化工学报 ›› 2024, Vol. 75 ›› Issue (2): 395-411.DOI: 10.11949/0438-1157.20230900
收稿日期:2023-08-31
修回日期:2023-12-12
出版日期:2024-02-25
发布日期:2024-04-10
通讯作者:
程芳琴
作者简介:王宝凤(1977—),女,博士,教授,wangbaofeng@sxu.edu.cn
基金资助:
Baofeng WANG( ), Shugao WANG, Fangqin CHENG(
), Shugao WANG, Fangqin CHENG( )
)
Received:2023-08-31
Revised:2023-12-12
Online:2024-02-25
Published:2024-04-10
Contact:
Fangqin CHENG
摘要:
含碳固废来源广、产量大,其大量堆存严重制约了环境可持续发展,因此含碳固废资源化利用意义重大。利用含碳固废制备多孔炭材料是其清洁高效利用的重要方式之一。对多孔炭进行硫原子掺杂不仅可使材料表面的亲水性得到改善,还可以改变材料表面的化学异质性,生成有利于CO2捕集的活性位点,强化材料对CO2分子的吸附作用,从而提高其CO2吸附容量。简述了固废基硫掺杂多孔炭材料的制备方法,总结了硫掺杂多孔炭材料用于CO2吸附的最新研究进展,并对硫掺杂多孔炭材料未来发展趋势及其在CO2吸附领域的工业化应用进行了展望。
中图分类号:
王宝凤, 王术高, 程芳琴. 固废基硫掺杂多孔炭材料制备及其对CO2吸附性能研究进展[J]. 化工学报, 2024, 75(2): 395-411.
Baofeng WANG, Shugao WANG, Fangqin CHENG. Progress in preparation and CO2 adsorption properties of solid waste-based sulfur-doped porous carbon materials[J]. CIESC Journal, 2024, 75(2): 395-411.
| 制备方法 | 反应温度/℃ | 优势 | 不足之处 | 硫掺杂多孔炭特性 | 文献 |
|---|---|---|---|---|---|
| 热解法 | 300~1000 | 操作简单、成本低 | 所需温度高、能耗大 | 掺杂量可控 | [ |
| 水热碳化法 | 160~280 | 绿色环保、原料适用广 | 反应时间长、存在高压风险 | 碳化程度低;比表面积较小 | [ |
| 活化法 | 600~1200 | 活化剂可选、制备成本低 | 腐蚀设备、污染环境 | 表面官能团丰富;比表面积大 | [ |
| 后处理掺杂法 | 取决于掺杂步骤 | 适用范围广、掺杂量易控 | 需要额外工艺、步骤复杂 | 掺杂不均匀 | [ |
表1 硫掺杂多孔炭材料制备方法比较
Table 1 Comparison of preparation methods of sulfur-doped porous carbon materials
| 制备方法 | 反应温度/℃ | 优势 | 不足之处 | 硫掺杂多孔炭特性 | 文献 |
|---|---|---|---|---|---|
| 热解法 | 300~1000 | 操作简单、成本低 | 所需温度高、能耗大 | 掺杂量可控 | [ |
| 水热碳化法 | 160~280 | 绿色环保、原料适用广 | 反应时间长、存在高压风险 | 碳化程度低;比表面积较小 | [ |
| 活化法 | 600~1200 | 活化剂可选、制备成本低 | 腐蚀设备、污染环境 | 表面官能团丰富;比表面积大 | [ |
| 后处理掺杂法 | 取决于掺杂步骤 | 适用范围广、掺杂量易控 | 需要额外工艺、步骤复杂 | 掺杂不均匀 | [ |
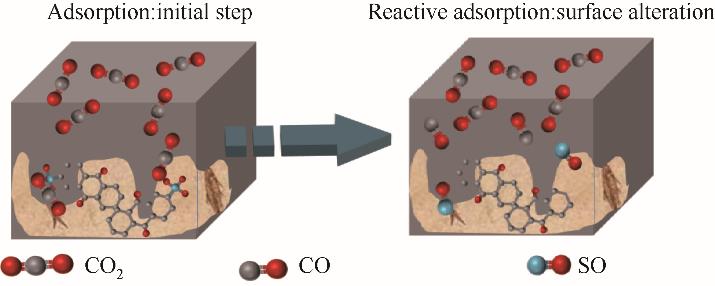
图5 硫掺杂多孔炭/rGO复合材料吸附CO2时的表面变化示意图[132]
Fig.5 A schematic diagram of the surface changes of sulfur-doped porous carbon / rGO composites when adsorbing CO2[132]
| 样品 | 硫含量/%(质量分数) | 比表面积/(cm3/g) | 总孔容/(cm3/g) | 测试条件 | CO2最大捕集量/(mmol/g) | 文献 |
|---|---|---|---|---|---|---|
| SCEMC | 6.56 | 1627 | 0.90 | 25℃,1 bar | 2.46 | [ |
| CSI-800 ox | 7.80 | 1893 | 1.16 | 20℃,1 bar | 2.90 | [ |
| CS-1 | 8.2 | 2865 | 2.3 | 25℃,1 bar | 2.06 | [ |
| S-CX-4-700-KOH | 1.23 | 2051 | 1.10 | 0℃,1 bar | 5.37 | [ |
| NSCS-4-700 | 2.93 | 1757 | 0.813 | 25℃,8bar | 11.68 | [ |
| CS2 | 2.4 | 979 | 0.47 | 0℃,1 bar | 5.63 | [ |
| NSPC-1-650 | 1.48 | 1927 | 0.36 | 25℃,5 bar | 5.56 | [ |
| C3800 | 4.42 | 1631 | 1.08 | 25℃,20 bar | 12.9 | [ |
| SNPC-F127-1 | 0.14 | 1512 | 0.71 | 25℃,1 bar | 4.29 | [ |
| NSOPC-1 | 0.26 | 1292 | 0.68 | 25℃,1 bar | 3.88 | [ |
| PFC-S | 2.89 | 488 | 0.36 | 30℃,0.125 bar | 1.25 | [ |
| LS-3 | 1 | 3626 | 1.741 | 25℃,757 Torr | 10.89 | [ |
| NSDCS2700 | 4.4 | 2125 | 0.97 | 0℃,1 bar | 5.6 | [ |
| GT-550-3 | 1.21 | 1213 | 0.48 | 25℃,1 bar | 5.54 | [ |
| NSPC-2-500 | 1.63 | 1131 | 0.62 | 25℃,1 bar | 3.73 | [ |
| HS-550-3 | 0.42 | 1600 | 0.61 | 25℃,1 bar | 4.30 | [ |
| WT-550-3 | 0.45 | 1729 | 0.69 | 25℃,1 bar | 4.31 | [ |
| NSAC-(1∶1)500(1) | 0.2 | 1800 | 0.98 | 0℃,1 bar | 7.02 | [ |
| PK3800 | 6.16 | 1673 | 0.76 | 25℃,20 bar | 17.29 | [ |
| NSPC-600-2.5 | 0.71 | 1412 | 0.77 | 25℃,1 bar | 5.51 | [ |
| ZTC-NS | 1.5 | 1006.5 | 0.556 | 25℃,1 bar | 6.03 | [ |
| S-PC-1 | 1.67 | 997 | 0.46 | 0℃,1 bar | 5.13 | [ |
| SSA1000 | 8.4 | 658.08 | 0.29 | 25℃,0.15 bar | 0.95 | [ |
表2 不同硫掺杂多孔炭对CO2的吸附性能
Table 2 Summary of CO2 adsorption performane of different sulfur-doped porous carbons
| 样品 | 硫含量/%(质量分数) | 比表面积/(cm3/g) | 总孔容/(cm3/g) | 测试条件 | CO2最大捕集量/(mmol/g) | 文献 |
|---|---|---|---|---|---|---|
| SCEMC | 6.56 | 1627 | 0.90 | 25℃,1 bar | 2.46 | [ |
| CSI-800 ox | 7.80 | 1893 | 1.16 | 20℃,1 bar | 2.90 | [ |
| CS-1 | 8.2 | 2865 | 2.3 | 25℃,1 bar | 2.06 | [ |
| S-CX-4-700-KOH | 1.23 | 2051 | 1.10 | 0℃,1 bar | 5.37 | [ |
| NSCS-4-700 | 2.93 | 1757 | 0.813 | 25℃,8bar | 11.68 | [ |
| CS2 | 2.4 | 979 | 0.47 | 0℃,1 bar | 5.63 | [ |
| NSPC-1-650 | 1.48 | 1927 | 0.36 | 25℃,5 bar | 5.56 | [ |
| C3800 | 4.42 | 1631 | 1.08 | 25℃,20 bar | 12.9 | [ |
| SNPC-F127-1 | 0.14 | 1512 | 0.71 | 25℃,1 bar | 4.29 | [ |
| NSOPC-1 | 0.26 | 1292 | 0.68 | 25℃,1 bar | 3.88 | [ |
| PFC-S | 2.89 | 488 | 0.36 | 30℃,0.125 bar | 1.25 | [ |
| LS-3 | 1 | 3626 | 1.741 | 25℃,757 Torr | 10.89 | [ |
| NSDCS2700 | 4.4 | 2125 | 0.97 | 0℃,1 bar | 5.6 | [ |
| GT-550-3 | 1.21 | 1213 | 0.48 | 25℃,1 bar | 5.54 | [ |
| NSPC-2-500 | 1.63 | 1131 | 0.62 | 25℃,1 bar | 3.73 | [ |
| HS-550-3 | 0.42 | 1600 | 0.61 | 25℃,1 bar | 4.30 | [ |
| WT-550-3 | 0.45 | 1729 | 0.69 | 25℃,1 bar | 4.31 | [ |
| NSAC-(1∶1)500(1) | 0.2 | 1800 | 0.98 | 0℃,1 bar | 7.02 | [ |
