化工学报 ›› 2023, Vol. 74 ›› Issue (11): 4739-4748.DOI: 10.11949/0438-1157.20230930
• 材料化学工程与纳米技术 • 上一篇
收稿日期:2023-09-06
修回日期:2023-10-24
出版日期:2023-11-25
发布日期:2024-01-22
通讯作者:
陈昌国
作者简介:高孝麟(1990—),男,博士,624972527@qq.com
Xiaolin GAO( ), Changguo CHEN(
), Changguo CHEN( )
)
Received:2023-09-06
Revised:2023-10-24
Online:2023-11-25
Published:2024-01-22
Contact:
Changguo CHEN
摘要:
化石燃料燃烧排放的大量CO2 造成了全球气候变暖。CO2矿化是近年来CO2末端减排最有效的技术之一。CO2矿化的本质是利用天然碱性矿物或工业碱性固废将酸性CO2气体转化、固定为碳酸盐的过程,但目前所报道的技术大多仍面临高能耗、高成本的限制。提出一种安全、环保、低能耗的空气驱动的膜电解技术,可在低能耗下促使硅灰石有效矿化CO2并产优质多孔白炭黑(二氧化硅)产品。核心技术为:电解条件下,阴极氧气还原反应(ORR)与阳极析氧反应(OER)同时进行实现低能耗下水的电离产生碱性和酸性液体。该电解技术比同电流密度下电解水低至少0.5 V的电解电压。电解所得酸性溶液溶解硅灰石后与电解所得碱性溶液混合可得优质多孔二氧化硅,CO2通入后可被有效吸收并得到矿化产物碳酸钙,实现了高效矿化利用CO2。
中图分类号:
高孝麟, 陈昌国. 空气驱动的膜电解技术促进硅灰石矿化CO2产白炭黑的研究[J]. 化工学报, 2023, 74(11): 4739-4748.
Xiaolin GAO, Changguo CHEN. A study on production of silica from CO2 mineralization by wollastonite promoted via air-driven membrane electrolysis technology[J]. CIESC Journal, 2023, 74(11): 4739-4748.
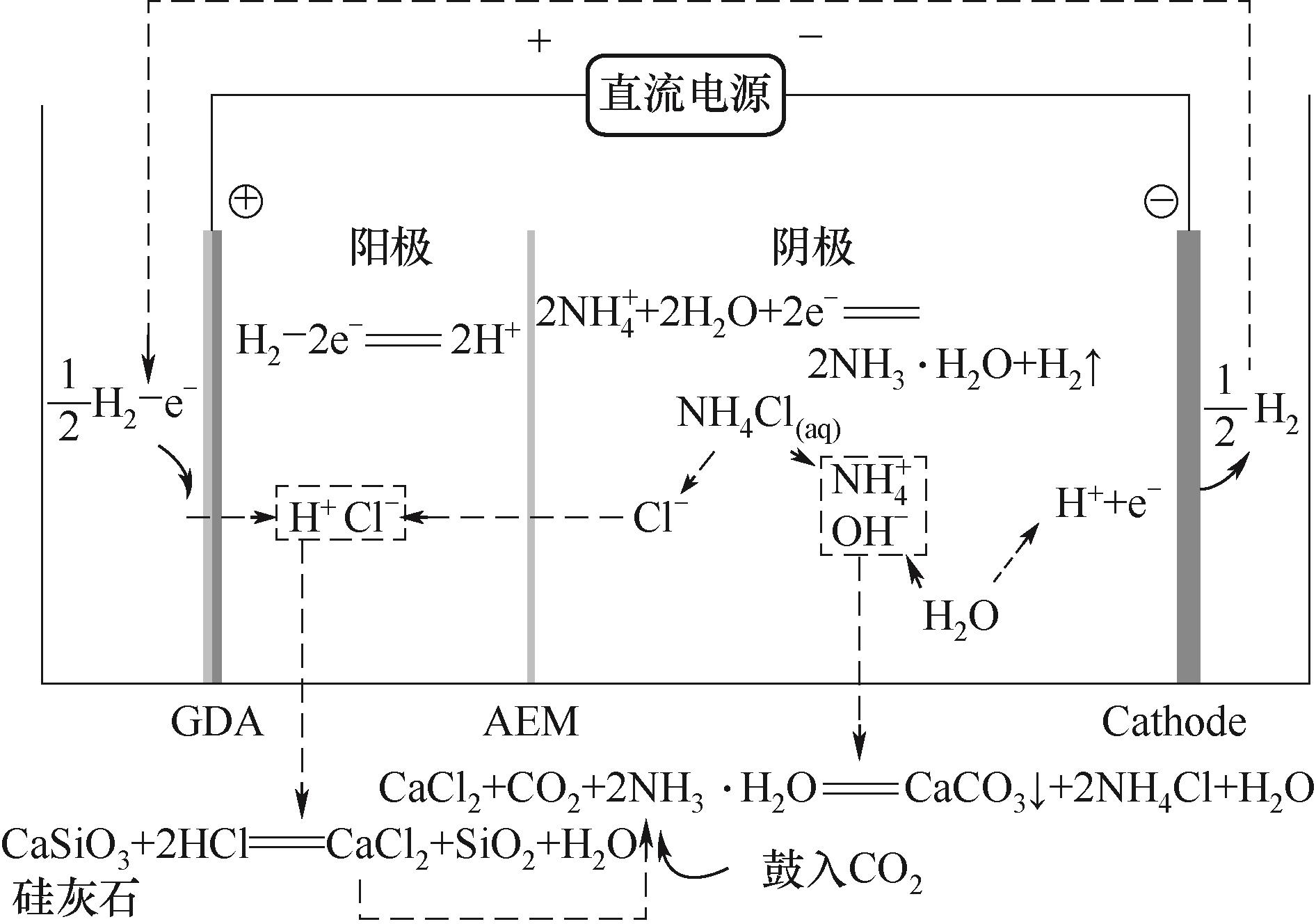
图2 氢气驱动的膜电解技术促进硅灰石矿化利用CO2产白炭黑工作原理[25]
Fig.2 The principle of silica production from CO2 mineralization by wollastonite promoted via H2-driven membrane electrolysis technology[25]
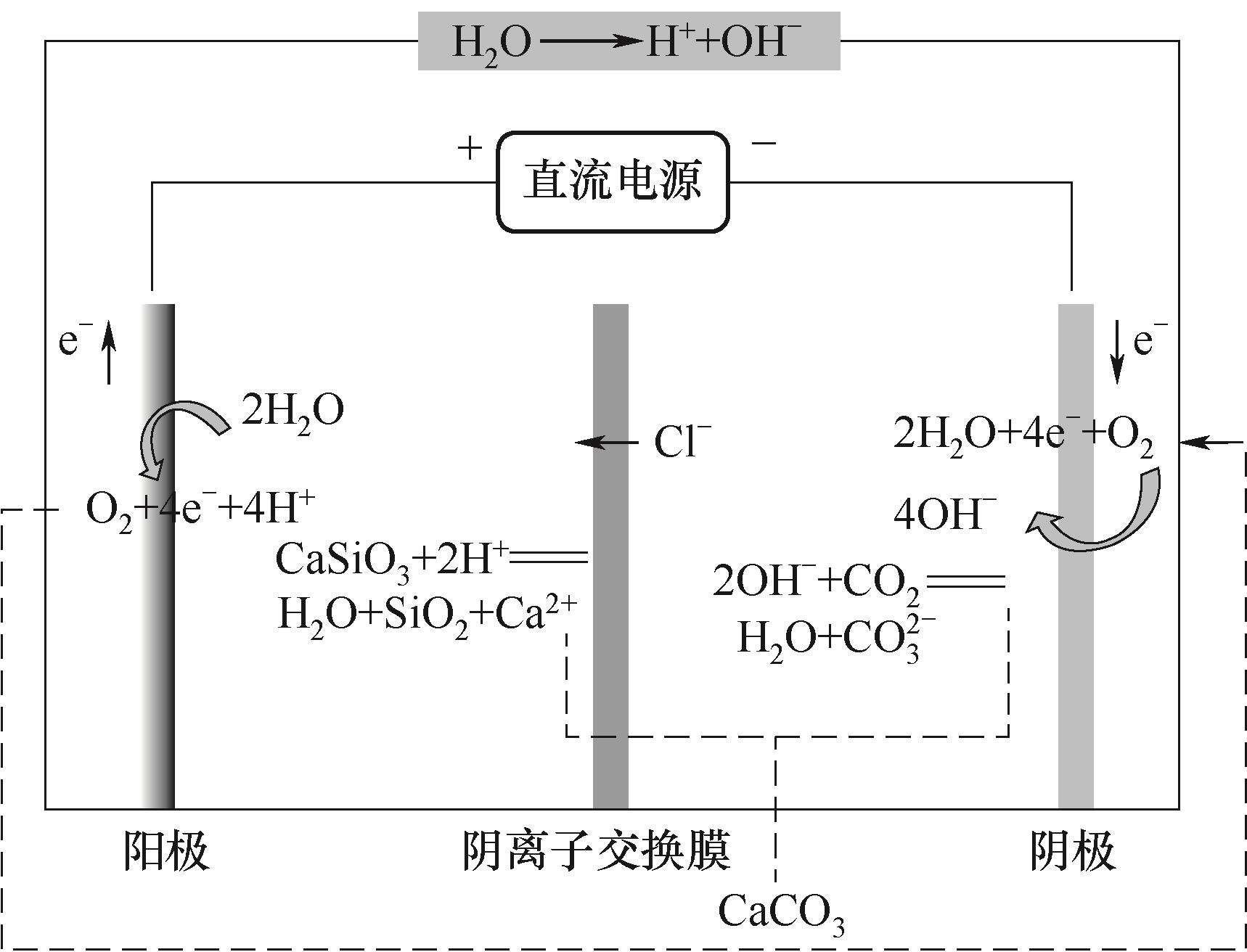
图4 空气驱动的膜电解技术促进硅灰石矿化CO2产白炭黑的原理
Fig.4 The principle of silica production from CO2 mineralization by wollastonite promoted via air-driven membrane electrolysis technology
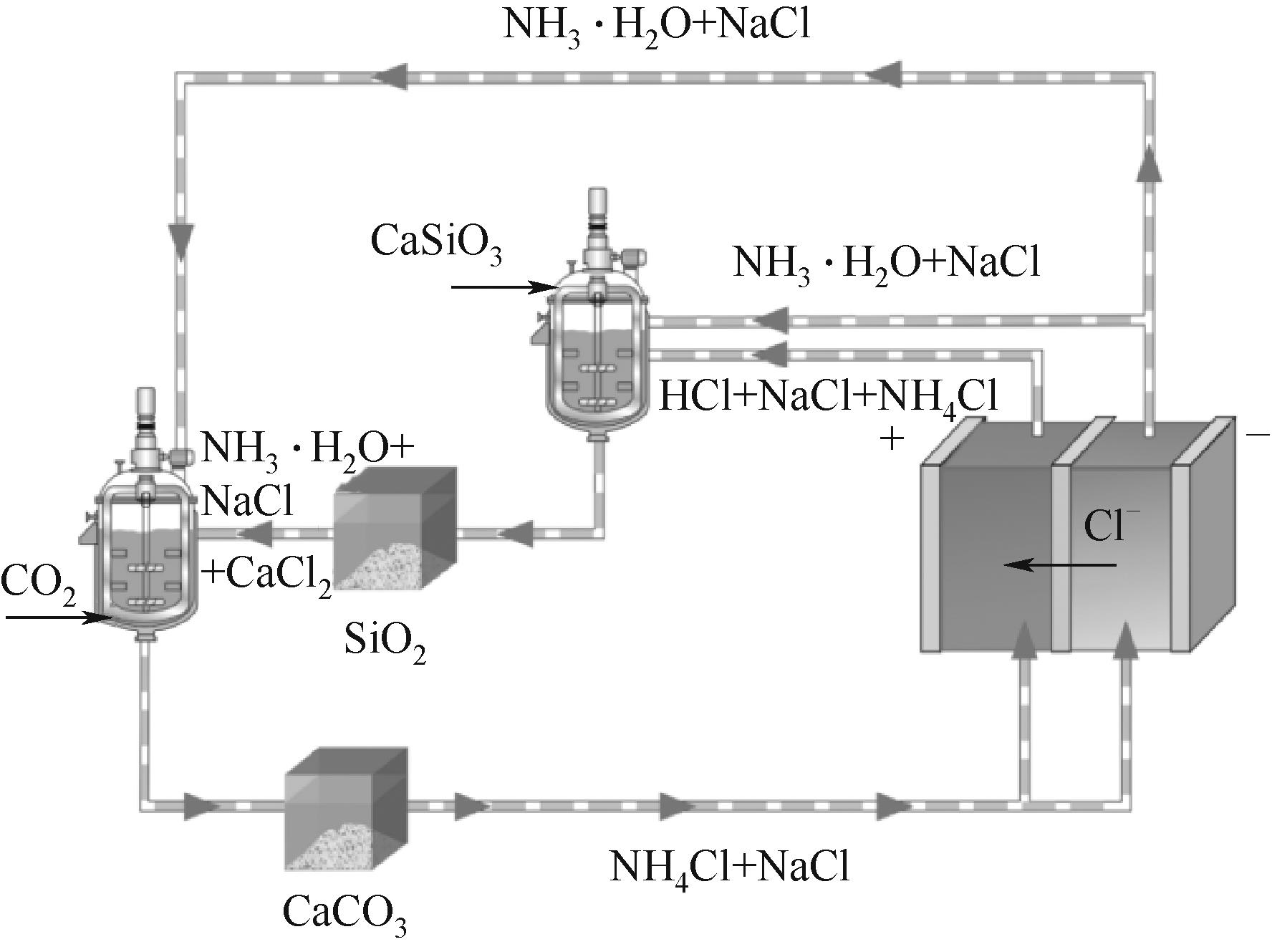
图5 空气驱动的硅灰石矿化利用CO2联产白炭黑工艺路线
Fig.5 The process route of silica production from CO2 mineralization by wollastonite promoted via air-driven membrane electrolysis technology
