化工学报 ›› 2024, Vol. 75 ›› Issue (11): 3911-3922.DOI: 10.11949/0438-1157.20240558
宋璟1( ), 王玉军1, 邓建1, 陈光文2, 骆广生1(
), 王玉军1, 邓建1, 陈光文2, 骆广生1( )
)
收稿日期:2024-05-26
修回日期:2024-06-20
出版日期:2024-11-25
发布日期:2024-12-26
通讯作者:
骆广生
作者简介:宋璟(1997—),男,博士研究生,songj23@mails.tsinghua.edu.cn
基金资助:
Jing SONG1( ), Yujun WANG1, Jian DENG1, Guangwen CHEN2, Guangsheng LUO1(
), Yujun WANG1, Jian DENG1, Guangwen CHEN2, Guangsheng LUO1( )
)
Received:2024-05-26
Revised:2024-06-20
Online:2024-11-25
Published:2024-12-26
Contact:
Guangsheng LUO
摘要:
芳烃的硝化反应作为工业生产中最重要的基本反应类型之一,随着化学品安全、绿色、高效生产的要求,其反应工艺向微尺度方向的发展已是必然的趋势。如何实现微尺度芳烃硝化反应的高质量发展,是重大工程前沿方向之一。本文全面综述并剖析了硝化微反应工艺的发展和面临的问题,从微尺度硝化反应动力学研究入手,为芳烃硝化微反应工艺设计及强化指明方向,并总结了当前硝化微反应工艺中的过程强化及安全性评价方法,最后对其未来发展方向进行了展望。
中图分类号:
宋璟, 王玉军, 邓建, 陈光文, 骆广生. 微反应器内芳烃硝化反应研究进展[J]. 化工学报, 2024, 75(11): 3911-3922.
Jing SONG, Yujun WANG, Jian DENG, Guangwen CHEN, Guangsheng LUO. Research progress of aromatic nitration reaction in microreactors[J]. CIESC Journal, 2024, 75(11): 3911-3922.
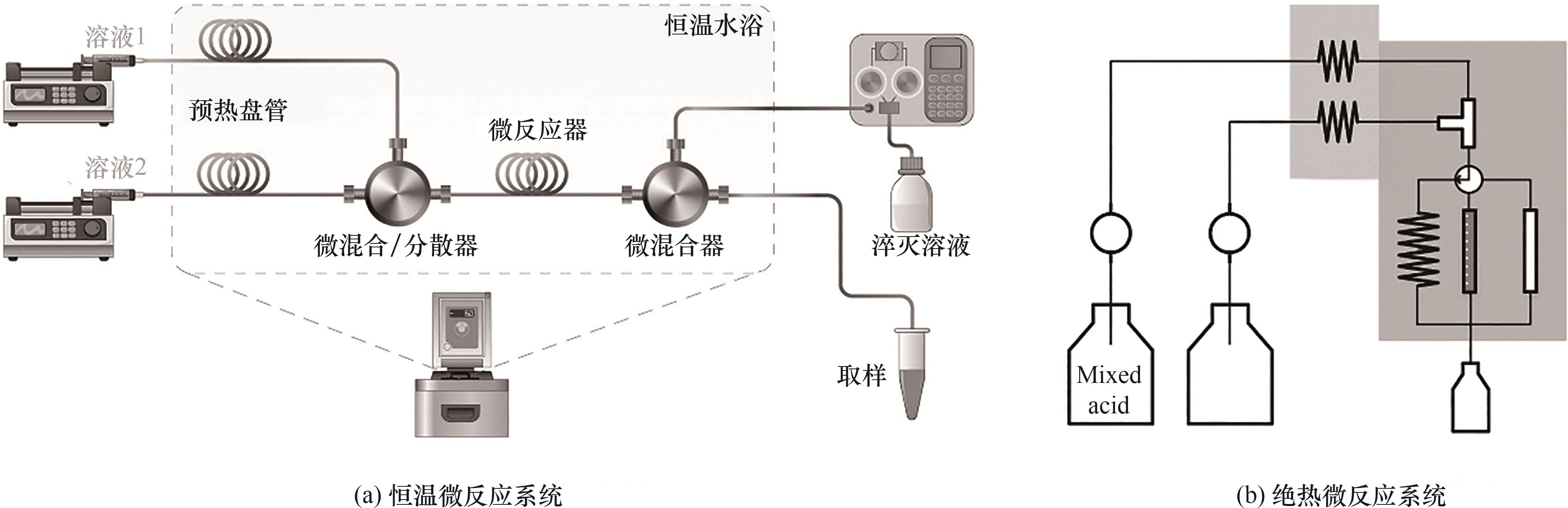
图2 芳烃硝化微尺度反应动力学研究实验装置示意图[32, 34-35, 40, 46]
Fig.2 Schematic diagram of experimental apparatus for the kinetic study of aromatics nitration at microscale[32, 34-35, 40, 46]
| 文献 | 硝化底物 | 相态 | Ea/(kJ/mol) | lnA |
|---|---|---|---|---|
| [ | 乙酰愈创木酚 | 均相 | 139 | — |
| [ | 2-乙基己醇 | 均相 | 42.67 | — |
| [ | 三氟甲氧基苯 | 非均相 | 29.3(邻位) | 8.75(邻位) |
| 26.9(对位) | 9.64(对位) | |||
| [ | 对硝基甲苯 | 均相 | 50.21 | 58.39 |
| 邻硝基甲苯 | 72.36 | 66.73 | ||
| [ | 邻二氯苯 | 非均相 | 30.96 | — |
| [ | 氯苯 | 均相 | 25.98 | — |
| [ | 甲苯 | 非均相 | 28.00 | 100.15 |
| [ | 硝基苯 | 非均相 | 71.23 | 7.81 |
| [ | 三氟甲基苯 | 非均相 | 86.33 | — |
表1 微尺度芳烃硝化反应动力学文献总结
Table 1 Summary of literatures on the kinetic study of microscale aromatic nitration
| 文献 | 硝化底物 | 相态 | Ea/(kJ/mol) | lnA |
|---|---|---|---|---|
| [ | 乙酰愈创木酚 | 均相 | 139 | — |
| [ | 2-乙基己醇 | 均相 | 42.67 | — |
| [ | 三氟甲氧基苯 | 非均相 | 29.3(邻位) | 8.75(邻位) |
| 26.9(对位) | 9.64(对位) | |||
| [ | 对硝基甲苯 | 均相 | 50.21 | 58.39 |
| 邻硝基甲苯 | 72.36 | 66.73 | ||
| [ | 邻二氯苯 | 非均相 | 30.96 | — |
| [ | 氯苯 | 均相 | 25.98 | — |
| [ | 甲苯 | 非均相 | 28.00 | 100.15 |
| [ | 硝基苯 | 非均相 | 71.23 | 7.81 |
| [ | 三氟甲基苯 | 非均相 | 86.33 | — |
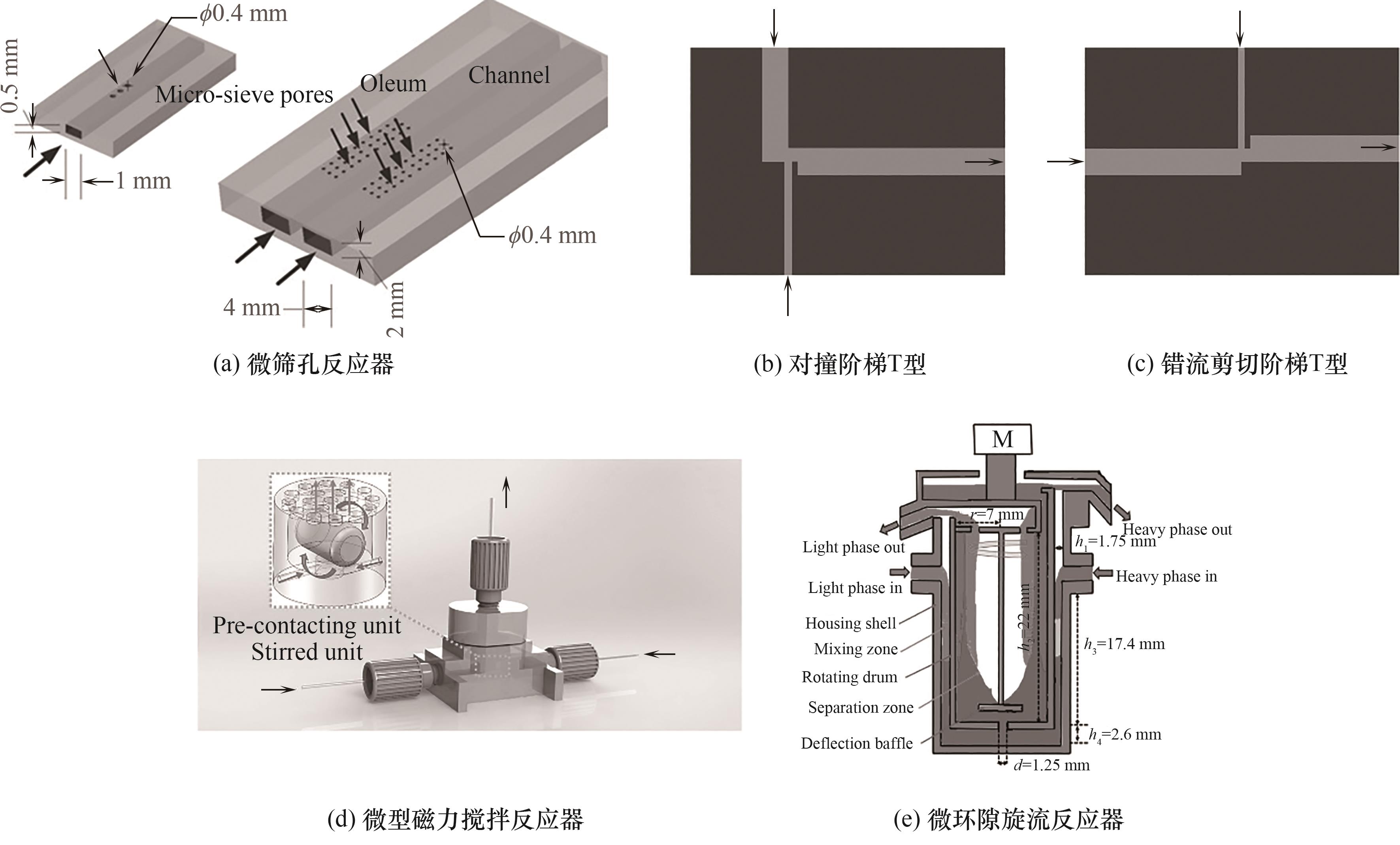
图4 几种具有优异分散和传质性能的新型微反应器结构[44, 54, 56-57, 63, 65]
