 19 | 黄国强, 孙帅帅, 王红星, 等. 一种用于多晶硅生产的隔壁热耦合精馏方法及设备: 102923714A[P]. 2013-02-13. |
| Huang G Q, Sun S S, Wang H X, et al. Next-door thermal coupling distillation method and equipment for producing polycrystalline silicon: 102923714A[P]. 2013-02-13. | |
| 20 | 刘春江, 郭凯, 袁希钢, 等. 三氯氢硅全热耦合集成多效精馏生产装置及生产方法: 103130227A[P]. 2013-06-05. |
| Liu C J, Guo K, Yuan X G, et al. Production device and production method for trichlorosilane full thermal coupling integration multi-effect distillation: 103130227A[P]. 2013-06-05. | |
| 21 | Brönsted J N. Einige bemerkungen über den begriff der Säuren und basen[J]. Recueil Des Travaux Chimiques Des Pays-Bas, 1923, 42(8): 718-728. |
| 22 | Kray W. Purification of chlorosilanes: US4481178A[P]. 1984-11-06. |
| 23 | Tzou M S. Phosphorous removal from chlorosilane: US5723644[P]. 1998-03-03. |
| 24 | Gmbh Licentia. Purifying silanes or chlorinated silanes: GB893495[P]. 1962-04-11. |
| 25 | Seliger B, Schladerbeck N, Pauli I, et al. Method for removing boron-containing impurities from halogen silanes and apparatus for performing said method: US20110150739[P]. 2011-06-23. |
| 26 | Benkeser R A. Chemistry of trichlorosilane-tertiary amine combinations[J]. Accounts of Chemical Research, 1971, 4(3): 94-100. |
| 27 | Wolff G A. Removal of boron trichloride from silicon tetrachloride: US2877097[P]. 1959-03-10. |
| 28 | Popov K K, Campbell J L P, Kysilka O, et al. Reductive amination revisited: reduction of aldimines with trichlorosilane catalyzed by dimethylformamide-functional group tolerance, scope, and limitations[J]. The Journal of Organic Chemistry, 2022, 87(2): 920-943. |
| 29 | Terauchi K, Sugimura S, Kobayashi M, et al. Purification of chlorosilane using amine compound: WO2011024257A1[P]. 2011-03-03. |
| 30 | Nozakura S. Addition of trichlorosilane to styrene, 2 vinylpyridine, allylcyanide and octene-1[J]. Bulletin of the Chemical Society of Japan, 1956, 29(7): 784-789. |
| 31 | Kotzsch H J, Vahlensieck H J. Purifying chlorosilanes: GB1241108[P]. 1971-07-28. |
| 32 | Koyanagi S, Iseki Y. Method to refine chloro silane: JP2005067979A[P]. 2005-3-17. |
| 33 | Hasegawa M, Aoyama T. Method for purification of chlorosilanes: JP2013001632A[P]. 2013-01-07. |
| 34 | Mueh E, Rauleder H, Schork R. Installation and method for the removal of boron and aluminum from halosilanes: WO2009089951A2[P]. 2009-07-23. |
| 35 | Ghetti G. Process and plant for the purification of trichlorosilane and silicon tetrachloride: WO2006054325A2[P]. 2006-05-26. |
| 36 | Hasegawa M, Tonomura Y, Kubota T, et al. Method for purifying chlorosilanes: US20130177492[P]. 2013-07-11. |
| 37 | Nagai N, Shimizu T, Uehara K, et al. Method for purifying chlorosilanes: US9193597[P]. 2015-11-24. |
| 38 | Darnell R D, Ingle W M. Purification of silicon source materials: US4409195[P]. 1983-10-11. |
| 39 | Uehara K, Kubota T, Osima M. Method for purifying chlorosilanes: US20090068081[P]. 2009-03-12. |
| 40 | Kishi R, Ishida M, Netsu S. Purification system for trichlorosilane in the manufacture of and silicon crystals: DE102018001359A1[P]. 2018-08-30. |
| 41 | Bradley H B. Purification of silicon compounds: US3188168[P]. 1965-06-08. |
| 42 | Wheland J M. Purification of silicon tetrachloride and germanium tetrachloride: US2821460[P]. 1958-01-28. |
| 43 | Sakai J, Iiyama S. Method for producing refined chlorosilane comprising boron compound and phosphorus compound: WO2020153342A1[P]. 2020-07-30. |