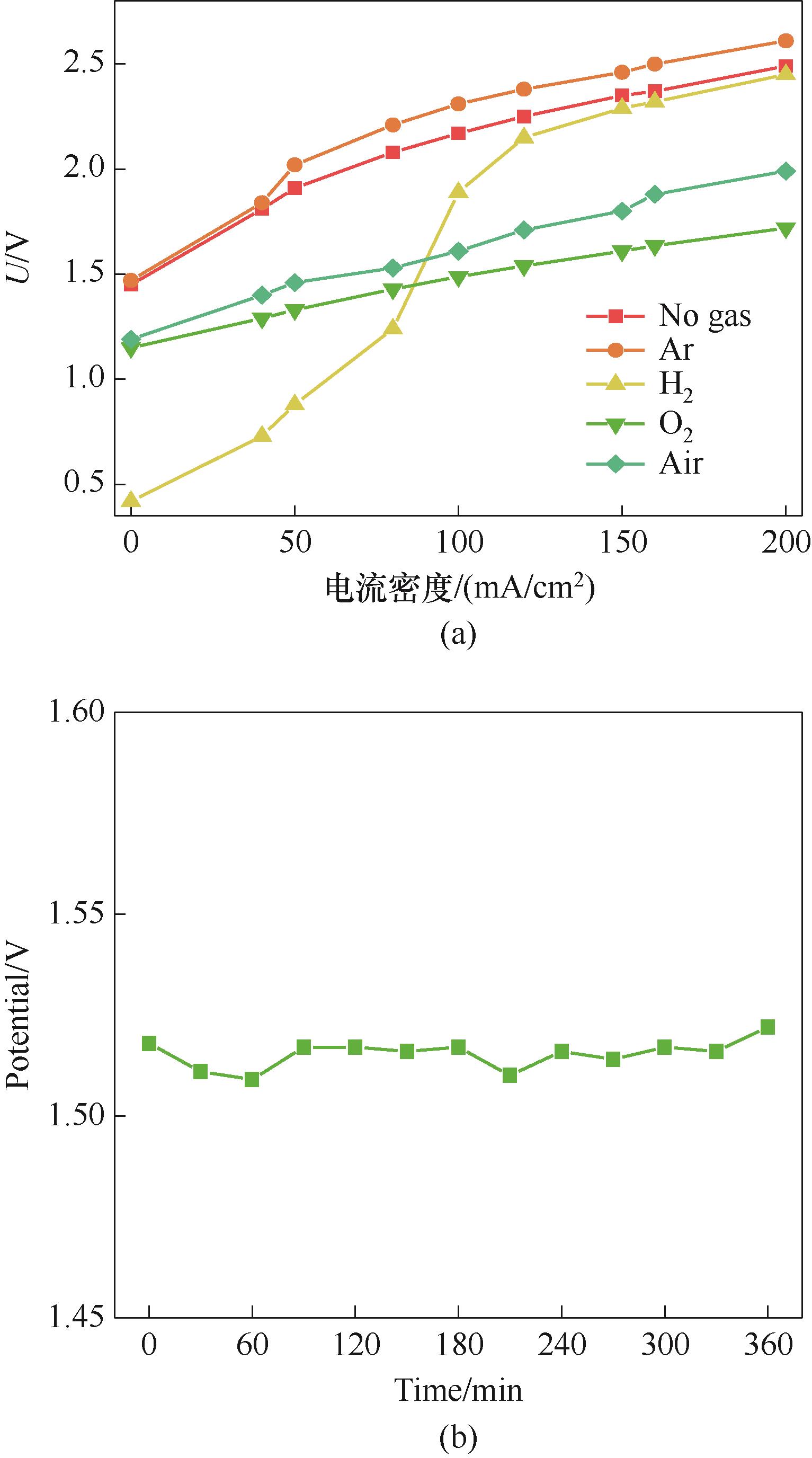
图7 不同气体通入对膜电解的影响(a)及空气驱动的膜电解稳定性运行电压变化(b)
Fig.7 The effect of different gas injected on membrane electrolysis (a) and the voltage change of the air-driven membrane electrolysis stability test (b)
| 电解时间/h | 阴极理论碱浓度/(mol/L) | 阴极实际碱浓度/(mol/L) | 阴极pH | 阴极电解效率/% | 阳极理论酸浓度/(mol/L) | 阳极实际酸浓度/(mol/L) | 阳极pH | 阳极电解效率/% |
|---|---|---|---|---|---|---|---|---|
| 0 | — | -0.0022 | 6.35 | — | — | 0.0022 | 6.35 | — |
| 1 | 0.0309 | 0.02977 | 8.24 | 96.34 | 0.0265 | 0.0263 | 1.86 | 99.25 |
| 2 | 0.0596 | 0.0564 | 8.54 | 94.63 | 0.0552 | 0.0537 | 1.17 | 97.28 |
| 3 | 0.0883 | 0.0804 | 8.69 | 91.05 | 0.0839 | 0.0770 | 1.02 | 93.78 |
| 4 | 0.1170 | 0.1009 | 8.83 | 86.24 | 0.1126 | 0.1002 | 0.95 | 88.99 |
| 5 | 0.1457 | 0.1150 | 8.92 | 78.93 | 0.1414 | 0.1182 | 0.77 | 83.59 |
| 6 | 0.1744 | 0.1218 | 9.01 | 69.84 | 0.1700 | 0.1293 | 0.69 | 76.06 |
表1 电解过程阴阳极酸碱性变化及电解效率
Table 1 Changes of pH of anode and cathode and electrolytic efficiency in electrolytic process
| 电解时间/h | 阴极理论碱浓度/(mol/L) | 阴极实际碱浓度/(mol/L) | 阴极pH | 阴极电解效率/% | 阳极理论酸浓度/(mol/L) | 阳极实际酸浓度/(mol/L) | 阳极pH | 阳极电解效率/% |
|---|---|---|---|---|---|---|---|---|
| 0 | — | -0.0022 | 6.35 | — | — | 0.0022 | 6.35 | — |
| 1 | 0.0309 | 0.02977 | 8.24 | 96.34 | 0.0265 | 0.0263 | 1.86 | 99.25 |
| 2 | 0.0596 | 0.0564 | 8.54 | 94.63 | 0.0552 | 0.0537 | 1.17 | 97.28 |
| 3 | 0.0883 | 0.0804 | 8.69 | 91.05 | 0.0839 | 0.0770 | 1.02 | 93.78 |
| 4 | 0.1170 | 0.1009 | 8.83 | 86.24 | 0.1126 | 0.1002 | 0.95 | 88.99 |
| 5 | 0.1457 | 0.1150 | 8.92 | 78.93 | 0.1414 | 0.1182 | 0.77 | 83.59 |
