化工学报 ›› 2024, Vol. 75 ›› Issue (4): 1552-1564.DOI: 10.11949/0438-1157.20231301
王瑞瑞1( ), 金颖2(
), 金颖2( ), 刘玉梅1, 李梦悦1, 朱胜文1, 闫瑞一1, 刘瑞霞1(
), 刘玉梅1, 李梦悦1, 朱胜文1, 闫瑞一1, 刘瑞霞1( )
)
收稿日期:2023-12-06
修回日期:2024-01-31
出版日期:2024-04-25
发布日期:2024-06-06
通讯作者:
刘瑞霞
作者简介:王瑞瑞(1989—),女,博士,wangruirui@ipe.ac.cn基金资助:
Ruirui WANG1( ), Ying JIN2(
), Ying JIN2( ), Yumei LIU1, Mengyue LI1, Shengwen ZHU1, Ruiyi YAN1, Ruixia LIU1(
), Yumei LIU1, Mengyue LI1, Shengwen ZHU1, Ruiyi YAN1, Ruixia LIU1( )
)
Received:2023-12-06
Revised:2024-01-31
Online:2024-04-25
Published:2024-06-06
Contact:
Ruixia LIU
摘要:
以乙烯基咪唑为单体,添加不同量的引发剂,采用常规热聚合中自由基聚合的方式,合成了不同分子量的聚乙烯基咪唑,热重(TG)分析表明该聚合物具有良好的热稳定性。采用季铵化反应,对最优聚合物进行1-氯代十八烷、1-氯代十二烷以及1-氯代正己烷三种不同烷基链长度的取代,合成系列聚合离子液体(PIL)。通过傅里叶红外光谱(FT-IR)、扫描电子显微镜(SEM)、X射线光电子能谱(XPS)、接触角等表征手段分析了聚合离子液体的化学结构及形貌特征,并考察了PIL-C18、PIL-C12、PIL-C6催化环己烷选择性氧化为己二酸的性能,进而选取PIL-C12深入研究了离子率对催化性能的影响规律。结果表明,烷基链段长度以及离子率可以有效调控亲疏水性和反应活性位点,进而提高反应性能,使得反应生成己二酸的转化率达到21.8%,选择性为41.5%。
中图分类号:
王瑞瑞, 金颖, 刘玉梅, 李梦悦, 朱胜文, 闫瑞一, 刘瑞霞. 聚合离子液体设计及催化环己烷选择性氧化性能研究[J]. 化工学报, 2024, 75(4): 1552-1564.
Ruirui WANG, Ying JIN, Yumei LIU, Mengyue LI, Shengwen ZHU, Ruiyi YAN, Ruixia LIU. Study on design of polymeric ionic liquids and the performance for selective oxidation of cyclohexane[J]. CIESC Journal, 2024, 75(4): 1552-1564.
