化工学报 ›› 2023, Vol. 74 ›› Issue (10): 4286-4301.DOI: 10.11949/0438-1157.20230687
收稿日期:2023-07-05
修回日期:2023-09-07
出版日期:2023-10-25
发布日期:2023-12-22
通讯作者:
张军社,魏进家
作者简介:杨柳青(1991—),男,博士研究生,4120116024@stu.xjtu.edu.cn
基金资助:
Liuqing YANG( ), Zirui ZHAO, Junshe ZHANG(
), Zirui ZHAO, Junshe ZHANG( ), Jinjia WEI(
), Jinjia WEI( )
)
Received:2023-07-05
Revised:2023-09-07
Online:2023-10-25
Published:2023-12-22
Contact:
Junshe ZHANG, Jinjia WEI
摘要:
化学链甲烷干重整可将CH4和CO2转化为各种增值产品,是一种具有低分离要求的高效反应技术,并可助力碳中和。目前,该技术面临的一个主要挑战是设计和开发具有良好反应性和稳定性的载氧体。合成了Ba取代的具有锚定纳米颗粒的(La0.5Sr0.5)1-x Ba x Fe0.6Co0.4O3钙钛矿氧化物,将其用作化学链甲烷干重整的载氧体,通过多种表征手段和性能评价,研究了该类材料的物化性质和氧化还原性能。结果表明,在所有样品中,La0.35Sr0.35Ba0.3Fe0.6Co0.4O3在甲烷部分氧化过程中可实现84.3%的甲烷转化率、15.23 mmol·g-1的合成气产量以及最高的氧消耗量(5.29 mmol·g-1),显示出优异的氧扩散速率,同时具有95.8%的合成气选择性、70.0%的CO选择性和1.36 mmol·g-1的积炭。对甲烷还原过程中气体生成速率的分析表明,Ba取代可以优化载氧体的晶格结构,导致高的离子迁移率,促进氧在体相中的快速扩散,进而提升CH4转化。此外,氧化还原循环测试表明该载氧体具有较优异的结构稳定性和较好的再生能力。
中图分类号:
杨柳青, 赵子瑞, 张军社, 魏进家. 钡含量对(La0.5Sr0.5)1-x Ba x Fe0.6Co0.4O3化学链甲烷干重整性能的影响[J]. 化工学报, 2023, 74(10): 4286-4301.
Liuqing YANG, Zirui ZHAO, Junshe ZHANG, Jinjia WEI. Effect of Ba content on chemical looping dry reforming of methane performance of (La0.5Sr0.5)1-x Ba x Fe0.6Co0.4O3[J]. CIESC Journal, 2023, 74(10): 4286-4301.
| 样品 | 缩写 |
|---|---|
| La0.45Sr0.45Ba0.1Fe0.6Co0.4O3 | LSB10FC |
| La0.4Sr0.4Ba0.2Fe0.6Co0.4O3 | LSB20FC |
| La0.35Sr0.35Ba0.3Fe0.6Co0.4O3 | LSB30FC |
| La0.3Sr0.3Ba0.4Fe0.6Co0.4O3 | LSB40FC |
| La0.25Sr0.25Ba0.5Fe0.6Co0.4O3 | LSB50FC |
表1 样品的化学组成与缩写
Table 1 Chemical compositions and abbreviations of the samples
| 样品 | 缩写 |
|---|---|
| La0.45Sr0.45Ba0.1Fe0.6Co0.4O3 | LSB10FC |
| La0.4Sr0.4Ba0.2Fe0.6Co0.4O3 | LSB20FC |
| La0.35Sr0.35Ba0.3Fe0.6Co0.4O3 | LSB30FC |
| La0.3Sr0.3Ba0.4Fe0.6Co0.4O3 | LSB40FC |
| La0.25Sr0.25Ba0.5Fe0.6Co0.4O3 | LSB50FC |
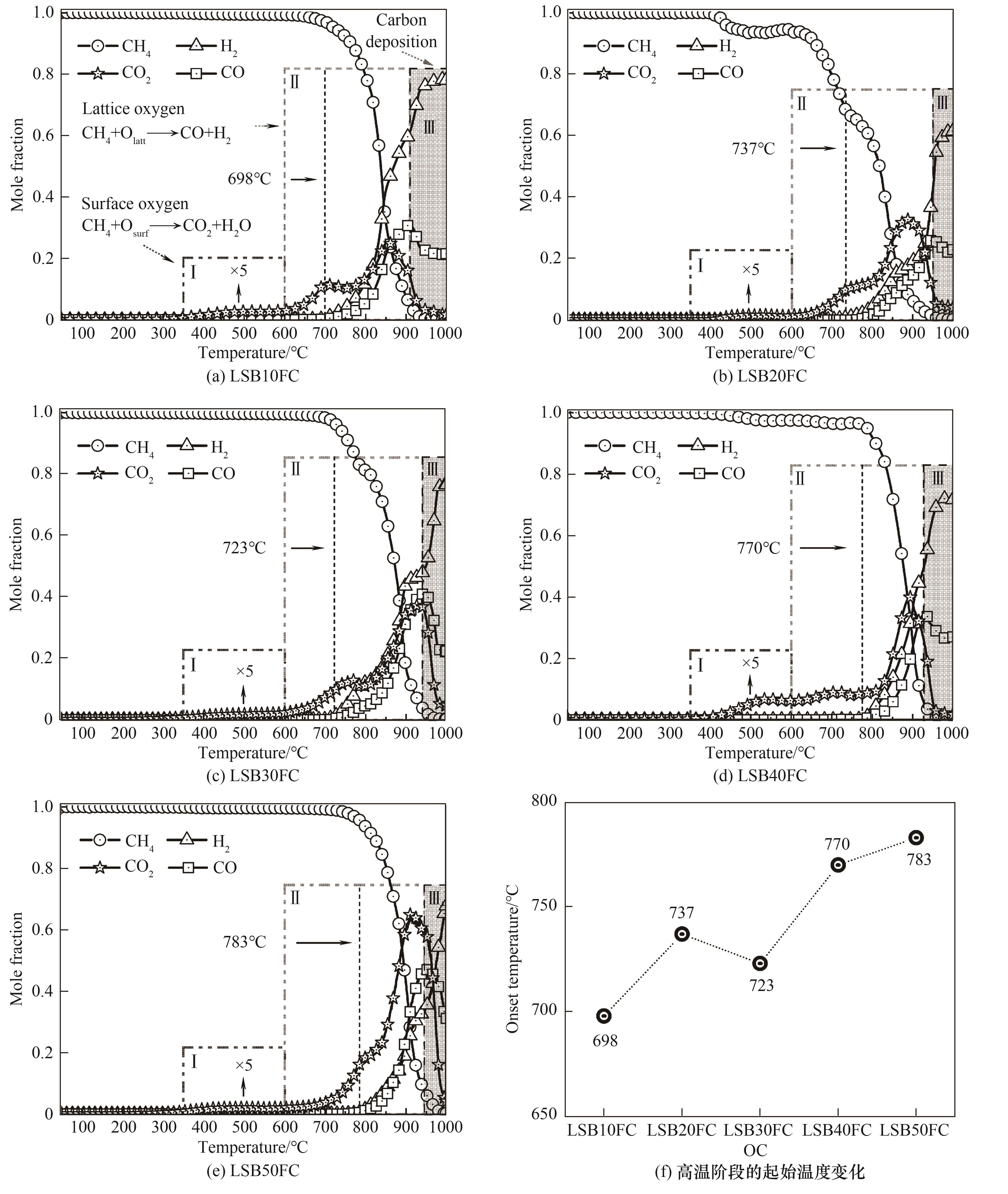
图6 不同Ba含量载氧体的CH4-TPR谱图及高温阶段的起始温度变化
Fig.6 CH4-TPR profiles over different oxygen carriers and onset reaction temperatures of high temperature phase during CH4-TPR
| 载氧体 | 无限定还原时间的CL-DRM过程 | 限定还原时间的CL-DRM过程 | ||||
|---|---|---|---|---|---|---|
| 转化率/% | 消耗氧量/(mmol·g-1) | 补充氧量/(mmol·g-1) | 转化率/% | 消耗氧量/(mmol·g-1) | 补充氧量/(mmol·g-1) | |
| LSB10FC | 67.1 | 8.00 | 4.82 | 83.0 | 4.12 | 3.91 |
| LSB20FC | 58.0 | 7.53 | 5.15 | 82.6 | 4.38 | 4.75 |
| LSB30FC | 68.9 | 6.68 | 5.54 | 84.3 | 5.29 | 5.24 |
| LSB40FC | 44.3 | 7.36 | 5.59 | 73.0 | 4.37 | 4.36 |