| 6 | 0.1744 | 0.1218 | 9.01 | 69.84 | 0.1700 | 0.1293 | 0.69 | 76.06 |
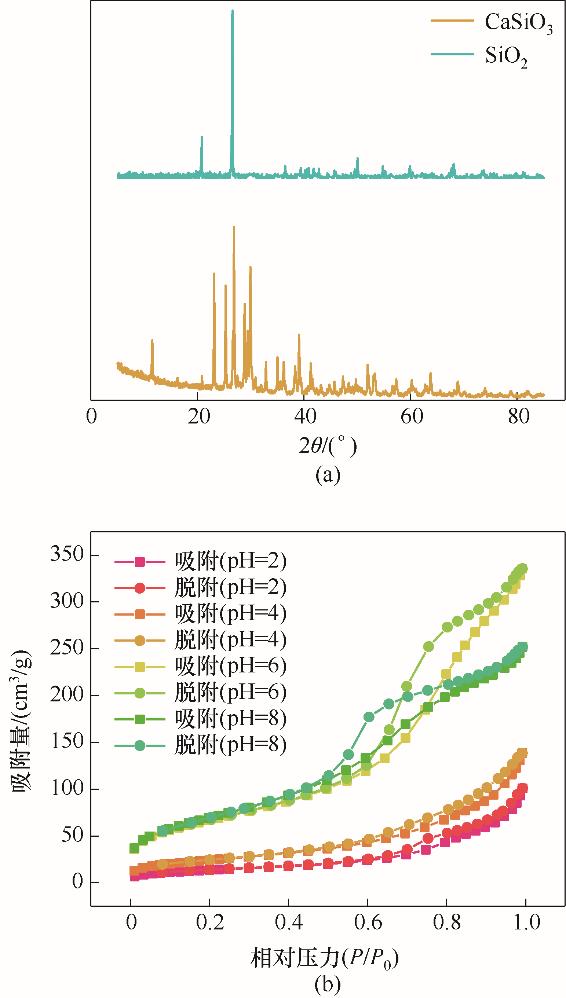
图8 CaSiO3原料与SiO2产品XRD谱图对照(a)及不同pH下得到SiO2产品的BET吸脱附曲线(b)
Fig.8 XRD patterns of the product SiO2 and the raw material CaSiO3 (a) and the BET absorption-desorption curve of SiO2 prepared in different pH (b)
| 制备过程pH | 比表面积/(m2/g) | 平均孔径/nm | 平均微粒尺寸/nm |
|---|---|---|---|
| 2 | 50 | 9.7 | 119.3 |
| 4 | 88 | 8.3 | 64.7 |
| 6 | 241 | 8.0 | 24.8 |
| 8 | 255 | 5.7 | 23.5 |
表2 不同pH下得到SiO2产品的比表面积、孔径和平均微粒尺寸
Table 2 The specific surface area, pore size and average particle size of SiO2 products obtained under different pH
| 制备过程pH | 比表面积/(m2/g) | 平均孔径/nm | 平均微粒尺寸/nm |
|---|---|---|---|
| 2 | 50 | 9.7 | 119.3 |
| 4 | 88 | 8.3 | 64.7 |
| 6 | 241 | 8.0 | 24.8 |
| 8 | 255 | 5.7 | 23.5 |
| 1 | IEA. CO2 emissions from fuel combustion-highlights 2020[R]. Paris: International Energy Agency, 2020. |
| 2 | IEA. CO2 emissions in 2022[R]. Paris: International Energy Agency, 2023. |
| 3 | IPCC. Climate change 2023: synthesis report[R]. Geneva: Intergovernmental Panel on Climate Change, 2023. |
| 4 | Jayanthakumaran K, Verma R, Liu Y. CO2 emissions, energy consumption, trade and income: a comparative analysis of China and India[J]. Energy Policy, 2012, 42: 450-460. |
| 5 | Parekh A, Chaturvedi G, Dutta A. Sustainability analyses of CO2 sequestration and CO2 utilization as competing options for mitigating CO2 emissions[J]. Sustainable Energy Technologies and Assessments, 2023, 55: 102942. |
| 6 | Zickfeld K, Azevedo D, Mathesius S, et al. Asymmetry in the climate-carbon cycle response to positive and negative CO2 emissions[J]. Nature Climate Change, 2021, 11(7): 613-617. |