| VIM/g | DMF/ml | AIBN/mg |
|---|---|---|
| 2 | 8 | 0.9 |
| 2 | 8 | 1.9 |
| 2 | 8 | 4.8 |
| 2 | 8 | 7.6 |
表1 VIM与AIBN的具体用量
Table 1 Specific consumption of VIM and AIBN
| VIM/g | DMF/ml | AIBN/mg |
|---|---|---|
| 2 | 8 | 0.9 |
| 2 | 8 | 1.9 |
| 2 | 8 | 4.8 |
| 2 | 8 | 7.6 |
| 聚合物 | Mn | PDI | Y/% |
|---|---|---|---|
| PVIM-M1 | 12320 | 1.8 | 94.3 |
| PVIM-M2 | 15320 | 2.2 | 93.4 |
| PVIM-M3 | 16040 | 2.7 | 83.2 |
| PVIM-M4 | 19830 | 2.9 | 80.9 |
表2 聚合物数据
Table 2 Data of polymer
| 聚合物 | Mn | PDI | Y/% |
|---|---|---|---|
| PVIM-M1 | 12320 | 1.8 | 94.3 |
| PVIM-M2 | 15320 | 2.2 | 93.4 |
| PVIM-M3 | 16040 | 2.7 | 83.2 |
| PVIM-M4 | 19830 | 2.9 | 80.9 |
| Entry | Catalysts | Con/% | Sel/% | ||||
|---|---|---|---|---|---|---|---|
| K | A | KA | AA | Others | |||
| 1 | blank | 0 | 0 | 0 | 0 | 0 | 0 |
| 2① | VIM | 2.8 | 51.9 | 35.4 | 87.4 | 10.1 | 2.6 |
| 3① | PVIM-M1 | 7.6 | 27.7 | 18.7 | 46.4 | 37.7 | 15.9 |
| 4① | PVIM- M2 | 8.1 | 28.1 | 21.6 | 49.7 | 31.2 | 19.1 |
| 5① | PVIM- M3 | 9.1 | 36.8 | 28.9 | 65.7 | 27.2 | 7.2 |
| 6① | PVIM- M4 | 10.9 | 35.0 | 19.7 | 54.7 | 34.3 | 11.1 |
| 7 ② | VIM | 4.4 | 24.3 | 16.4 | 40.7 | 40.0 | 19.3 |
| 8② | PVIM-M1 | 11.3 | 23.1 | 16.6 | 39.7 | 47.7 | 15.9 |
| 9② | PVIM- M2 | 11.9 | 34.0 | 20.7 | 54.7 | 37.2 | 7.2 |
| 10② | PVIM- M3 | 13.5 | 22.7 | 13.7 | 36.4 | 34.3 | 11.1 |
| 11② | PVIM- M4 | 12.3 | 24.4 | 16.3 | 40.7 | 41.2 | 19.2 |
表3 不同分子量聚合物对环己烷氧化的影响
Table 3 Effect of different molecular weights on cyclohexane oxidation
| Entry | Catalysts | Con/% | Sel/% | ||||
|---|---|---|---|---|---|---|---|
| K | A | KA | AA | Others | |||
| 1 | blank | 0 | 0 | 0 | 0 | 0 | 0 |
| 2① | VIM | 2.8 | 51.9 | 35.4 | 87.4 | 10.1 | 2.6 |
| 3① | PVIM-M1 | 7.6 | 27.7 | 18.7 | 46.4 | 37.7 | 15.9 |
| 4① | PVIM- M2 | 8.1 | 28.1 | 21.6 | 49.7 | 31.2 | 19.1 |
| 5① | PVIM- M3 | 9.1 | 36.8 | 28.9 | 65.7 | 27.2 | 7.2 |
| 6① | PVIM- M4 | 10.9 | 35.0 | 19.7 | 54.7 | 34.3 | 11.1 |
| 7 ② | VIM | 4.4 | 24.3 | 16.4 | 40.7 | 40.0 | 19.3 |
| 8② | PVIM-M1 | 11.3 | 23.1 | 16.6 | 39.7 | 47.7 | 15.9 |
| 9② | PVIM- M2 | 11.9 | 34.0 | 20.7 | 54.7 | 37.2 | 7.2 |
| 10② | PVIM- M3 | 13.5 | 22.7 | 13.7 | 36.4 | 34.3 | 11.1 |
| 11② | PVIM- M4 | 12.3 | 24.4 | 16.3 | 40.7 | 41.2 | 19.2 |