Fig.4 Several novel microreactors with excellent microdispersion and mass transfer performance[44, 54, 56-57, 63, 65]
| 1 | Badgujar D M, Talawar M B, Mahulikar P P. Review on greener and safer synthesis of nitro compounds[J]. Propellants, Explosives, Pyrotechnics, 2016, 41(1): 24-34. |
| 2 | Patel S S, Patel D B, Patel H D. Synthetic protocols for aromatic nitration: a review[J]. ChemistrySelect, 2021, 6(6): 1337-1356. |
| 3 | Gillespie R J, Hughes E D, Ingold C K, et al. Kinetics and mechanism of aromatic nitration[J]. Nature, 1949, 163(4146): 599-600. |
| 4 | Barduhn A J, Kobe K A. Toluene nitration kinetics[J]. Industrial & Engineering Chemistry, 1956, 48(8): 1305-1315. |
| 5 | Coombes R G, Moodie R B, Schofield K. Electrophilic aromatic substitution (part Ⅰ): The nitration of some reactive aromatic compounds in concentrated sulphuric and perchloric acids[J]. Journal of the Chemical Society B: Physical Organic, 1968: 800-804. |
| 6 | Barnett J W, Moodie R B, Schofield K, et al. Electrophilic aromatic substitution (part ): Kinetics, isomer yields, and the consequences of ipso-attack in the nitration of toluene and polymethylbenzenes in aqueous sulphuric acid, and their significance for the mechanism of aromatic nitration[J]. Journal of the Chemical Society, Perkin Transactions 2, 1975(6): 648-654. |
| 7 | Sreedhar I, Singh M, Raghavan K V. Scientific advances in sulfuric acid free toluene nitration[J]. Catalysis Science & Technology, 2013, 3(10): 2499-2508. |
| 8 | Marziano N C, Traverso P G, Cimino G G. Thermodynamic nitration rates of aromatic compounds (part Ⅰ): The nitration of benzene and some benzene derivatives in aqueous sulphuric and perchloric acids. A comparison of the results referred to water as standard state[J]. Journal of the Chemical Society, Perkin Transactions 2, 1980(4): 574-578. |
| 9 | Marziano N C, Sampoli M, Pinna F, et al. Thermodynamic nitration rates of aromatic compounds (part Ⅱ): Linear description of rate profiles for the nitration of aromatic compounds in the range 40—98 wt% sulphuric acid[J]. Journal of the Chemical Society, Perkin Transactions 2, 1984(7): 1163-1166. |
| 10 | Marziano N C, Tortato C, Sampoli M. Thermodynamic nitration rates of aromatic compounds (part Ⅲ): Nitration of aromatic compounds in concentrated aqueous trifluoromethanesulphonic acid[J]. Journal of the Chemical Society, Perkin Transactions 2, 1991(3): 423-429. |