| 7 | Liu H J, Were P, Li Q, et al. Worldwide status of CCUS technologies and their development and challenges in China[J]. Geofluids, 2017, 2017: 1-25. |
| 8 | Geerlings H, Zevenhoven R. CO2 mineralization—bridge between storage and utilization of CO2 [J]. Annual Review of Chemical and Biomolecular Engineering, 2013, 4: 103-117. |
| 9 | Faruque Hasan M M, First E L, Boukouvala F, et al. A multi-scale framework for CO2 capture, utilization, and sequestration: CCUS and CCU[J]. Computers & Chemical Engineering, 2015, 81: 2-21. |
| 10 | Ostovari H, Müller L, Mayer F, et al. A climate-optimal supply chain for CO2 capture, utilization, and storage by mineralization[J]. Journal of Cleaner Production, 2022, 360: 131750. |
| 11 | Jiang K, Ashworth P, Zhang S Y, et al. China's carbon capture, utilization and storage (CCUS) policy: a critical review[J]. Renewable and Sustainable Energy Reviews, 2020, 119: 109601. |
| 12 | Xie H P, Yue H R, Zhu J H, et al. Scientific and engineering progress in CO2 mineralization using industrial waste and natural minerals[J]. Engineering, 2015, 1(1): 150-157. |
| 13 | Xie H P, Wang Y F, He Y, et al. Generation of electricity from CO2 mineralization: principle and realization[J]. Science China Technological Sciences, 2014, 57(12): 2335-2343. |
| 14 | Wang C, Yue H R, Li C, et al. Mineralization of CO2 using natural K-feldspar and industrial solid waste to produce soluble potassium[J]. Industrial & Engineering Chemistry Research, 2014, 53(19): 7971-7978. |