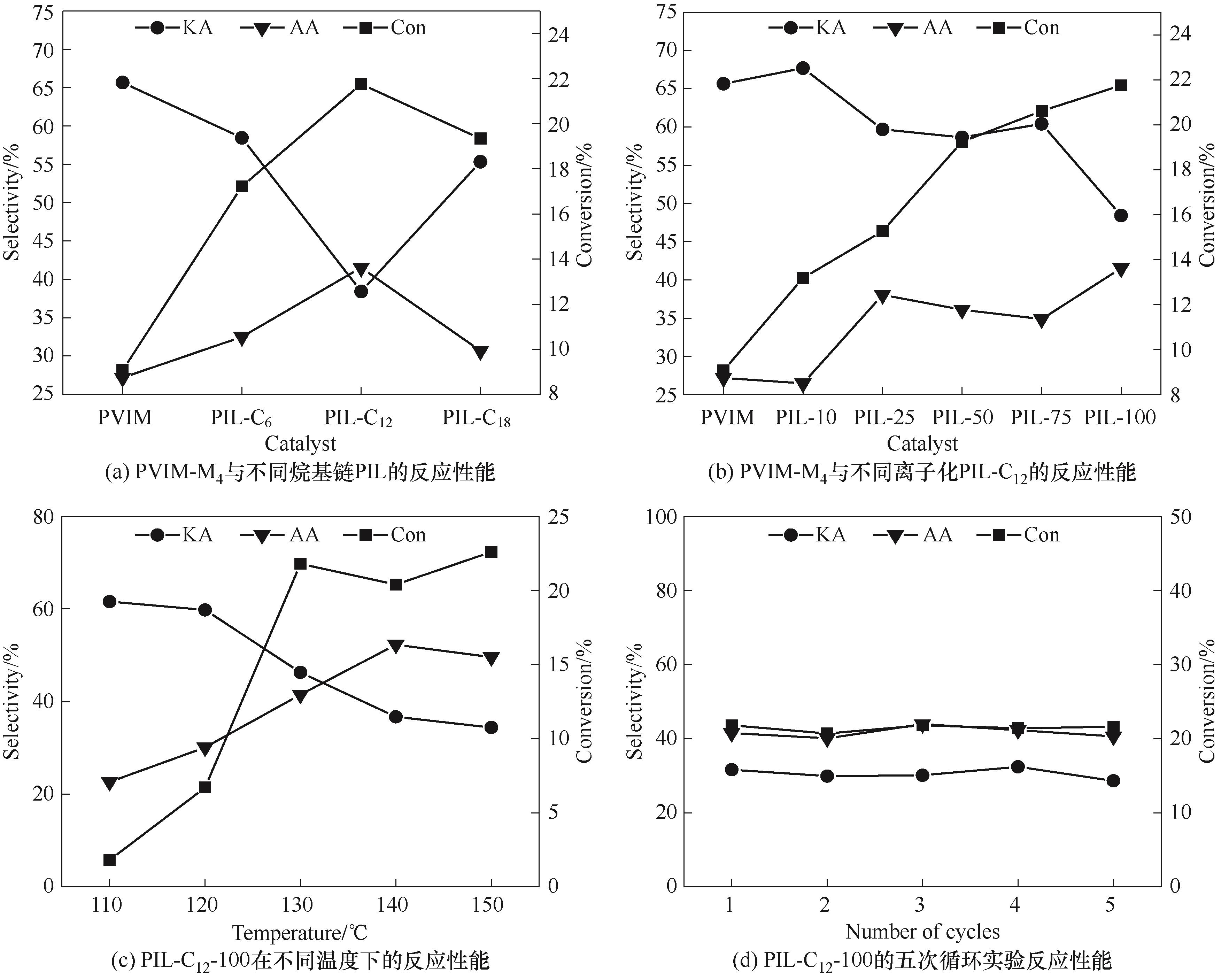
图6 不同催化剂的反应性能对比,温度对反应性能的影响及其循环实验反应性能对比(反应条件:25 mmol CH,0.02 g PIL,1.5 MPa O2,2 ml MeCN,130℃,2 h)
Fig.6 The performance of different catalysts, effect of the temperature on the performance, and the cycle experiment of PIL-C12-100
| Entry | Catalyst | Reaction conditions | Cyclohexane conversion/% | Selectivity of AA/% | Ref. |
|---|---|---|---|---|---|
| 1 | 1% Au-Pd/TiO2 | 150℃, 1.0 MPa O2, 4 h | 21.0 | 34.0 | [ |
| 2 | FeALPO-31 | 130℃, 1.5 MPa air, 24 h | 7.7 | 31.3 | [ |
| 3 | CoCl16Pc | 70℃, 5.5 MPa O2, 8 h | 18.0 | 23.9 | [ |
| 4 | [(C18H37)2N(CH3)2]6Mo7O24 | 160℃, 1.0 MPa O2, 6 h | 10.2 | 87.1 | [ |
| 5 | CeO2 | 110℃, 2.0 MPa O2, 6 h | 18.2 | 35.0 | [ |
| 6 | 1% Mn-HTS | 140℃, 1.0 MPa O2, 6 h | 13.4 | 57.5 | [ |
| 7 | Cu-20 min/a-C3N4 | 140℃, 20.0 bar O2, 4 h | 17.7 | 55.0 | [ |
| 8 | Co/C3N4-0.02 | 130℃, 1.0 MPa O2, 6 h | 9.1 | 38.0 | [ |
| 10 | PIL-C12-100 | 130℃, 1.5 MPa O2, 2 h | 21.7 | 41.2 | this work |
表4 本工作催化剂与已报道催化剂的环己烷氧化性能比较
Table 4 Comparison of the cyclohexane oxidation performance of catalysts in this work and other previously reported catalysts
| Entry | Catalyst | Reaction conditions | Cyclohexane conversion/% | Selectivity of AA/% | Ref. |
|---|---|---|---|---|---|
| 1 | 1% Au-Pd/TiO2 | 150℃, 1.0 MPa O2, 4 h | 21.0 | 34.0 | [ |
| 2 | FeALPO-31 | 130℃, 1.5 MPa air, 24 h | 7.7 | 31.3 | [ |
| 3 | CoCl16Pc | 70℃, 5.5 MPa O2, 8 h | 18.0 | 23.9 | [ |
| 4 | [(C18H37)2N(CH3)2]6Mo7O24 | 160℃, 1.0 MPa O2, 6 h | 10.2 | 87.1 | [ |
| 5 | CeO2 | 110℃, 2.0 MPa O2, 6 h | 18.2 | 35.0 | [ |
| 6 | 1% Mn-HTS | 140℃, 1.0 MPa O2, 6 h | 13.4 | 57.5 | [ |
| 7 | Cu-20 min/a-C3N4 | 140℃, 20.0 bar O2, 4 h | 17.7 | 55.0 | [ |
| 8 | Co/C3N4-0.02 | 130℃, 1.0 MPa O2, 6 h | 9.1 | 38.0 | [ |
| 10 | PIL-C12-100 | 130℃, 1.5 MPa O2, 2 h | 21.7 | 41.2 | this work |
| 1 | 刘长军, 唐盛伟, 谭平华, 等. 环己烷液相非催化/催化氧化反应动力学特性比较[J]. 化工学报, 2008, 59(8): 1992-1999. |
| Liu C J, Tang S W, Tan P H, et al. Comparison of kinetic behavior of non-catalytic/catalytic liquid-phase oxidation of cyclohexane[J]. Journal of Chemical Industry and Engineering (China), 2008, 59(8): 1992-1999. | |
| 2 | Schuchardt U, Cardoso D, Sercheli R, et al. Cyclohexane oxidation continues to be a challenge[J]. Applied Catalysis A: General, 2001, 211(1): 1-17. |