| PK3800 | 6.16 | 1673 | 0.76 | 25℃,20 bar | 17.29 | [ |
| NSPC-600-2.5 | 0.71 | 1412 | 0.77 | 25℃,1 bar | 5.51 | [ |
| ZTC-NS | 1.5 | 1006.5 | 0.556 | 25℃,1 bar | 6.03 | [ |
| S-PC-1 | 1.67 | 997 | 0.46 | 0℃,1 bar | 5.13 | [ |
| SSA1000 | 8.4 | 658.08 | 0.29 | 25℃,0.15 bar | 0.95 | [ |
| 1 | Pang Z F, Jiang S K, Zhu C Y, et al. Mass transfer of chemical absorption of CO2 in a serpentine minichannel[J]. Chemical Engineering Journal, 2021, 414: 128791. |
| 2 | Eskandari M, Khaksar S A N, Keshavarz P. CO2 absorption using benzylamine as absorbent and promoter in a hollow fiber membrane contactor: a numerical study[J]. Journal of CO2 Utilization, 2022, 66: 102287. |
| 3 | Yuan J C, Wang Y, Tang M F, et al. Preparation of N, O co-doped carbon nanotubes and activated carbon composites with hierarchical porous structure for CO2 adsorption by coal pyrolysis[J]. Fuel, 2023, 333: 126465. |
| 4 | Dehkordi S S R, Delavar Q, Ebrahim H A, et al. CO2 adsorption by coal-based activated carbon modified with sodium hydroxide[J]. Materials Today Communications, 2022, 33: 104776. |
| 5 | Liu B C, Qiao Y S, Li Q, et al. CO2 separation from CO2-EOR associated gas using hollower fiber membranes: a process design and simulation study[J]. Journal of Natural Gas Science and Engineering, 2022, 100: 104451. |
| 6 | Tu Z H, Shi M Z, Zhang X M, et al. Selective membrane separation of CO2 using novel epichlorohydrin-amine-based crosslinked protic ionic liquids: crosslinking mechanism and enhanced salting-out effect[J]. Journal of CO2 Utilization, 2021, 46: 101473. |
| 7 | Shen M H, Tong L G, Yin S W, et al. Cryogenic technology progress for CO2 capture under carbon neutrality goals: a review[J]. Separation and Purification Technology, 2022, 299: 121734. |
| 8 | Yousef A M, El-Maghlany W M, Eldrainy Y A, et al. New approach for biogas purification using cryogenic separation and distillation process for CO2 capture[J]. Energy, 2018, 156: 328-351. |
| 9 | Shi J S, Cui H, Xu J G, et al. Synthesis of nitrogen and sulfur co-doped carbons with chemical blowing method for CO2 adsorption[J]. Fuel, 2021, 305: 121505. |
| 10 | Yan H Y, Zhang G J, Xu Y, et al. High CO2 adsorption on amine-functionalized improved macro-/ mesoporous multimodal pore silica[J]. Fuel, 2022, 315: 123195. |
| 11 | Wawrzyńczak D, Panowski M, Majchrzak-Kucęba I. Possibilities of CO2 purification coming from oxy-combustion for enhanced oil recovery and storage purposes by adsorption method on activated carbon[J]. Energy, 2019, 180: 787-796. |
| 12 | Wei R P, Dai X C, Shi F. Enhanced CO2 adsorption on nitrogen-doped carbon materials by salt and base co-activation method[J]. Materials, 2019, 12(8): 1207. |
| 13 | Liu H R, Wang S Y, Wang X Q, et al. A stable solid amine adsorbent with interconnected open-cell structure for rapid CO2 adsorption and CO2/CH4 separation[J]. Energy, 2022, 258: 124899. |
| 14 | Yang H Y, Wang X Z, Liu J, et al. Amine-impregnated polymeric resin with high CO2 adsorption capacity for biogas upgrading[J]. Chemical Engineering Journal, 2022, 430: 132899. |
| 15 | Wang Y S, Du T, Qiu Z Y, et al. CO2 adsorption on polyethylenimine-modified ZSM-5 zeolite synthesized from rice husk ash[J]. Materials Chemistry and Physics, 2018, 207: 105-113. |
| 16 | Kodasma R, Fermoso J, Sanna A. Li-LSX-zeolite evaluation for post-combustion CO2 capture[J]. Chemical Engineering Journal, 2019, 358: 1351-1362. |
| 17 | Karka S, Kodukula S, Nandury S V, et al. Polyethylenimine-modified zeolite 13X for CO2 capture: adsorption and kinetic studies[J]. ACS Omega, 2019, 4(15): 16441-16449. |
| 18 | Danish M, Parthasarthy V, Al Mesfer M K. CO2 capture by low-cost date pits-based activated carbon and silica gel[J]. Materials, 2021, 14(14): 3885. |
| 19 | Sladekova K, Campbell C, Grant C, et al. The effect of atomic point charges on adsorption isotherms of CO2 and water in metal organic frameworks[J]. Adsorption, 2020, 26(5): 663-685. |
| 20 | 张所瀛, 刘红, 刘朋飞, 等. 金属有机骨架材料在CO2/CH4吸附分离中的研究进展[J]. 化工学报, 2014, 65(5): 1563-1570. |
| Zhang S Y, Liu H, Liu P F, et al. Progress of adsorption-based CO2/CH4 separation by metal organic frameworks[J]. CIESC Journal, 2014, 65(5): 1563-1570. | |
| 21 | Khan J, Iqbal N, Asghar A, et al. Novel amine functionalized metal organic framework synthesis for enhanced carbon dioxide capture[J]. Materials Research Express, 2019, 6(10): 105539. |
| 22 | Shin G J, Rhee K, Park S J. Improvement of CO2 capture by graphite oxide in presence of polyethylenimine[J]. International Journal of Hydrogen Energy, 2016, 41(32): 14351-14359. |