| 11 | Marziano N C, Tomasin A, Tortato C, et al. Thermodynamic nitration rates of aromatic compounds (part Ⅳ): Temperature dependence in sulfuric acid of HNO3→NO2 + equilibrium, nitration rates and acidic properties of the solvent [J]. Journal of the Chemical Society, Perkin Transactions 2, 1998(9): 1973-1982. |
| 12 | Wang K, Li L T, Xie P, et al. Liquid-liquid microflow reaction engineering[J]. Reaction Chemistry & Engineering, 2017, 2(5): 611-627. |
| 13 | Wang K, Luo G S. Microflow extraction: a review of recent development[J]. Chemical Engineering Science, 2017, 169: 18-33. |
| 14 | Luo G S, Du L, Wang Y J, et al. Recent developments in microfluidic device-based preparation, functionalization, and manipulation of nano- and micro-materials[J]. Particuology, 2019, 45: 1-19. |
| 15 | Kulkarni A A. Continuous flow nitration in miniaturized devices[J]. Beilstein Journal of Organic Chemistry, 2014, 10: 405-424. |
| 16 | Burns J R, Ramshaw C. A microreactor for the nitration of benzene and toluene[J]. Chemical Engineering Communications, 2002, 189(12): 1611-1628. |
| 17 | Chen Y Z, Zhao Y C, Han M, et al. Safe, efficient and selective synthesis of dinitro herbicides via a multifunctional continuous-flow microreactor: one-step dinitration with nitric acid as agent[J]. Green Chemistry, 2013, 15(1): 91-94. |
| 18 | Panke G, Schwalbe T, Stirner W, et al. A practical approach of continuous processing to high energetic nitration reactions in microreactors[J]. Synthesis, 2003(18): 2827-2830. |
| 19 | Ducry L, Roberge D M. Controlled autocatalytic nitration of phenol in a microreactor[J]. Angewandte Chemie International Edition, 2005, 44(48): 7972-7975. |
| 20 | Dummann G, Quittmann U, Gröschel L, et al. The capillary-microreactor: a new reactor concept for the intensification of heat and mass transfer in liquid-liquid reactions[J]. Catalysis Today, 2003, 79/80: 433-439. |
| 21 | Fu G, Ni L, Wei D, et al. Scale-up and safety of toluene nitration in a meso-scale flow reactor[J]. Process Safety and Environmental Protection, 2022, 160: 385-396. |