| 15 | Zhang R X, Arrigoni A, Panesar D. Could reactive MgO cement be a green solution? The effect of CO2 mineralization and manufacturing route on the potential global warming impact[J]. Cement and Concrete Composites, 2021, 124: 104263. |
| 16 | Wang J J, Watanabe N, Inomoto K, et al. Sustainable process for enhanced CO2 mineralization of calcium silicates using a recyclable chelating agent under alkaline conditions[J]. Journal of Environmental Chemical Engineering, 2022, 10(1): 107055. |
| 17 | Scott A, Oze C, Shah V, et al. Transformation of abundant magnesium silicate minerals for enhanced CO2 sequestration[J]. Communications Earth & Environment, 2021, 2: 25. |
| 18 | Wang F, Dreisinger D. Status of CO2 mineralization and its utilization prospects[J]. Minerals and Mineral Materials, 2022, 1: 4. |
| 19 | Xu H, van Deventer J S J. The geopolymerisation of alumino-silicate minerals[J]. International Journal of Mineral Processing, 2000, 59(3): 247-266. |
| 20 | Yip C K, Lukey G C, Provis J L, et al. Effect of calcium silicate sources on geopolymerisation[J]. Cement and Concrete Research, 2008, 38(4): 554-564. |
| 21 | Flanagan. 2015 minerals yearbook. Asbestos [advance release][R]. Reston: U.S. Geological Survey, 2016. |
| 22 | Zhang S, DePaolo D J. Rates of CO2 mineralization in geological carbon storage[J]. Accounts of Chemical Research, 2017, 50(9): 2075-2084. |
| 23 | Gadikota G, Matter J, Kelemen P, et al. Elucidating the differences in the carbon mineralization behaviors of calcium and magnesium bearing alumino-silicates and magnesium silicates for CO2 storage[J]. Fuel, 2020, 277: 117900. |