| 23 | Tiwari D, Goel C, Bhunia H, et al. Melamine-formaldehyde derived porous carbons for adsorption of CO2 capture[J]. Journal of Environmental Management, 2017, 197: 415-427. |
| 24 | Chiang Y C, Chin W T, Huang C C. The application of hollow carbon nanofibers prepared by electrospinning to carbon dioxide capture[J]. Polymers, 2021, 13(19): 3275. |
| 25 | Singh G, Lakhi K S, Sil S, et al. Biomass derived porous carbon for CO2 capture[J]. Carbon, 2019, 148: 164-186. |
| 26 | Wu Z X, Webley P A, Zhao D Y. Post-enrichment of nitrogen in soft-templated ordered mesoporous carbon materials for highly efficient phenol removal and CO2 capture[J]. Journal of Materials Chemistry, 2012, 22(22): 11379. |
| 27 | Hao G P, Mondin G, Zheng Z K, et al. Unusual ultra-hydrophilic, porous carbon cuboids for atmospheric-water capture[J]. Angewandte Chemie International Edition, 2015, 54(6): 1941-1945. |
| 28 | Oschatz M, Antonietti M. A search for selectivity to enable CO2 capture with porous adsorbents[J]. Energy & Environmental Science, 2018, 11(1): 57-70. |
| 29 | Tang S Y, Wang Y S, Yuan Y F, et al. Hydrophilic carbon monoliths derived from metal-organic frameworks@resorcinol-formaldehyde resin for atmospheric water harvesting[J]. New Carbon Materials, 2022, 37(1): 237-244. |
| 30 | Zhu W F, Wang Y Q, Yao F, et al. One-pot synthesis of N-doped petroleum coke-based microporous carbon for high-performance CO2 adsorption and supercapacitors[J]. Journal of Environmental Sciences (China), 2024, 139: 93-104. |
| 31 | Sun H Q, Zhou G L, Wang Y X, et al. A new metal-free carbon hybrid for enhanced photocatalysis[J]. ACS Applied Materials & Interfaces, 2014, 6(19): 16745-16754. |
| 32 | Wang Y F, Suo Y G, Xu Y S, et al. Enhancing CO2 adsorption performance of porous nitrogen-doped carbon materials derived from ZIFs: insights into pore structure and surface chemistry[J]. Separation and Purification Technology, 2024, 335: 126117. |
| 33 | Luo J Y, Liu B G, Shi R, et al. The effects of nitrogen functional groups and narrow micropore sizes on CO2 adsorption onto N-doped biomass-based porous carbon under different pressure[J]. Microporous and Mesoporous Materials, 2021, 327: 111404. |
| 34 | Cui H M, Xu J G, Shi J S, et al. Synthesis of sulfur doped carbon from dipotassium anthraquinone-1, 8-disulfonate for CO2 adsorption[J]. Journal of CO2 Utilization, 2021, 50: 101582. |
| 35 | Zhang C C, Huang M Y, Zhong S, et al. Controllable construction of boron and nitrogen co-doping honeycomb porous carbon as promising materials for CO2 capture and supercapacitors[J]. Journal of Energy Storage, 2022, 55: 105687. |
| 36 | Wu R, Hang Y P, Li J H, et al. Preparation of biomass-derived phosphorus-doped microporous carbon material and its application in dye adsorption and CO2 capture[J]. Surface and Interface Analysis, 2022, 54(8): 881-891. |
| 37 | Medha S, Romisher Z, van Bramer S, et al. Enhanced adsorption of perfluorooctanesulfonic acid (PFOS) in fluorine doped mesoporous carbon: experiment and simulation[J]. Carbon, 2024, 218: 118745. |
| 38 | dos Reis G S, Thivet J, Laisné E, et al. Synthesis of novel mesoporous selenium-doped biochar with high-performance sodium diclofenac and reactive orange 16 dye removals[J]. Chemical Engineering Science, 2023, 281: 119129. |
| 39 | Cao Y L, Mao S J, Li M M, et al. Metal/porous carbon composites for heterogeneous catalysis: old catalysts with improved performance promoted by N-doping[J]. ACS Catalysis, 2017, 7(12): 8090-8112. |
| 40 | Wang W, Wang P P, Wu C, et al. Adsorption of acetochlor-contaminated water systems using novel P-doped biochar: effects, application, and mechanism[J]. Chemosphere, 2024, 350: 141027. |
| 41 | Ye J Q, Zhao H Q, Song W, et al. Enhanced electronic conductivity and sodium-ion adsorption in N/S co-doped ordered mesoporous carbon for high-performance sodium-ion battery anode[J]. Journal of Power Sources, 2019, 412: 606-614. |
| 42 | Ma G X, Ning G Q, Wei Q. S-doped carbon materials: synthesis, properties and applications[J]. Carbon, 2022, 195: 328-340. |
| 43 | Paraknowitsch J P, Thomas A, Schmidt J. Microporous sulfur-doped carbon from thienyl-based polymer network precursors[J]. Chemical Communications, 2011, 47(29): 8283-8285. |
| 44 | Paraknowitsch J P, Thomas A. Doping carbons beyond nitrogen: an overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications[J]. Energy & Environmental Science, 2013, 6(10): 2839-2855. |