| 3 | 蹇建, 张嘉明, 佘祥, 等. V4+和V5+比例对钒磷氧催化NO2氧化环己烷性能的影响[J]. 化工学报, 2023, 74(4): 1570-1577. |
| Jian J, Zhang J M, She X, et al. Correlation with the redox V4+/V5+ ratio in VPO catalysts for oxidation of cyclohexane by NO2 [J]. CIESC Journal, 2023, 74(4): 1570-1577. | |
| 4 | Wang C H, Chen L F, Qi Z W. One-pot synthesis of gold nanoparticles embedded in silica for cyclohexane oxidation[J]. Catalysis Science & Technology, 2013, 3(4): 1123-1128. |
| 5 | 梁学博, 胡伯羽, 袁永军, 等. 金属卟啉催化空气氧化环己烷反应的工艺优化[J]. 化工学报, 2007, 58(3): 794-800. |
| Liang X B, Hu B Y, Yuan Y J, et al. Optimization of aerobic oxidation of cyclohexane catalyzed by metalloporphyrins[J]. Journal of Chemical Industry and Engineering (China), 2007, 58(3): 794-800. | |
| 6 | Lawal N S, Ibrahim H, Bala M D. Facile peroxidation of cyclohexane catalysed by in situ generated triazole-functionalised schiff base copper complexes[J]. Catalysis Letters, 2022, 152(5): 1264-1275. |
| 7 | Chavan S A, Srinivas D, Ratnasamy P. Oxidation of cyclohexane, cyclohexanone, and cyclohexanol to adipic acid by a non-HNO3 route over Co/Mn cluster complexes[J]. Journal of Catalysis, 2002, 212(1): 39-45. |
| 8 | Meireles A M, Guimarães A S, Querino G R, et al. Exploring manganese pyridylporphyrin isomers for cyclohexane oxidation: first-generation catalysts are better than third-generation ones[J]. Applied Organometallic Chemistry, 2021, 35(11): e6400. |
| 9 | Liu D C, Zhang B Q, Liu X F, et al. Cyclohexane oxidation over AFI molecular sieves: effects of Cr, Co incorporation and crystal size[J]. Catalysis Science & Technology, 2015, 5(6): 3394-3402. |
| 10 | Xie C J, Wang W, Yang Y P, et al. Enhanced stability and activity for solvent-free selective oxidation of cyclohexane over Cu2O/CuO fabricated by facile alkali etching method[J]. Molecular Catalysis, 2020, 495: 111134. |
| 11 | Shen H M, Wang X L, Ning L, et al. Efficient oxidation of cycloalkanes with simultaneously increased conversion and selectivity using O2 catalyzed by metalloporphyrins and boosted by Zn(AcO)2: a practical strategy to inhibit the formation of aliphatic diacids[J]. Applied Catalysis A: General, 2021, 609: 117904. |