| 24 | Ghoorah M, Dlugogorski B Z, Balucan R D, et al. Selection of acid for weak acid processing of wollastonite for mineralisation of CO2 [J]. Fuel, 2014, 122: 277-286. |
| 25 | Xie H P, Wang F H, Wang Y F, et al. CO2 mineralization of natural wollastonite into porous silica and CaCO3 powders promoted via membrane electrolysis[J]. Environmental Earth Sciences, 2018, 77(4): 149. |
| 26 | Rashid M M, Al Mesfer M K, Naseem H, et al. Hydrogen production by water electrolysis: a review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis[J]. International Journal of Engineering and Advanced Technology(IJEAT), 2015, 4(3): 80-93. |
| 27 | Chandran P, Bakshi S, Chatterjee D. Study on the characteristics of hydrogen bubble formation and its transport during electrolysis of water[J]. Chemical Engineering Science, 2015, 138: 99-109. |
| 28 | Christensen J, Albertus P, Sanchez-Carrera R S, et al. A critical review of Li/air batteries[J]. Journal of the Electrochemical Society, 2011, 159(2): R1-R30. |
| 29 | Olabi A G, Sayed E T, Wilberforce T, et al. Metal-air batteries—a review[J]. Energies, 2021, 14(21): 7373. |
| 30 | Pan J, Xu Y Y, Yang H A, et al. Advanced architectures and relatives of air electrodes in Zn-air batteries[J]. Advanced Science, 2018, 5(4): 1700691. |
| 31 | Ahn I K, Joo W, Lee J H, et al. Metal-organic framework-driven porous cobalt disulfide nanoparticles fabricated by gaseous sulfurization as bifunctional electrocatalysts for overall water splitting[J]. Scientific Reports, 2019, 9(1): 19539. |
| 32 | Reier T, Oezaslan M, Strasser P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: a comparative study of nanoparticles and bulk materials[J]. ACS Catalysis, 2012, 2(8): 1765-1772. |