| 45 | Li M, Wang B, Yang M Q, et al. Promoting mercury removal from desulfurization slurry via S-doped carbon nitride/graphene oxide 3D hierarchical framework[J]. Separation and Purification Technology, 2020, 239: 116515. |
| 46 | Liu Y, Wang J X, Wang T, et al. Removing mercury from flue gas by sulfur-doped zeolite-templated carbon: synthesize and adsorption mechanism[J]. Separation and Purification Technology, 2022, 294: 121228. |
| 47 | Wang M W, Su K M, Zhang M L, et al. Advanced trifunctional electrocatalysis with Cu-, N-, S-doped defect-rich porous carbon for rechargeable Zn-air batteries and self-driven water splitting[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(39): 13324-13336. |
| 48 | Balasubramanian P, Jansirani A, He S B, et al. Rational construction of N, S-doped carbon wrapped MnFe2O4 nanospheres with copious oxygen deficiency as extremely efficient and robust electrocatalyst for urea electrocatalysis[J]. Journal of Power Sources, 2021, 494: 229757. |
| 49 | Liang Z J, Peng Y H, Zhang X, et al. Sulfur-doped CMK-5 with expanded lattice for high-performance lithium ion batteries[J]. Chinese Chemical Letters, 2023, 34(7): 108054. |
| 50 | Zhao L, Ning G Q, Zhang S C. Green synthesis of S-doped carbon nanotubes via gaseous post-treatment and their application as conductive additive in Li ion batteries[J]. Carbon, 2021, 179: 425-434. |
| 51 | Huang Z Y, Jiang J J, Li W Y, et al. Stabilizing sulfur doped manganese oxide active sites with phosphorus doped hierarchical nested square carbon for efficient asymmetric supercapacitor[J]. Chemical Engineering Journal, 2023, 468: 143574. |
| 52 | Zhao G Y, Yu D F, Zhang H, et al. Sulphur-doped carbon nanosheets derived from biomass as high-performance anode materials for sodium-ion batteries[J]. Nano Energy, 2020, 67: 104219. |
| 53 | Yang X Y, Wang B F, Song X T, et al. Co-hydrothermal carbonization of sewage sludge and coal slime with sulfuric acid for N, S doped hydrochar[J]. Journal of Cleaner Production, 2022, 354: 131615. |
| 54 | Bai J L, Huang J M, Yu Q Y, et al. One-pot synthesis of self S-doped porous carbon for efficient CO2 adsorption[J]. Fuel Processing Technology, 2023, 244: 107700. |
| 55 | Wang H, Bo X, Zhang Y F, et al. Sulfur-doped ordered mesoporous carbon with high electrocatalytic activity for oxygen reduction[J]. Electrochimica Acta, 2013, 108: 404-411. |
| 56 | Yang S B, Zhi L J, Tang K, et al. Efficient synthesis of heteroatom (N or S)-doped graphene based on ultrathin graphene oxide-porous silica sheets for oxygen reduction reactions[J]. Advanced Functional Materials, 2012, 22(17): 3634-3640. |
| 57 | Poh H L, Šimek P, Sofer Z, et al. Sulfur-doped graphene via thermal exfoliation of graphite oxide in H2S, SO2, or CS2 gas[J]. ACS Nano, 2013, 7(6): 5262-5272. |
| 58 | Aristote N T, Liu C, Deng X L, et al. Sulfur-doping biomass based hard carbon as high performance anode material for sodium-ion batteries[J]. Journal of Electroanalytical Chemistry, 2022, 923: 116769. |
| 59 | Ren Q Q, Wu Z Y, Hu S, et al. Sulfur self-doped char with high specific capacitance derived from waste tire: effects of pyrolysis temperature[J]. The Science of the Total Environment, 2020, 741: 140193. |
| 60 | Hegazy M A, Mohammedy M M, Dhmees A S. Phosphorous and sulfur doped asphaltene derived activated carbon for supercapacitor application[J]. Journal of Energy Storage, 2021, 44: 103331. |
| 61 | Wohlgemuth S A, Vilela F, Titirici M M, et al. A one-pot hydrothermal synthesis of tunable dual heteroatom-doped carbon microspheres[J]. Green Chemistry, 2012, 14(3): 741-749. |
| 62 | 郑明涛, 肖勇, 张浩然, 等. 单分散掺硫碳微球的水热制备及其表征[J]. 无机化学学报, 2013, 29(7): 1391-1399. |
| Zheng M T, Xiao Y, Zhang H R, et al. Hydrothermal synthesis and characterization of sulfur-doped carbon microspheres[J]. Chinese Journal of Inorganic Chemistry, 2013, 29(7): 1391-1399. | |
| 63 | Liu S M, Cai Y J, Zhao X, et al. Sulfur-doped nanoporous carbon spheres with ultrahigh specific surface area and high electrochemical activity for supercapacitor[J]. Journal of Power Sources, 2017, 360: 373-382. |
| 64 | Hao E C, Liu W, Liu S, et al. Rich sulfur doped porous carbon materials derived from ginkgo leaves for multiple electrochemical energy storage devices[J]. Journal of Materials Chemistry A, 2017, 5(5): 2204-2214. |
| 65 | Navarro R M, Peña M A, Fierro J L G. Hydrogen production reactions from carbon feedstocks: fossil fuels and biomass[J]. Chemical Reviews, 2007, 107(10): 3952-3991. |