| 12 | Wan Y L, Guo Q, Wang K, et al. Efficient and selective photocatalytic oxidation of cyclohexane using O2 as oxidant in VOCl2 solution and mechanism insight[J]. Chemical Engineering Science, 2019, 203: 163-172. |
| 13 | Hereijgers B P C, Weckhuysen B M. Aerobic oxidation of cyclohexane by gold-based catalysts: new mechanistic insight by thorough product analysis[J]. Journal of Catalysis, 2010, 270(1): 16-25. |
| 14 | Guo C C, Chu M F, Liu Q, et al. Effective catalysis of simple metalloporphyrins for cyclohexane oxidation with air in the absence of additives and solvents[J]. Applied Catalysis A: General, 2003, 246(2): 303-309. |
| 15 | Wu M Z, Zhan W C, Guo Y L, et al. An effective Mn-Co mixed oxide catalyst for the solvent-free selective oxidation of cyclohexane with molecular oxygen[J]. Applied Catalysis A: General, 2016, 523: 97-106 |
| 16 | Alkordi M H, Liu Y L, Larsen R W, et al. Zeolite-like metal-organic frameworks as platforms for applications: on metalloporphyrin-based catalysts[J]. Journal of the American Chemical Society, 2008, 130(38): 12639-12641. |
| 17 | Prabu M, Sharma S, Raja A, et al. Nitric acid free cyclohexane to adipic acid production using nickel and vanadium incorporated AlPO-5 molecular sieve[J]. Molecular Catalysis, 2023, 540: 113051. |
| 18 | Zhang S Y, Zhuang Q, Zhang M, et al. Poly(ionic liquid) composites[J]. Chemical Society Reviews, 2020, 49(6): 1726-1755. |
| 19 | Yan X C, Zhang F F, Zhang H N, et al. Improving oxygen reduction performance by using protic poly(ionic liquid) as proton conductors[J]. ACS Applied Materials & Interfaces, 2019, 11(6): 6111-6117. |
| 20 | 陈艺飞, 王佳铭, 阮雪华, 等. 聚离子液体二氧化碳分离膜材料的研究进展[J]. 化工学报, 2021, 72(12): 6062-6072. |
| Chen Y F, Wang J M, Ruan X H, et al. Research progress in poly(ionic liquids) materials for CO2 membrane separation[J]. CIESC Journal, 2021, 72(12): 6062-6072. | |
| 21 | Chen S X, An R, Li Y W, et al. Strategy of regulating the electrophilic/nucleophilic ability by ionic ratio in poly(ionic liquid)s to control the coupling reaction of epoxide[J]. Catalysis Science & Technology, 2021, 11(19): 6498-6506. |
| 22 | 吴岳峰, 曲永芳, 李大欢, 等. 聚离子液体载MoO2/Ag催化分子氧氧化苯乙烯的研究[J]. 化工学报, 2020, 71(11): 4990-4998. |