| 44 | Li T Q, Lin N, Han Y, et al. Metallothermic reduction of molten adduct [PCl 4 + ][AlCl 4 - ] at 50℃ to amorphous phosphorus or crystallized phosphides[J]. ACS Applied Materials & Interfaces, 2018, 10(49): 42469-42474. |
| 45 | Lang W, Schmidt D, Hofer J, et al. Purification of halosilanes: DE2546957A1[P]. 1977-04-21. |
| 46 | Miyao S, Ishida M, Yoshida A, et al. Method for purifying chlorosilane with high efficiency at low cost: JP2016017023A[P]. 2016-02-01. |
| 47 | 刘见华, 赵雄, 万烨, 等. 氯硅化物的纯化方法和纯化系统: 106882808B[P]. 2019-09-10. |
| Liu J H, Zhao X, Wan Y, et al. Purification method and purification system of chlorosilicate: 106882808[P]. 2019-09-10. | |
| 48 | Rad A S, Ayub K. DFT study of boron trichloride adsorption on the surface of Al12N12 nanocluster[J]. Molecular Physics, 2017, 115(7): 879-884. |
| 49 | Kudzin M H, Giełdowska M, Krata A A, et al. Phosphorylation of chitosan (chitin) surface with PCl3 [J]. Phosphorus, Sulfur, and Silicon and the Related Elements, 2022, 197(5): 625-629. |
| 50 | Li F S, Wu X F, Guo F Z, et al. One-step conversion of amides and esters to acid chlorides with PCl3 [J]. European Journal of Organic Chemistry, 2021, 2021(30): 4314-4317. |
| 51 | Domaracka A, Ptasińska-Denga E, Szmytkowski C. Electron collisions with boron trichloride (BCl3) molecules[J]. Physical Review A, 2005, 71(5): 052711. |
| 52 | 张茜, 任其龙, 杨亦文, 等. 硼吸附材料及其性能[J]. 化学进展, 2015, 27(1): 125-134. |
| Zhang X, Ren Q L, Yang Y W, et al. Materials for boron adsorption[J]. Progress in Chemistry, 2015, 27(1): 125-134. | |
| 53 | Lu J R, Wu X N, Li Y, et al. Modified silica gel surface with chelating ligand for effective mercury ions adsorption[J]. Surfaces and Interfaces, 2018, 12: 108-115. |
| 54 | Doornbos R S. Purification of chlorosilanes: US4713230[P]. 1987-12-15. |
| 55 | Park J H, Kang G H, Park S H, et al. Method for removing phosphorus compounds from chlorosilane: KR2016143994A[P]. 2016-12-15. |
| 56 | Hou S Y, Huang Z H, Zhu T L, et al. Adsorption removal of styrene on C—Cl grafted silica gel adsorbents[J]. Chemosphere, 2023, 315: 137679. |
| 57 | 郭婷, 赵长森, 牛强, 等. 一种多晶硅生产过程中的三氯氢硅提纯工艺: 115259165A[P]. 2022-11-01. |
| Guo T, Zhao C S, Niu Q, et al. Trichlorosilane purification process in polycrystalline silicon production process: 115259165A[P]. 2022-11-01. | |
| 58 | Hazeltine B, Fahrenbruck S, Qin W J. Purification of trichlorosilane: US20130121907[P]. 2013-05-16. |
| 59 | Chu S Y, Feng X F, Liu C C, et al. Advances in chelating resins for adsorption of heavy metal ions[J]. Industrial & Engineering Chemistry Research, 2022, 61(31): 11309-11328. |
| 60 | Stephan K, Hans-Dieter B, Hans-Joachim L, et al. Method for purifying trichlorosilane: US6843972[P]. 2005-01-18. |
| 61 | Jeon M G, Kang G H, Park S H, et al. Purification method of chlorosilane using anion exchange resin: KR2016143973A[P]. 2016-12-15. |
| 62 | 曹玲玲, 宗冰, 鲍守珍, 等. 用于氯硅烷除杂的吸附树脂及其制备方法: 113402640A[P]. 2021-09-17. |
| Cao L L, Zong B, Bao S Z, et al. Adsorbing resin for removing impurities from chlorosilane and preparation method of adsorbing resin: 113402640A[P]. 2021-09-17. | |
| 63 | Sugimura S, Matsuoto K, Matsuoto Y, et al. Method for purifying chlorosilane: US20120148471[P]. 2012-06-14. |
| 64 | 官健, 刘逸枫, 罗轩, 等. 一种提高三氯氢硅品质的方法: 113716570A[P]. 2021-11-30. |
| Guan J, Liu Y F, Luo X, et al. Method for improving quality of trichlorosilane: 113716570A[P]. 2021-11-30. | |
| 65 | Tarancon G. Purification of silane: DE2755824A1[P]. 1978-06-22. |