| 66 | Shi J S, Yan N F, Cui H M, et al. Sulfur doped microporous carbons for CO2 adsorption[J]. Journal of Environmental Chemical Engineering, 2017, 5(5): 4605-4611. |
| 67 | Kim D W, Kil H S, Nakabayashi K, et al. Structural elucidation of physical and chemical activation mechanisms based on the microdomain structure model[J]. Carbon, 2017, 114: 98-105. |
| 68 | Contreras M S, Páez C A, Zubizarreta L, et al. A comparison of physical activation of carbon xerogels with carbon dioxide with chemical activation using hydroxides[J]. Carbon, 2010, 48(11): 3157-3168. |
| 69 | Horax K M, Bao S J, Wang M Q, et al. Analysis of graphene-like activated carbon derived from rice straw for application in supercapacitor[J]. Chinese Chemical Letters, 2017, 28(12): 2290-2294. |
| 70 | Demiral İ, Aydın Şamdan C, Demiral H. Production and characterization of activated carbons from pumpkin seed shell by chemical activation with ZnCl2 [J]. Desalination and Water Treatment, 2016, 57(6): 2446-2454. |
| 71 | Pang L Y, Zou B, Zou Y C, et al. A new route for the fabrication of corn starch-based porous carbon as electrochemical supercapacitor electrode material[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2016, 504: 26-33. |
| 72 | Peng H, Ma G F, Sun K J, et al. Nitrogen-doped interconnected carbon nanosheets from pomelo mesocarps for high performance supercapacitors[J]. Electrochimica Acta, 2016, 190: 862-871. |
| 73 | Mossfika E, Syukri S, Aziz H. Preparation of activated carbon from tea waste by NaOH activation as a supercapacitor material[J]. Journal of Aceh Physics Society, 2020, 9(2): 42-47. |
| 74 | Zhang C, Sun S Z, He S, et al. Direct air capture of CO2 by KOH-activated bamboo biochar[J]. Journal of the Energy Institute, 2022, 105: 399-405. |
| 75 | 苏文韬, 蔡铭, 车美红, 等. 由聚苯硫醚废料制备硫掺杂多孔碳处理含重金属废水[J]. 工业技术创新, 2022, 9(3): 83-90. |
| Su W T, Cai M, Che M H, et al. Preparing the sulfur-doped porous carbon from polyphenylene sulfide waste to treat wastewater contained heavy metals[J]. Industrial Technology Innovation, 2022, 9(3): 83-90. | |
| 76 | Yaglikci S, Gokce Y, Yagmur E, et al. The performance of sulphur doped activated carbon supercapacitors prepared from waste tea[J]. Environmental Technology, 2020, 41(1): 36-48. |
| 77 | Bai J L, Huang J M, Jiang Q, et al. Synthesis and characterization of polyphenylene sulfide resin-derived S-doped porous carbons for efficient CO2 capture[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2023, 674: 131916. |
| 78 | Ren Q Q, Hu S, Li Q, et al. Treating waste tire to prepare high-yield sulfur-doped porous char via ZnCl2-KOH heat treatment method[J]. Journal of Cleaner Production, 2022, 372: 133672. |
| 79 | 王宝凤, 杨晓阳, 郭彦霞, 等. 一种硫氮原位掺杂多孔炭材料的制备方法: 115520864A[P]. 2022-12-27. |
| Wang B F, Yang X Y, Guo Y X, et al. A preparation method for sulfur nitrogen in-situ doped porous carbon material: 115520864A[P]. 2022-12-27. | |
| 80 | Zhao G G, Zou G Q, Hou H S, et al. Sulfur-doped carbon employing biomass-activated carbon as a carrier with enhanced sodium storage behavior[J]. Journal of Materials Chemistry A, 2017, 5(46): 24353-24360. |
| 81 | de Yuso A M, de Fina M, Nita C, et al. Synthesis of sulfur-doped porous carbons by soft and hard templating processes for CO2 and H2 adsorption[J]. Microporous and Mesoporous Materials, 2017, 243: 135-146. |
| 82 | Bandosz T J, Ren T Z. Porous carbon modified with sulfur in energy related applications[J]. Carbon, 2017, 118: 561-577. |
| 83 | Saha D, Orkoulas G, Chen J H, et al. Adsorptive separation of CO2 in sulfur-doped nanoporous carbons: selectivity and breakthrough simulation[J]. Microporous and Mesoporous Materials, 2017, 241: 226-237. |
| 84 | Hu L, Lu Y, Zhang T W, et al. Ultramicroporous carbon through an activation-free approach for Li-S and Na-S batteries in carbonate-based electrolyte[J]. ACS Applied Materials & Interfaces, 2017, 9(16): 13813-13818. |
| 85 | Wang H Z, Guo W Q, Liu B H, et al. Sludge-derived biochar as efficient persulfate activators: sulfurization-induced electronic structure modulation and disparate nonradical mechanisms[J]. Applied Catalysis B: Environmental, 2020, 279: 119361. |
| 86 | Bear J C, McGettrick J D, Parkin I P, et al. Porous carbons from inverse vulcanised polymers[J]. Microporous and Mesoporous Materials, 2016, 232: 189-195. |
| 87 | Lee J S, Parker D J, Cooper A I, et al. High surface area sulfur-doped microporous carbons from inverse vulcanised polymers[J]. Journal of Materials Chemistry A, 2017, 5(35): 18603-18609. |
| 88 | 谢金明, 庄容, 杜宇轩, 等. 硫掺杂炭材料在钠离子电池负极中的研究进展[J]. 新型炭材料, 2023, 38(2): 305-316. |