| 22 | Yang A M, Yue J C, Zheng S Q, et al. Experimental investigation of mononitrotoluene preparation in a continuous-flow microreactor[J]. Research on Chemical Intermediates, 2022, 48(10): 4373-4390. |
| 23 | Han B C, Chen Y D, Zou H W, et al. Study on characteristics of toluene/chlorobenzene nitrification in different microreactors[J]. Chemical Engineering Research and Design, 2024, 205: 343-353. |
| 24 | Fischer A, Fyles D L, Henderson G N, et al. Ipso nitration (Ⅹ Ⅹ Ⅷ): Nitration of 4-substituted toluenes: 1, 2 adducts[J]. Canadian Journal of Chemistry, 1986, 64(9): 1764-1770. |
| 25 | Hirose A, Matsui K, Sekiguchi S. Aromatic nitration (Ⅲ): Competitive nitration of toluene and benzene in carbon tetrachloride[J]. Bulletin of the Chemical Society of Japan, 1972, 45(9): 2955-2956. |
| 26 | Yan Z F, Tian J X, Du C C, et al. Reaction kinetics determination based on microfluidic technology[J]. Chinese Journal of Chemical Engineering, 2022, 41: 49-72. |
| 27 | Rahaman M, Mandal B P, Ghosh P. Nitration of nitrobenzene at high-concentrations of sulfuric acid[J]. AIChE Journal, 2007, 53(9): 2476-2480. |
| 28 | Rahaman M, Mandal B, Ghosh P. Nitration of nitrobenzene at high-concentrations of sulfuric acid: mass transfer and kinetic aspects[J]. AIChE Journal, 2010, 56(3): 737-748. |
| 29 | Ravikumar Bandaru S V, Ghosh P. Mass transfer of chlorobenzene in concentrated sulfuric acid[J]. International Journal of Heat and Mass Transfer, 2011, 54(11/12): 2245-2252. |
| 30 | Jin N, Song Y B, Yue J, et al. Heterogeneous nitration of nitrobenzene in microreactors: process optimization and modelling[J]. Chemical Engineering Science, 2023, 281: 119198. |
| 31 | Wen Z H, Yang M, Zhao S N, et al. Kinetics study of heterogeneous continuous-flow nitration of trifluoromethoxybenzene[J]. Reaction Chemistry & Engineering, 2018, 3(3): 379-387. |
| 32 | Song J, Cui Y J, Wang Y J, et al. Accurate determination of the kinetics of toluene nitration in a liquid-liquid microflow system[J]. Journal of Flow Chemistry, 2023, 13(3): 311-323. |
| 33 | Li L, Yao C Q, Jiao F J, et al. Experimental and kinetic study of the nitration of 2-ethylhexanol in capillary microreactors[J]. Chemical Engineering and Processing: Process Intensification, 2017, 117: 179-185. |