| Wu Y F, Qu Y F, Li D H, et al. Study on oxidation of styrene with molecular oxygen catalyzed by MoO2/Ag on polyionic liquid[J]. CIESC Journal, 2020, 71(11): 4990-4998. | |
| 23 | Gou H B, Ma X F, Su Q, et al. Hydrogen bond donor functionalized poly(ionic liquid)s for efficient synergistic conversion of CO2 to cyclic carbonates[J]. Physical Chemistry Chemical Physics, 2021, 23(3): 2005-2014. |
| 24 | Chen S H, Li Y W, Wang Z W, et al. Poly(ionic liquid)s hollow spheres nanoreactor for enhanced cyclohexane catalytic oxidation[J]. Journal of Catalysis, 2022, 411, 135-148. |
| 25 | Santanakrishnan S, Hutchinson R A. Free-radical polymerization of N-vinylimidazole and quaternized vinylimidazole in aqueous solution[J]. Macromolecular Chemistry and Physics, 2013, 214(10): 1140-1146. |
| 26 | Bunaciu A A, Udriştioiu E G, Aboul-Enein H Y. X-Ray diffraction: instrumentation and applications[J]. Critical Reviews in Analytical Chemistry, 2015, 45(4): 289-299. |
| 27 | 李慧慧, 姚开胜, 赵亚南, 等. 离子液体调控合成Pt-Pd双金属纳米材料及其催化氨硼烷水解释氢[J]. 应用化学, 2023, 40: 597-609. |
| Li H H, Yao K S, Zhao Y N, et al. Ionic liquid controlled synthesis of Pt-Pd bimetallic nanomaterials and their catalytic hydrogen interpretation by ammonia borane water[J]. Applied Chemistry, 2023, 40: 597-609 | |
| 28 | An R, Chen S X, Zhang R R, et al. Synthesis of propylene glycol methyl ether catalyzed by imidazole polymer catalyst: performance evaluation, kinetics study, and process simulation[J]. Chemical Engineering Journal, 2021, 405, 126636. |
| 29 | Allen M H, Hemp S T, Smith A E, et al. Controlled radical polymerization of 4-vinylimidazole[J]. Macromolecules, 2012, 45(9): 3669-3676. |
| 30 | Tang C W, Wang C B, Chien S H. Characterization of cobalt oxides studied by FT-IR, Raman, TPR and TG-MS[J]. Thermochimica Acta, 2008, 473(1/2): 68-73. |
| 31 | Silva A R, Mourão T, Rocha J. Oxidation of cyclohexane by transition-metal complexes with biomimetic ligands[J]. Catalysis Today, 2013, 203: 81-86. |
| 32 | Uno K, Tsutsumi O. Crystal structure and phase transition behavior of dioctadecyldimethylammonium chloride monohydrate[J]. Molecular Crystals and Liquid Crystals, 2012, 563(1): 58-66. |