| Xie J M, Zhuang R, Du Y X, et al. Advances in sulfur-doped carbon materials for use as anodes in sodium-ion batteries[J]. New Carbon Materials, 2023, 38(2): 305-316. | |
| 89 | Gu W T, Sevilla M, Magasinski A, et al. Sulfur-containing activated carbons with greatly reduced content of bottle neck pores for double-layer capacitors: a case study for pseudocapacitance detection[J]. Energy & Environmental Science, 2013, 6(8): 2465-2476. |
| 90 | Guo Y P, Zeng Z Q, Liu Y J, et al. One-pot synthesis of sulfur doped activated carbon as a superior metal-free catalyst for the adsorption and catalytic oxidation of aqueous organics[J]. Journal of Materials Chemistry A, 2018, 6(9): 4055-4067. |
| 91 | Tian J Y, Zhang H Y, Liu Z M, et al. One-step synthesis of 3D sulfur-doped porous carbon with multilevel pore structure for high-rate supercapacitors[J]. International Journal of Hydrogen Energy, 2018, 43(3): 1596-1605. |
| 92 | Seredych M, Jagiello J, Bandosz T J. Complexity of CO2 adsorption on nanoporous sulfur-doped carbons — is surface chemistry an important factor?[J]. Carbon, 2014, 74: 207-217. |
| 93 | Xia Y D, Zhu Y Q, Tang Y. Preparation of sulfur-doped microporous carbons for the storage of hydrogen and carbon dioxide[J]. Carbon, 2012, 50(15): 5543-5553. |
| 94 | Kiciński W, Szala M, Bystrzejewski M. Sulfur-doped porous carbons: synthesis and applications[J]. Carbon, 2014, 68: 1-32 |
| 95 | Sevilla M, Fuertes A B. Sustainable porous carbons with a superior performance for CO2 capture[J]. Energy & Environmental Science, 2011, 4(5): 1765-1771. |
| 96 | Liu B G, Ma X C, Wei D, et al. Development of ultramicropore-mesopore interconnected pore architectures for boosting carbon dioxide capture at low partial pressure[J]. Carbon, 2022, 192: 41-49. |
| 97 | Marco-Lozar J P, Kunowsky M, Suárez-García F, et al. Sorbent design for CO2 capture under different flue gas conditions[J]. Carbon, 2014, 72: 125-134. |
| 98 | Zaman A C. Pyrolysis of sulfonic acid substituted benzenes and investigation of CO2 capture capability of resulting carbons[J]. Journal of Solid State Chemistry, 2021, 303: 122546. |
| 99 | Rehman A, Park S J. From chitosan to urea-modified carbons: tailoring the ultra-microporosity for enhanced CO2 adsorption[J]. Carbon, 2020, 159: 625-637. |
| 100 | Tang Z P, Gao J M, Zhang Y, et al. Ultra-microporous biochar-based carbon adsorbents by a facile chemical activation strategy for high-performance CO2 adsorption[J]. Fuel Processing Technology, 2023, 241: 107613. |
| 101 | Bai J L, Shao J W, Yu Q Y, et al. Sulfur-doped porous carbon adsorbent: a promising solution for effective and selective CO2 capture[J]. Chemical Engineering Journal, 2024, 479: 147667. |
| 102 | Shi W W, Wang R Z, Liu H L, et al. Biowaste-derived 3D honeycomb-like N and S dual-doped hierarchically porous carbons for high-efficient CO2 capture[J]. RSC Advances, 2019, 9(40): 23241-23253. |
| 103 | Shao J W, Ma C D, Zhao J J, et al. Effective nitrogen and sulfur co-doped porous carbonaceous CO2 adsorbents derived from amino acid[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 632: 127750. |
| 104 | Cui H M, Xu J G, Shi J S, et al. N, S co-doped carbon spheres synthesized from glucose and thiourea as efficient CO2 adsorbents[J]. Journal of the Taiwan Institute of Chemical Engineers, 2022, 138: 104441. |
| 105 | Li Y, Wang Y G, Liu N, et al. Nitrogen and sulfur co-doped microporous carbon prepared by a couple of activating and functionalized reagents for efficient CO2 capture and selective CO2/CH4 separation[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2023, 658: 130732. |
| 106 | Ma C D, Lu T Y, Shao J W, et al. Biomass derived nitrogen and sulfur co-doped porous carbons for efficient CO2 adsorption[J]. Separation and Purification Technology, 2022, 281: 119899. |
| 107 | Ma C D, Bai J L, Demir M, et al. Water chestnut shell-derived N/S-doped porous carbons and their applications in CO2 adsorption and supercapacitor[J]. Fuel, 2022, 326: 125119. |
| 108 | Montes-Morán M A, Suárez D, Menéndez J A, et al. On the nature of basic sites on carbon surfaces: an overview[J]. Carbon, 2004, 42(7): 1219-1225. |
| 109 | Ma X C, Yang Y H, Wu Q D, et al. Underlying mechanism of CO2 uptake onto biomass-based porous carbons: do adsorbents capture CO2 chiefly through narrow micropores?[J]. Fuel, 2020, 282: 118727. |
| 110 | Zhang P Y, Sui Z Y, Wang E, et al. Preparation of hierarchically porous sulfur- and oxygen-co-doped carbon for gas uptake and lithium-ion battery[J]. Microporous and Mesoporous Materials, 2018, 264: 118-124. |