| 34 | Song J, Cui Y J, Luo G S, et al. Kinetic study of o-nitrotoluene nitration in a homogeneously continuous microflow[J]. Reaction Chemistry & Engineering, 2022, 7(1): 111-122. |
| 35 | Song J, Cui Y J, Sheng L, et al. Determination of nitration kinetics of p-nitrotoluene with a homogeneously continuous microflow[J]. Chemical Engineering Science, 2022, 247: 117041. |
| 36 | Marziano N C, Cimino G M, Passerini R C. The MC activity coefficient function for acid-base equilibria (part Ⅰ): New methods for estimating pKa values for weak bases[J]. J. Chem. Soc., Perkin Trans. 2, 1973(14): 1915-1922. |
| 37 | Marziano N C, Traverso P G, Passerini R C. The MC activity coefficient function for acid-base equilibria (part Ⅱ): A critical analysis of acidity functions and the incompatibility amongst proposed empirical correlations[J]. J. Chem. Soc., Perkin Trans. 2, 1977(3): 306-309. |
| 38 | Marziano N C, Traverso P G, Tomasin A, et al. The MC activity coefficient function for acid-base equilibria (part Ⅲ): Improvement on the MC function by mathematical treatment[J]. J. Chem. Soc., Perkin Trans 2, 1977(3): 309-313. |
| 39 | Traverso P G, Marziano N C, Passerini R C. The MC activity coefficient function for acid-base equilibria (part Ⅳ): Limitations of empirical relationships involving observed nitration rates and acidity functions[J]. J. Chem. Soc., Perkin Trans 2, 1977(6): 845-847. |
| 40 | Cui Y J, Song J, Du C C, et al. Determination of the kinetics of chlorobenzene nitration using a homogeneously continuous microflow[J]. AIChE Journal, 2022, 68(4): e17564. |
| 41 | Zhang J S, Wang K, Lu Y C, et al. Characterization and modeling of micromixing performance in micropore dispersion reactors[J]. Chemical Engineering and Processing: Process Intensification, 2010, 49(7): 740-747. |
| 42 | Chen Q C, Wang Y B, Du C C, et al. Micromixing performance of a miniaturized annular rotating flow mixer (MARFM)[J]. Chemical Engineering and Processing-Process Intensification, 2022, 182: 109181. |
| 43 | Chen Q C, Wang Y B, Deng J, et al. Micromixing intensification by gas introduction in a miniaturized annular rotating flow mixer (MARFM)[J]. Chemical Engineering Science, 2023, 272: 118610. |