| 33 | Gorjian H, Fahim H, Ghaffari Khaligh N. Poly(N-vinylimidazole): a biocompatible and biodegradable functional polymer, metal-free, and highly recyclable heterogeneous catalyst for the mechanochemical synthesis of oximes[J]. Turkish Journal of Chemistry, 2021, 45(6): 2007-2012. |
| 34 | Alshammari A, Kalevaru V, Martin A. Bimetallic catalysts containing gold and palladium for environmentally important reactions[J]. Catalysts, 2016, 6(7): 97. |
| 35 | Dugal M, Sankar G, Raja R, et al. Designing a heterogeneous catalyst for the production of adipic acid by aerial oxidation of cyclohexane[J]. Angewandte Chemie International Edition, 2000, 39(13): 2310-2313. |
| 36 | Raja R, Ratnasamy P. Oxidation of cyclohexane over copper phthalocyanines encapsulated in zeolites[J]. Catalysis Letters, 1997, 48(1/2): 1-10. |
| 37 | Lv H Y, Ren W Z, Liu P F, et al. One-step aerobic oxidation of cyclohexane to adipic acid using an Anderson-type catalyst [(C18H37)2N(CH3)2]6Mo7O24 [J]. Applied Catalysis A: General, 2012, 441/442: 136-141. |
| 38 | Dandapat S, Rao J L, Likhar P R, et al. One-step production of adipic acid from cyclohexane over stable oxides (CeO2 & ZrO2) using O2: enhanced oxidation activity in acidic medium[J]. Catalysis Communications, 2022, 172: 106555. |
| 39 | Zou G Q, Zhong W Z, Xu Q, et al. Oxidation of cyclohexane to adipic acid catalyzed by Mn-doped titanosilicate with hollow structure[J]. Catalysis Communications, 2015, 58: 46-52. |
| 40 | Shahzeydi A, Ghiaci M, Farrokhpour H, et al. Facile and green synthesis of copper nanoparticles loaded on the amorphous carbon nitride for the oxidation of cyclohexane[J]. Chemical Engineering Journal, 2019, 370: 1310-1321. |
| 41 | Yuan E X, Zhou M X, Jian P M, et al. Atomically dispersed Co/C3N4 for boosting aerobic cyclohexane oxidation[J].Applied Surface Science, 2023, 613: 155886. |
| 42 | Cremer T, Kolbeck C, Lovelock K R J, et al. Towards a molecular understanding of cation-anion interactions: probing the electronic structure of imidazolium ionic liquids by NMR spectroscopy, X-ray photoelectron spectroscopy and theoretical calculations[J]. Chemistry, 2010, 16(30): 9018-9033. |
| 43 | Yu H, Peng F, Tan J, et al. Selective catalysis of the aerobic oxidation of cyclohexane in the liquid phase by carbon nanotubes[J]. Angewandte Chemie International Edition, 2011, 50(17): 3978-3982. |