| LSB50FC | 45.2 | 7.17 | 5.17 | 67.3 | 4.64 | 4.90 |
表2 不同载氧体的甲烷转化率、消耗氧量和补充氧量
Table 2 Methane conversion, the amount of O consumed and replenished for different oxygen carriers
| 载氧体 | 无限定还原时间的CL-DRM过程 | 限定还原时间的CL-DRM过程 | ||||
|---|---|---|---|---|---|---|
| 转化率/% | 消耗氧量/(mmol·g-1) | 补充氧量/(mmol·g-1) | 转化率/% | 消耗氧量/(mmol·g-1) | 补充氧量/(mmol·g-1) | |
| LSB10FC | 67.1 | 8.00 | 4.82 | 83.0 | 4.12 | 3.91 |
| LSB20FC | 58.0 | 7.53 | 5.15 | 82.6 | 4.38 | 4.75 |
| LSB30FC | 68.9 | 6.68 | 5.54 | 84.3 | 5.29 | 5.24 |
| LSB40FC | 44.3 | 7.36 | 5.59 | 73.0 | 4.37 | 4.36 |
| LSB50FC | 45.2 | 7.17 | 5.17 | 67.3 | 4.64 | 4.90 |

图10 不同Ba含量载氧体的化学链甲烷干重整过程(30 min的还原过程)
Fig.10 Evolution of the temporal gaseous product over as-prepared oxygen carriers during the CL-DRM process with 30 min reduction
| 载氧体 | 反应温度/℃ | CH4还原步骤 | 氧变化量/ (mmol·g-1) | CO2分解步骤 | 文献 | ||
|---|---|---|---|---|---|---|---|
| CH4转化率/% | 合成气选择性/% | 合成气产率/(mmol·g-1) | 转化率/% | ||||
| La0.5Ce0.5FeO3 | 850 | 82 | 93 | 4.2 | — | 约92 | [ |
| LaFe0.8Al0.2O3 | 900 | 约85 | >95 | 3.4 | — | 约85 | [ |
| La0.85Sr0.15Fe0.95Al0.05O3-δ | 900 | 约80 (20%XOC) | >99 | — | — | 约97 | [ |
| LSB30FC | 900 | 84.3 | 95.8 | 15.23 | 5.29 | — | 本文 |
表3 不同载氧体的性能比较
Table 3 Comparison of performance for different oxygen carriers
| 载氧体 | 反应温度/℃ | CH4还原步骤 | 氧变化量/ (mmol·g-1) | CO2分解步骤 | 文献 | ||
|---|---|---|---|---|---|---|---|
| CH4转化率/% | 合成气选择性/% | 合成气产率/(mmol·g-1) | 转化率/% | ||||
| La0.5Ce0.5FeO3 | 850 | 82 | 93 | 4.2 | — | 约92 | [ |
| LaFe0.8Al0.2O3 | 900 | 约85 | >95 | 3.4 | — | 约85 | [ |
| La0.85Sr0.15Fe0.95Al0.05O3-δ | 900 | 约80 (20%XOC) | >99 | — | — | 约97 | [ |
| LSB30FC | 900 | 84.3 | 95.8 | 15.23 | 5.29 | — | 本文 |
| 1 | Li X Y, Pei C L, Gong J L. Shale gas revolution: catalytic conversion of C1—C3 light alkanes to value-added chemicals[J]. Chem, 2021, 7(7): 1755-1801. |
| 2 | 王保文, 张港, 刘同庆, 等. CeO2/CuFe2O4氧载体CH4化学链重整耦合CO2热催化还原研究[J]. 化工学报, 2022, 73(12): 5414-5426. |
| Wang B W, Zhang G, Liu T Q, et al. Research on chemical looping reforming of CH4 by CeO2 doped CuFe2O4 oxygen carrier coupled with CO2 thermocatalytic reduction[J]. CIESC Journal, 2022, 73(12): 5414-5426. | |
| 3 | Song S, Song H, Li L M, et al. A selective Au-ZnO/TiO2 hybrid photocatalyst for oxidative coupling of methane to ethane with dioxygen[J]. Nature Catalysis, 2021, 4(12): 1032-1042. |