| 111 | Querejeta N, Gil M V, Pevida C, et al. Standing out the key role of ultramicroporosity to tailor biomass-derived carbons for CO2 capture[J]. Journal of CO2 Utilization, 2018, 26: 1-7. |
| 112 | Guo Z, Lu X, Xin Z. N, S, O co-doped porous carbons derived from bio-based polybenzoxazine for efficient CO2 capture[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 646: 128845. |
| 113 | Wang Y H, Wang M H, Wang Z W, et al. Tunable-quaternary (N, S, O, P)-doped porous carbon microspheres with ultramicropores for CO2 capture[J]. Applied Surface Science, 2020, 507(C): 145130. |
| 114 | Tian Z H, Lai F L, Tobias H, et al. Synthesis of carbon frameworks with N, O and S-lined pores from Gallic acid and thiourea for superior CO2 adsorption and supercapacitors[J]. Science China Materials, 2020, 63(5): 748-757. |
| 115 | 邵健, 冯军宗, 柳凤琦, 等. 酚醛树脂基炭微球结构调控与功能化制备研究进展[J]. 化工学报, 2022, 73(9): 3787-3801. |
| Shao J, Feng J Z, Liu F Q, et al. Research progress on structural modulation and functionalized preparation of phenolic resin-based carbon microspheres[J]. CIESC Journal, 2022, 73(9): 3787-3801. | |
| 116 | Sharma M, Snyder M A. Facile synthesis of flower-like carbon microspheres for carbon dioxide capture[J]. Microporous and Mesoporous Materials, 2022, 335: 111801. |
| 117 | Xu W L, Chen H J, Wang Y C, et al. Chitin-derived fibrous carbon microspheres as support of polyamine for remarkable CO2 capture[J]. Green Chemical Engineering, 2022, 3(3): 267-279. |
| 118 | Hong J Y, Huh S. Hollow S-doped carbon spheres from spherical CT/PEDOT composite particles and their CO₂ sorption properties[J]. Journal of Colloid and Interface Science, 2014, 436: 77-82. |
| 119 | Sun Y H, Zhao J H, Wang J L, et al. Sulfur-doped millimeter-sized microporous activated carbon spheres derived from sulfonated poly(styrene-divinylbenzene) for CO2 capture[J]. The Journal of Physical Chemistry C, 2017, 121(18): 10000-10009. |
| 120 | 周毅, 王永洪, 张新儒, 等. PEBA/氮硫共掺杂多孔炭球混合基质膜的制备及CO2分离性能研究[J]. 化工学报, 2021, 72(10): 5237-5246. |
| Zhou Z, Wang Y H, Zhang X R, et al. Preparation of PEBA/N,S co-doped porous carbon sphere mixed matrix membrane for CO2 separation [J]. CIESC Journal, 2021, 72(10): 5237-5246. | |
| 121 | Wang X Y, Liu Z, Liu X F, et al. Ultralight and multifunctional PVDF/SiO2@GO nanofibrous aerogel for efficient harsh environmental oil-water separation and crude oil absorption[J]. Carbon, 2022, 193: 77-87. |
| 122 | 牛卉芳, 闫伦靖, 吕鹏, 等. 煤焦油沥青基碳气凝胶微球的制备及分析[J]. 化工学报, 2022, 73(12): 5605-5614. |
| Niu H F, Yan L J, Lyu P, et al. Preparation and analysis of carbon aerogel microspheres based on coal tar pitch[J]. CIESC Journal, 2022, 73(12): 5605-5614. | |
| 123 | Dan H B, Ji K D, Gao Y, et al. Fabrication of superhydrophobic Enteromorpha-derived carbon aerogels via NH4H2PO4 modification for multi-behavioral oil/water separation[J]. Science of the Total Environment, 2022, 837: 155869. |
| 124 | Wohlgemuth S A, White R J, Willinger M G, et al. A one-pot hydrothermal synthesis of sulfur and nitrogen doped carbon aerogels with enhanced electrocatalytic activity in the oxygen reduction reaction[J]. Green Chemistry, 2012, 14(5): 1515-1523. |
| 125 | Li D H, Chang G J, Zong L, et al. From double-helix structured seaweed to S-doped carbon aerogel with ultra-high surface area for energy storage[J]. Energy Storage Materials, 2018, 17: 22-30. |
| 126 | Kiciński W, Dziura A. Heteroatom-doped carbon gels from phenols and heterocyclic aldehydes: sulfur-doped carbon xerogels[J]. Carbon, 2014, 75: 56-67. |
| 127 | 肖弦, 徐文昊, 沈亮, 等. 氧化石墨烯与剩余活性污泥聚合制备多孔碳材料及其电化学性能[J]. 化工学报, 2021, 72(7): 3869-3879. |
| Xiao X, Xu W H, Shen L, et al. Preparation and electrochemical properties of new porous carbon materials by synthesizing graphene oxide and waste activated sludge[J]. CIESC Journal, 2021, 72(7): 3869-3879. | |
| 128 | Wood B C, Bhide S Y, Dutta D, et al. Methane and carbon dioxide adsorption on edge-functionalized graphene: a comparative DFT study[J]. The Journal of Chemical Physics, 2012, 137(5): 054702. |
| 129 | Seema H, Kemp K C, Le N H, et al. Highly selective CO2 capture by S-doped microporous carbon materials[J]. Carbon, 2014, 66: 320-326. |
| 130 | Li J Y, Hou M L, Chen Y Q, et al. Enhanced CO2 capture on graphene via N, S dual-doping[J]. Applied Surface Science, 2017, 399: 420-425. |
| 131 | Li X F, Xue Q Z, Chang X, et al. Effects of sulfur doping and humidity on CO2 capture by graphite split pore: a theoretical study[J]. ACS Applied Materials & Interfaces, 2017, 9(9): 8336-8343. |