| 33 | Zhang K X, Zou R Q. Advanced transition metal-based OER electrocatalysts: current status, opportunities, and challenges[J]. Small, 2021, 17(37): 2100129. |
| 34 | Su P P, Pei W, Wang X W, et al. Exceptional electrochemical HER performance with enhanced electron transfer between Ru nanoparticles and single atoms dispersed on a carbon substrate[J]. Angewandte Chemie, 2021, 133(29): 16180-16186. |
| 35 | Yu Q M, Zhang Z Y, Qiu S Y, et al. A Ta-TaS2 monolith catalyst with robust and metallic interface for superior hydrogen evolution[J]. Nature Communications, 2021, 12(1): 6051. |
| 36 | Sadeghi E, Peighambardoust N S, Khatamian M, et al. Metal doped layered MgB2 nanoparticles as novel electrocatalysts for water splitting[J]. Scientific Reports, 2021, 11: 3337. |
| 37 | Lazaro A, Brouwers H J H, Quercia G, et al. The properties of amorphous nano-silica synthesized by the dissolution of olivine[J]. Chemical Engineering Journal, 2012, 211/212: 112-121. |
| 38 | 陈天虎. 硅灰石酸溶法制取白炭黑工艺研究[J]. 非金属矿, 1995, 18(3): 45-46, 59. |
| Chen T H. Study on preparation of white carbon black by acid dissolution of wollastonite[J]. Non-Metallic Mines, 1995, 18(3): 45-46, 59. | |
| 39 | Crundwell F K. The mechanism of dissolution of forsterite, olivine and minerals of the orthosilicate group[J]. Hydrometallurgy, 2014, 150: 68-82. |
| 40 | Oelkers E H, Declercq J, Saldi G D, et al. Olivine dissolution rates: a critical review[J]. Chemical Geology, 2018, 500: 1-19. |