| 4 | Koolen C D, Luo W, Züttel A. From single crystal to single atom catalysts: structural factors influencing the performance of metal catalysts for CO2 electroreduction[J]. ACS Catalysis, 2023, 13(2): 948-973. |
| 5 | 王峰, 张顺鑫, 余方博, 等. 光催化CO2还原制碳氢燃料系统优化策略研究[J]. 化工学报, 2023, 74(1): 29-44. |
| Wang F, Zhang S X, Yu F B, et al. Optimization strategy for producing carbon based fuels by photocatalytic CO2 reduction[J]. CIESC Journal, 2023, 74(1): 29-44. | |
| 6 | 刘彦铄, 王新赫, 张军社, 等. 太阳能甲烷重整反应器研究进展[J]. 化工进展, 2019, 38(12): 5339-5350. |
| Liu Y S, Wang X H, Zhang J S, et al. Progress in solar methane reforming reactors[J]. Chemical Industry and Engineering Progress, 2019, 38(12): 5339-5350. | |
| 7 | Huang Y, Sun W J, Qin Z C, et al. The role of China’s terrestrial carbon sequestration 2010—2060 in offsetting energy-related CO2 emissions[J]. National Science Review, 2022, 9(8): nwac057. |
| 8 | Hu Q Q, Li Y Z, Wu J C, et al. Extraordinary catalytic performance of nickel half-metal clusters for light-driven dry reforming of methane[J]. Advanced Energy Materials, 2023, 13(21): 2300071. |
| 9 | Buelens L C, Galvita V V, Poelman H, et al. Super-dry reforming of methane intensifies CO2 utilization via Le Chatelier’s principle[J]. Science, 2016, 354(6311): 449-452. |
| 10 | Yang L Q, Zhao Z R, Cui C Y, et al. Effect of nickel and cobalt doping on the redox performance of SrFeO3- δ toward chemical looping dry reforming of methane[J]. Energy & Fuels, 2023, 37(16): 12045-12057. |
| 11 | 蔡润夏, 李凡星. 复杂氧化物载氧体的调变策略及在过程强化中的应用[J]. 化工学报, 2021, 72(12): 6122-6130. |
| Cai R X, Li F X. Tailoring the thermodynamic properties of complex oxides for thermochemical air separation and beyond[J]. CIESC Journal, 2021, 72(12): 6122-6130. | |
| 12 | Zhu Q Y, Zhou H, Wang L, et al. Enhanced CO2 utilization in dry reforming of methane achieved through nickel-mediated hydrogen spillover in zeolite crystals[J]. Nature Catalysis, 2022, 5(11): 1030-1037. |
| 13 | Luo M, Shen R C, Qin Y J, et al. Conversion and syngas production of toluene as a biomass tar model compound in chemical looping reforming[J]. Fuel, 2023, 345: 128203. |
| 14 | Zhu X, Imtiaz Q, Donat F, et al. Chemical looping beyond combustion—a perspective[J]. Energy & Environmental Science, 2020, 13(3): 772-804. |
| 15 | Li D Y, Xu R D, Gu Z H, et al. Chemical-looping conversion of methane: a review[J]. Energy Technology, 2020, 8(8): 1900925. |