| 66 | Haghpanah R, Depierro M, Ketola B. Process for purifying halogenated silicon compounds: WO2021034526A1[P]. 2021-02-25. |
| 67 | 沈峰, 杨伟强, 姜洪涛, 等. 一种吸附去除氯硅烷中硼磷的装置: 217972616U[P]. 2022-12-06. |
| Shen F, Yang W Q, Jiang H T, et al. A device for removing boron and phosphorus impurities from chlorosilanes through adsorption: 217972616U[P]. 2022-12-06. | |
| 68 | 童贵, 何瑞, 鲁林武. 一种氯硅烷吸附树脂填充装置和树脂填充方法: 110613955B[P]. 2021-08-13. |
| Tong G, He R, Lu L W, et al. Chlorosilane adsorption resin filling device and resin filling method: 110613955B[P]. 2021-08-13. | |
| 69 | 宋高杰, 武珠峰, 夏进京, 等. 电子级三氯氢硅提纯装置及方法: 115105850A[P]. 2022-09-27. |
| Song G J, Wu Z F, Xia J J, et al. Device and method for purifying electronic-grade trichlorosilane: 115105850A[P]. 2022-09-27. | |
| 70 | Rauleder H, Mueh E. Process for purification of alkoxysilanes and siloxanes from metal-containing impurities by adsorption and filtration: WO2010066487A1[P]. 2010-06-17. |
| 71 | 李群生. 一种精馏、吸附、膜分离联合生产电子级三氯氢硅的装置及方法: 114735709A[P]. 2022-07-12. |
| Li Q S. Device and method for producing electronic grade trichlorosilane through combination of rectification and membrane separation: 114735709A[P]. 2022-07-12. | |
| 72 | 梁晓阳, 季建波, 王宁, 等. 一种多晶硅生产装置尾气处理系统: 218853865U[P]. 2023-04-14. |
| Liang X Y, Ji J B, Wang N, et al. The utility model relates to a tail gas treatment system of a polysilicon production production unit: 218853865U[P]. 2023-04-14. | |
| 73 | Greenwood N N, Greatrex R. Kinetics and mechanism of the thermolysis and photolysis of binary boranes[J]. Pure and Applied Chemistry, Pure and Applied Chemistry, 1987, 59(7): 857-868. |
| 74 | Jones N B, Gibbons B, Morris A J, et al. Reversible dissociation for effective storage of diborane gas within the UiO-66-NH2 metal-organic framework[J]. ACS Applied Materials & Interfaces, 2022, 14(6): 8322-8332. |
| 75 | Jones N B, Sharp C H, Troya D, et al. Bifurcated dihydrogen bonding in the uptake of gas-phase diborane on silica[J]. The Journal of Physical Chemistry Letters, 2021, 12(20): 4987-4992. |
| 76 | Wang J, Liu W, Luo G, et al. Synergistic effect of well-defined dual sites boosting the oxygen reduction reaction[J]. Energy & Environmental Science, 2018, 11(12): 3375-3379. |
| 77 | Hardwick S J, Mailloux J C. Waste minimization in semiconductor processing[J]. MRS Online Proceedings Library, 1994, 344(1): 273-279. |
| 78 | Watanabe T, Suzuki T. Reaction mechanisms of B2H6, Si2H6, PH3 and H2Se with CuO[J]. Journal of the Ceramic Society of Japan, 1997, 105(1225): 779-783. |
| 79 | Feng J Y, Ma L X, Wang C, et al. Catalytic decomposition mechanism of PH3 on 3DCuO/C and high value utilization of deactivated catalysts[J]. Small, 2023, 19(28): 2370206. |
| 80 | El-Shobaky G A, Fagal G A, Mokhtar M. Effect of ZnO on surface and catalytic properties of CuO/Al2O3 [J]. Applied Catalysis A: General, 1997, 155(2): 167-178. |
| 81 | Yang L P, Yi H H, Tang X L, et al. Effect of rare earth addition on Cu-Fe/AC adsorbents for phosphine adsorption from yellow phosphorous tail gas[J]. Journal of Rare Earths, 2010, 28: 322-325. |
| 82 | Ning P, Yi H H, Yu Q F, et al. Effect of zinc and cerium addition on property of copper-based adsorbents for phosphine adsorption[J]. Journal of Rare Earths, 2010, 28(4): 581-586. |
| 83 | Ren Z D, Quan S S, Zhu Y C, et al. Purification of yellow phosphorus tail gas for the removal of PH3 on the spot with flower-shaped CuO/AC[J]. RSC Advances, 2015, 5(38): 29734-29740. |