| 132 | Seredych M, Rodriguez-castellon E, Bandosz T. Alterations of S-doped porous carbon-rGO composites surface features upon CO2 adsorption at ambient conditions[J]. Carbon, 2016, 107: 501-509. |
| 133 | Bandosz T J, Seredych M, Rodríguez-Castellón E, et al. Evidence for CO2 reactive adsorption on nanoporous S- and N-doped carbon at ambient conditions[J]. Carbon, 2016, 96: 856-863. |
| 134 | 郭宁宁, 王宇, 王润伟, 等. 常温合成硫掺杂微孔碳及其二氧化碳的吸附性能[J]. 无机化学学报, 2017, 33(11): 2147-2152. |
| Guo N N, Wang Y, Wang R W, et al. Synthesis of sulfur doped porous carbon at room temperature for CO2 adsorption[J]. Chinese Journal of Inorganic Chemistry, 2017, 33(11): 2147-2152. | |
| 135 | 叶剑波. 活性炭改性及其吸附CO2/H2O性能研究[D]. 沈阳: 东北大学, 2014. |
| Ye J B. Study on modification of activated carbon and its adsorption performance for CO2/H2O[D]. Shenyang: Northeastern University, 2014. | |
| 136 | Plaza M G, González A S, Rubiera F, et al. Water vapour adsorption by a coffee-based microporous carbon: effect on CO2 capture[J]. Journal of Chemical Technology & Biotechnology, 2015, 90(9): 1592-1600. |
| 137 | Wang J T, Chen H C, Zhou H H, et al. Carbon dioxide capture using polyethylenimine-loaded mesoporous carbons[J]. Journal of Environmental Sciences, 2013, 25(1): 124-132. |
| 138 | Sun Y H, Li K X, Zhao J H, et al. Nitrogen and sulfur co-doped microporous activated carbon macro-spheres for CO2 capture[J]. Journal of Colloid and Interface Science, 2018, 526: 174-183. |
| 139 | Xiao J F, Wang Y, Zhang T C, et al. N, S-containing polycondensate-derived porous carbon materials for superior CO2 adsorption and supercapacitor[J]. Applied Surface Science, 2021, 562: 150128. |
| 140 | Jin Z E, Wang J L, Zhao R J, et al. Synthesis of S, N co-doped porous carbons from polybenzoxazine for CO2 capture[J]. New Carbon Materials, 2018, 33(5): 392-401. |
| 141 | Singh J, Bhunia H, Basu S M. Development of sulphur-doped carbon monolith derived from phenol-formaldehyde resin for fixed bed CO2 adsorption[J]. Environmental Technology & Innovation, 2020, 20: 101104. |
| 142 | Saha D, Orkoulas G, Bates D. One-step synthesis of sulfur-doped nanoporous carbons from lignin with ultra-high surface area, sulfur content and CO2 adsorption capacity[J]. Materials, 2023, 16(1): 455. |
| 143 | Luo L, Yang C L, Liu F, et al. Heteroatom-N, S co-doped porous carbons derived from waste biomass as bifunctional materials for enhanced CO2 adsorption and conversion[J]. Separation and Purification Technology, 2023, 320: 124090. |
| 144 | Cui H M, Shi J S, Xu J G, et al. Direct synthesis of N, S co-doped porous carbons using novel organic potassium salts as activators for efficient CO2 adsorption[J]. Fuel, 2023, 342: 127824. |
| 145 | Cao M, Shu Y, Bai Q H, et al. Design of biomass-based N, S co-doped porous carbon via a straightforward post-treatment strategy for enhanced CO2 capture performance[J]. The Science of the Total Environment, 2023, 884: 163750. |
| 146 | Cao W T, Huang Y F, Li D, et al. N/S co-doped microporous zeolite-templated carbon for efficient CO2 adsorption and separation[J]. Journal of the Energy Institute, 2023, 106: 101159. |
| [1] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [2] | 韩晨, 司徒友珉, 朱斌, 许建良, 郭晓镭, 刘海峰. 协同处理废液的多喷嘴粉煤气化炉内反应流动研究[J]. 化工学报, 2023, 74(8): 3266-3278. |
| [3] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [4] | 张曼铮, 肖猛, 闫沛伟, 苗政, 徐进良, 纪献兵. 危废焚烧处理耦合有机朗肯循环系统工质筛选与热力学优化[J]. 化工学报, 2023, 74(8): 3502-3512. |
| [5] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [6] | 陈朝光, 贾玉香, 汪锰. 以低浓度废酸驱动中和渗析脱盐的模拟与验证[J]. 化工学报, 2023, 74(6): 2486-2494. |
| [7] | 肖川宝, 李林洋, 刘武锋, 钟年丙, 解泉华, 钟登杰, 常海星. 光催化与离子交换吸附耦合有效去除2,4,6-三氯苯酚[J]. 化工学报, 2023, 74(4): 1587-1597. |
| [8] | 刘定平, 陈爱桦, 张向阳, 何文浩, 王海. 铝灰半干法水解脱氮研究[J]. 化工学报, 2023, 74(3): 1294-1302. |
| [9] | 潘煜, 王子航, 王佳韵, 王如竹, 张华. 基于可得然-氯化锂复合吸附剂的除湿换热器热湿性能研究[J]. 化工学报, 2023, 74(3): 1352-1359. |
| [10] | 李敏, 阎雪茹, 刘新磊. 苯并咪唑连接聚合物吸附剂和膜研究进展[J]. 化工学报, 2023, 74(2): 599-616. |
| [11] | 孔德齐, 张莹莹, 武文玲, 马军, 宋振兴, 张东辉, 张彦军. 六塔变压吸附制氧工艺的模拟与分析[J]. 化工学报, 2023, 74(12): 4934-4944. |
| [12] | 李彦乐, 刘宜林, 霍俊杰, 孙艳霞, 董生德, 贺欣, 许琪, 马路祥, 周园, 海春喜. 层状结构铝系吸附剂在盐湖提锂领域的研究[J]. 化工学报, 2023, 74(12): 4777-4791. |
| [13] | 童文华, 李义隆, 张永奎, 王雅博. 碱辅助四氧化三锰降解磷酸三丁酯及磷元素回收研究[J]. 化工学报, 2023, 74(10): 4277-4285. |
| [14] | 张鑫琦, 张宸, 张舵咏, 宣涛, 干桌臻, 朱炫灿, 王丽伟. 高选择性PEI@MOF-808吸附剂在潮湿烟气中的碳捕集性能研究[J]. 化工学报, 2023, 74(10): 4330-4342. |
| [15] | 党迎喜, 谈朋, 刘晓勤, 孙林兵. 辐射冷却和太阳能加热驱动的CO2变温捕获[J]. 化工学报, 2023, 74(1): 469-478. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号