| 16 | Zeng L, Cheng Z, Fan J A, et al. Metal oxide redox chemistry for chemical looping processes[J]. Nature Reviews Chemistry, 2018, 2(11): 349-364. |
| 17 | Sun Z, Russell C K, Whitty K J, et al. Chemical looping-based energy transformation via lattice oxygen modulated selective oxidation[J]. Progress in Energy and Combustion Science, 2023, 96: 101045. |
| 18 | Zeng D W, Kang F W, Qiu Y, et al. Iron oxides with gadolinium-doped cerium oxides as active supports for chemical looping hydrogen production[J]. Chemical Engineering Journal, 2020, 396: 125153. |
| 19 | Luo C Q, Dou B L, Zhang H, et al. Co-production of hydrogen and syngas from chemical looping water splitting coupled with decomposition of glycerol using Fe-Ce-Ni based oxygen carriers[J]. Energy Conversion and Management, 2021, 238: 114166. |
| 20 | Zhang R J, Cao Y, Li H B, et al. The role of CuO modified La0.7Sr0.3FeO3 perovskite on intermediate-temperature partial oxidation of methane via chemical looping scheme[J]. International Journal of Hydrogen Energy, 2020, 45(7): 4073-4083. |
| 21 | 马源, 肖晴月, 岳君容, 等. CeO2-Al2O3复合载体负载Ni基催化剂催化CO x 共甲烷化性能[J]. 化工进展, 2023, 42(5): 2421-2428. |
| Ma Y, Xiao Q Y, Yue J R, et al. CO x co-methanation over a Ni-based catalyst supported on CeO2-Al2O3 composite[J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2421-2428. | |
| 22 | Hwang J, Rao R R, Giordano L, et al. Perovskites in catalysis and electrocatalysis[J]. Science, 2017, 358(6364): 751-756. |
| 23 | Wang Y H, Robson M J, Manzotti A, et al. High-entropy perovskites materials for next-generation energy applications[J]. Joule, 2023, 7(5): 848-854. |
| 24 | Zhang J S, Haribal V, Li F X. Perovskite nanocomposites as effective CO2-splitting agents in a cyclic redox scheme[J]. Science Advances, 2017, 3(8): e1701184. |
| 25 | Xia X, Chang W X, Cheng S W, et al. Oxygen activity tuning via FeO6 octahedral tilting in perovskite ferrites for chemical looping dry reforming of methane[J]. ACS Catalysis, 2022, 12(12): 7326-7335. |
| 26 | Chang H, Bjørgum E, Mihai O, et al. Effects of oxygen mobility in La-Fe-based perovskites on the catalytic activity and selectivity of methane oxidation[J]. ACS Catalysis, 2020, 10(6): 3707-3719. |
| 27 | Joo S, Kim K, Kwon O, et al. Enhancing thermocatalytic activities by upshifting the d-band center of exsolved Co-Ni-Fe ternary alloy nanoparticles for the dry reforming of methane[J]. Angewandte Chemie International Edition, 2021, 60(29): 15912-15919. |