| 44 | Wang Z H, Wu Y C, Wu C C, et al. Electrophilic oxygen on defect-rich carbon nanotubes for selective oxidation of cyclohexane[J]. Catalysis Science & Technology, 2020, 10(2): 332-336. |
| 45 | Su K Y, Zhang C F, Wang Y H, et al. Unveiling the highly disordered NbO6 units as electron-transfer sites in Nb2O5 photocatalysis with N-hydroxyphthalimide under visible light irradiation[J]. Chinese Journal of Catalysis, 2022, 43(7): 1894-1905. |
| 46 | Fu Y, Zhan W C, Guo Y L, et al. Highly efficient cobalt-doped carbon nitride polymers for solvent-free selective oxidation of cyclohexane[J]. Green Energy and Environment, 2017, 2: 142-150. |
| [1] | 董霄, 白志山, 杨晓勇, 殷伟, 刘宁普, 于启凡. CHPPO工艺氧化液耦合除杂技术的研究与工业应用[J]. 化工学报, 2024, 75(4): 1630-1641. |
| [2] | 李昂, 赵振宇, 李洪, 高鑫. 微波诱导高分散Pd/FeP催化剂构筑及其电催化性能研究[J]. 化工学报, 2024, 75(4): 1594-1606. |
| [3] | 臧雅晴, 张益钧, 王金钊, 王倩, 李殿卿, 冯俊婷, 段雪. 基于反应耦合的低能耗水合氯化钙脱水制无水氯化钙[J]. 化工学报, 2024, 75(4): 1508-1518. |
| [4] | 李添翼, 武玉泰, 王永胜, 顾佳锐, 宋沂恒, 杨丰铖, 郝广平. 轻同位素分离纯化与催化标记研究进展[J]. 化工学报, 2024, 75(4): 1284-1301. |
| [5] | 孙铭泽, 黄鹤来, 牛志强. 铂基氧还原催化剂:从单晶电极到拓展表面纳米材料[J]. 化工学报, 2024, 75(4): 1256-1269. |
| [6] | 蒋方涛, 钱刚, 周兴贵, 段学志, 张晶. 基于[bmim][BF4]相转移催化的氟代碳酸乙烯酯高效合成[J]. 化工学报, 2024, 75(4): 1543-1551. |
| [7] | 申州洋, 薛康, 刘青, 史成香, 邹吉军, 张香文, 潘伦. 吸热型纳米流体燃料研究进展[J]. 化工学报, 2024, 75(4): 1167-1182. |
| [8] | 程骁恺, 历伟, 王靖岱, 阳永荣. 镍催化可控/活性自由基聚合反应研究进展[J]. 化工学报, 2024, 75(4): 1105-1117. |
| [9] | 程婷, 焦纬洲, 刘有智. 功能性填料在超重力旋转填料床中的应用和研究进展[J]. 化工学报, 2024, 75(4): 1414-1428. |
| [10] | 李云璇, 刘新悦, 陈熙, 刘文, 周明月, 蓝兴英. 基于固液氧化还原靶向反应的能量存储技术:材料、器件及动力学[J]. 化工学报, 2024, 75(4): 1222-1240. |
| [11] | 范以薇, 刘威, 李盈盈, 王培霞, 张吉松. 有机液体储氢中全氢化乙基咔唑催化脱氢研究进展[J]. 化工学报, 2024, 75(4): 1198-1208. |
| [12] | 贾旭东, 杨博龙, 程前, 李雪丽, 向中华. 分步负载金属法制备铁钴双金属位点高效氧还原电催化剂[J]. 化工学报, 2024, 75(4): 1578-1593. |
| [13] | 徐安冉, 刘凯, 王娜, 赵振宇, 李洪, 高鑫. 强吸波催化剂协同微波能强化果糖脱水制5-羟甲基糠醛[J]. 化工学报, 2024, 75(4): 1565-1577. |
| [14] | 严孝清, 赵瑛, 张宇哲, 欧鸿辉, 黄起中, 胡华贵, 杨贵东. 五重孪晶铜纳米线@聚吡咯制备及其电催化硝酸盐还原制氨[J]. 化工学报, 2024, 75(4): 1519-1532. |
| [15] | 韩宇, 周乐, 张鑫, 罗勇, 孙宝昌, 邹海魁, 陈建峰. 高黏附性Pd/SiO2/NF整体式催化剂的制备及加氢性能研究[J]. 化工学报, 2024, 75(4): 1533-1542. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号