| 41 | Diao Y F, Zheng X Y, He B S, et al. Experimental study on capturing CO2 greenhouse gas by ammonia scrubbing[J]. Energy Conversion and Management, 2004, 45(13/14): 2283-2296. |
| 42 | Bai H, Yeh A C. Removal of CO2 greenhouse gas by ammonia scrubbing[J]. Industrial & Engineering Chemistry Research, 1997, 36(6): 2490-2493. |
| 43 | Li X N, Hagaman E, Tsouris C, et al. Removal of carbon dioxide from flue gas by ammonia carbonation in the gas phase[J]. Energy & Fuels, 2003, 17(1): 69-74. |
| 44 | 张昀, 李振中, 李成之, 等. 电站烟气中CO2减排新技术双重效益的研究[J]. 现代电力, 2002, 19(3): 14-15. |
| Zhang Y, Li Z Z, Li C Z, et al. Dual benefits of a new CO2 sequestration technology from fossil fuel power plants[J]. Modern Electric Power, 2002, 19(3): 14-15. |
| [1] | 黄琮琪, 吴一梅, 陈建业, 邵双全. 碱性电解水制氢装置热管理系统仿真研究[J]. 化工学报, 2023, 74(S1): 320-328. |
| [2] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [3] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [4] | 康飞, 吕伟光, 巨锋, 孙峙. 废锂离子电池放电路径与评价研究[J]. 化工学报, 2023, 74(9): 3903-3911. |
| [5] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [6] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [7] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [8] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [9] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [10] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [11] | 尹刚, 李伊惠, 何飞, 曹文琦, 王民, 颜非亚, 向禹, 卢剑, 罗斌, 卢润廷. 基于KPCA和SVM的铝电解槽漏槽事故预警方法[J]. 化工学报, 2023, 74(8): 3419-3428. |
| [12] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [13] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [14] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [15] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号