| 84 | Yi H H, Yu Q F, Tang X L, et al. Phosphine adsorption removal from yellow phosphorus tail gas over CuO-ZnO-La2O3/activated carbon[J]. Industrial & Engineering Chemistry Research, 2011, 50(7): 3960-3965. |
| 85 | 吴锋, 田新, 吴鹏. 一种电子级多晶硅生产用氢气提纯的方法: 113233420B[P]. 2022-02-01. |
| Wu F, Tian X, Wu P. Method for purifying hydrogen for electronic grade polycrystalline silicon production: 113233420B[P]. 2022-02-01. | |
| 86 | 张鹏, 田洪先, 刘强, 等. 一种循环氢气再纯化的方法及装置: 105293438A[P]. 2016-02-03. |
| Zhang P, Tian H X, Liu Q, et al. Method and device for circulating hydrogen repurification: 105293438A[P]. 2016-02-03. | |
| 87 | Xu X, Miao X, Liao N, et al. Breakthrough analysis for adsorption of phosphine on 5A molecular sieve[J]. Chemical Engineering & Technology, 2011, 34(1): 140-145. |
| 88 | Bush E L. Purification of silane: GB831216 [P]. 1960-03-23. |
| 89 | Yu Q F, Tang X L, Yi H H, et al. Equilibrium and heat of adsorption of phosphine on CaCl2-modified molecular sieve[J]. Asia-Pacific Journal of Chemical Engineering, 2009, 4(5): 612-617. |
| 90 | 黄小凤, 谭娟, 宁平. 改性5A分子筛吸附净化PH3的实验研究[J]. 西安建筑科技大学学报(自然科学版), 2011, 43(2): 220-223. |
| Huang X F, Tan J, Ning P. Adsorbing purification of PH3 by modified 5A molecular sieves[J]. Journal of Xi’an University of Architecture & Technology (Natural Science Edition), 2011, 43(2): 220-223. | |
| 91 | Xu X W, Huang G Q. Effect of 13X zeolite modified with CuCl2 and ZnCl2 for removing phosphine from circular hydrogen of a polysilicon chemical vapor deposition stove[J]. Industrial & Engineering Chemistry Research, 2016, 55(5): 1380-1386. |
| 92 | Li W C, Bai H, Hsu J N, et al. Metal loaded zeolite adsorbents for phosphine removal[J]. Industrial & Engineering Chemistry Research, 2008, 47(5): 1501-1505. |
| 93 | Li S, Li K, Hao J M, et al. Acid modified mesoporous Cu/SBA-15 for simultaneous adsorption/oxidation of hydrogen sulfide and phosphine[J]. Chemical Engineering Journal, 2016, 302: 69-76. |
| 94 | 吴锋, 徐玲锋, 梁帅军. 用于电子级多晶硅尾气纯化的多层吸附塔: 106512644A[P]. 2017-03-22. |
| Wu F, Xu L F, Liang S J. Multilayer adsorption tower for purification of electronic grade polycrystalline silicon tail gas: 106512644A[P]. 2017-03-22. | |
| 95 | 黄国强, 黄琪琪, 王乃治, 等. 用于生产高品质多晶硅的还原循环氢气深冷除杂的装置: 215439670U[P]. 2022-01-07. |
| Huang G Q, Huang Q Q, Wang N Z, et al. Reduction cycle hydrogen cryogenic impurity removal device for producing high-quality polycrystalline silicon: 215439670U[P]. 2022-01-07. |
| [1] | 王尤佳, 赵亮, 高金森, 徐春明. 柴油烃类族组成分离技术研究进展[J]. 化工学报, 2024, 75(1): 20-32. |
| [2] | 王雪杰, 崔国庆, 王文涵, 杨扬, 王淙恺, 姜桂元, 徐春明. 电内加热Pt/NPC催化剂高效催化甲基环己烷脱氢反应研究[J]. 化工学报, 2024, 75(1): 292-301. |
| [3] | 齐元帅, 彭文朝, 李阳, 张凤宝, 范晓彬. 电化学脱盐机理及相关研究进展[J]. 化工学报, 2024, 75(1): 171-189. |
| [4] | 郑雨婷, 方冠东, 张梦波, 张浩淼, 王靖岱, 阳永荣. 微化工精馏分离技术研究进展[J]. 化工学报, 2024, 75(1): 47-59. |
| [5] | 朱娇, 栾丽萍, 从深震, 刘新磊. 氢气分离有机膜[J]. 化工学报, 2024, 75(1): 138-158. |
| [6] | 孟祥军, 花莹曦, 张长金, 张弛, 杨林睿, 杨若昔, 刘鉴漪, 许春建. 6N电子级氘气的制备与纯化技术研究[J]. 化工学报, 2024, 75(1): 377-390. |
| [7] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [8] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [9] | 黄琮琪, 吴一梅, 陈建业, 邵双全. 碱性电解水制氢装置热管理系统仿真研究[J]. 化工学报, 2023, 74(S1): 320-328. |
| [10] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [11] | 李科, 文键, 忻碧平. 耦合蒸气冷却屏的真空多层绝热结构对液氢储罐自增压过程的影响机制研究[J]. 化工学报, 2023, 74(9): 3786-3796. |
| [12] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [13] | 曹跃, 余冲, 李智, 杨明磊. 工业数据驱动的加氢裂化装置多工况切换过渡状态检测[J]. 化工学报, 2023, 74(9): 3841-3854. |
| [14] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [15] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号