| 28 | Zhang D W, De Santiago H A, Xu B Y, et al. Compositionally complex perovskite oxides for solar thermochemical water splitting[J]. Chemistry of Materials, 2023, 35(5): 1901-1915. |
| 29 | Shannon R D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides[J]. Acta Crystallographica Section A, 1976, 32(5): 751-767. |
| 30 | Yang L Q, Zhang J S, Wei J J. Highly active La0.35Sr0.35Ba0.3Fe1- x Co x O3 oxygen carriers with the anchored nanoparticles for chemical looping dry reforming of methane[J]. Fuel, 2023, 349: 128771. |
| 31 | Klaas L, Bulfin B, Kriechbaumer D, et al. Impact of the Sr content on the redox thermodynamics and kinetics of Ca1– x Sr x MnO3– δ for tailored properties[J]. Physical Chemistry Chemical Physics, 2023, 25(13): 9188-9197. |
| 32 | Sifontes Á B, Del Toro R S, Ávila E, et al. Chitosan templated synthesis of strontium-iron-oxygen nanocrystalline system[J]. Ceramics International, 2015, 41(10): 13250-13256. |
| 33 | Srilakshmi C, Saraf R, Shivakumara C. Effective degradation of aqueous nitrobenzene using the SrFeO3- δ photocatalyst under UV illumination and its kinetics and mechanistic studies[J]. Industrial & Engineering Chemistry Research, 2015, 54(32): 7800-7810. |
| 34 | Naveenkumar A, Kuruva P, Shivakumara C, et al. Mixture of fuels approach for the synthesis of SrFeO3– δ nanocatalyst and its impact on the catalytic reduction of nitrobenzene[J]. Inorganic Chemistry, 2014, 53(22): 12178-12185. |
| 35 | Yang L Q, Li X F, Zhang X Z, et al. Supercritical solvothermal synthesis and formation mechanism of V2O3 microspheres with excellent catalytic activity on the thermal decomposition of ammonium perchlorate[J]. Journal of Alloys and Compounds, 2019, 806: 1394-1402. |
| 36 | 杨柳青, 何志帅, 张雄志, 等. 纳米Fe3O4的制备及其催化高氯酸铵热分解性能的研究[J]. 现代化工, 2016, 36(11): 94-97. |
| Yang L Q, He Z S, Zhang X Z, et al. Preparation of Fe3O4 nanoparticles and its catalytic performance for thermal decomposition of ammonium perchlorate[J]. Modern Chemical Industry, 2016, 36(11): 94-97. | |
| 37 | Li Y F, Zhang W Q, Zheng Y, et al. Controlling cation segregation in perovskite-based electrodes for high electro-catalytic activity and durability[J]. Chemical Society Reviews, 2017, 46(20): 6345-6378. |
| 38 | Barbero B P, Cadús L E, Marchetti S G. Determination of Fe(Ⅳ) species in partially substituted perovskite La0.6Ca0.4FeO3 [J]. Hyperfine Interactions, 2009, 194(1): 367-379. |
| 39 | Feng N J, Wu Y, Meng J, et al. Catalytic combustion of soot over Ce and Co substituted three-dimensionally ordered macroporous La1- x Ce x Fe1- y Co y O3 perovskite catalysts[J]. RSC Advances, 2015, 5(111): 91609-91618. |
| 40 | Zhang L, Xu W B, Wu J A, et al. Identifying the role of A-site cations in modulating oxygen capacity of iron-based perovskite for enhanced chemical looping methane-to-syngas conversion[J]. ACS Catalysis, 2020, 10(16): 9420-9430. |
| 41 | Neal L M, Shafiefarhood A, Li F X. Dynamic methane partial oxidation using a Fe2O3@La0.8Sr0.2FeO3- δ core-shell redox catalyst in the absence of gaseous oxygen[J]. ACS Catalysis, 2014, 4(10): 3560-3569. |
| 42 | Zhang X H, Pei C L, Chang X, et al. FeO6 octahedral distortion activates lattice oxygen in perovskite ferrite for methane partial oxidation coupled with CO2 splitting[J]. Journal of the American Chemical Society, 2020, 142(26): 11540-11549. |
| 43 | Donat F, Müller C R. CO2-free conversion of CH4 to syngas using chemical looping[J]. Applied Catalysis B: Environmental, 2020, 278: 119328. |
| [1] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [2] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [3] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [4] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [5] | 傅予, 刘兴翀, 王瀚雨, 李海敏, 倪亚飞, 邹文静, 雷月, 彭永姗. F3EACl修饰层对钙钛矿太阳能电池性能提升的研究[J]. 化工学报, 2023, 74(8): 3554-3563. |
| [6] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [7] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [8] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [9] | 牛超, 沈胜强, 杨艳, 潘泊年, 李熠桥. 甲烷BOG喷射器流动过程计算与性能分析[J]. 化工学报, 2023, 74(7): 2858-2868. |
| [10] | 刘晓洋, 喻健良, 侯玉洁, 闫兴清, 张振华, 吕先舒. 螺旋微通道对掺氢甲烷爆轰传播的影响[J]. 化工学报, 2023, 74(7): 3139-3148. |
| [11] | 周小文, 杜杰, 张战国, 许光文. 基于甲烷脉冲法的Fe2O3-Al2O3载氧体还原特性研究[J]. 化工学报, 2023, 74(6): 2611-2623. |
| [12] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [13] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [14] | 葛泽峰, 吴雨青, 曾名迅, 查振婷, 马宇娜, 侯增辉, 张会岩. 灰化学成分对生物质气化特性的影响规律[J]. 化工学报, 2023, 74(5): 2136-2146. |
| [15] | 李晨曦, 刘永峰, 张璐, 刘海峰, 宋金瓯, 何旭. O2/CO2氛围下正庚烷的燃烧机理研究[J]. 化工学报, 2023, 74(5): 2157-2169. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号