| 44 | Song J, Cheng B, Wang Y J, et al. A microfluidic chip structure with ultra-high liquid-liquid mass transfer performance[J]. Separation and Purification Technology, 2023, 324: 124440. |
| 45 | Russo D, Tomaiuolo G, Andreozzi R, et al. Heterogeneous benzaldehyde nitration in batch and continuous flow microreactor[J]. Chemical Engineering Journal, 2019, 377: 120346. |
| 46 | Lan Z, Lu Y C. Continuous nitration of o-dichlorobenzene in micropacked-bed reactor: process design and modelling[J]. Journal of Flow Chemistry, 2021, 11(2): 171-179. |
| 47 | Du C C, Wang P C, Hu Y P, et al. Liquid-liquid mass transfer enhancement in milliscale packed beds[J]. Industrial & Engineering Chemistry Research, 2020, 59(9): 4048-4057. |
| 48 | Zhang C Y, Zhang J S, Luo G S. Kinetic study and intensification of acetyl guaiacol nitration with nitric acid-acetic acid system in a microreactor[J]. Journal of Flow Chemistry, 2016, 6(4): 309-314. |
| 49 | Guo S, Cao J Y, Liu M Q, et al. Intensification and kinetic study of trifluoromethylbenzen nitration with mixed acid in the microreactor[J]. Chemical Engineering and Processing-Process Intensification, 2023, 183: 109239. |
| 50 | Yu Z Q, Lv Y W, Yu C M, et al. A high-output, continuous selective and heterogeneous nitration of p-difluorobenzene[J]. Organic Process Research & Development, 2013, 17(3): 438-442. |
| 51 | Kulkarni A A, Kalyani V S, Joshi R A, et al. Continuous flow nitration of benzaldehyde[J]. Organic Process Research & Development, 2009, 13(5): 999-1002. |
| 52 | Knapkiewicz P, Skowerski K, Jaskólska D E, et al. Nitration under continuous flow conditions: convenient synthesis of 2-isopropoxy-5-nitrobenzaldehyde, an important building block in the preparation of nitro-substituted Hoveyda-Grubbs metathesis catalyst[J]. Organic Process Research & Development, 2012, 16(8): 1430-1435. |
| 53 | Mittal A K, Prakash G, Pathak P, et al. Continuous flow synthesis of tert-butyl nitrite and its applications as nitrating agent[J]. Organic Process Research & Development, 2024, 28(5): 1510-1514. |
| 54 | Wang K, Lu Y C, Luo G S. Strategy for scaling-up of a microsieve dispersion reactor[J]. Chemical Engineering & Technology, 2014, 37(12): 2116-2122. |
| 55 | Shao H W, Lu Y C, Wang K, et al. Liquid-liquid flow and mass transfer characteristics in micro-sieve array device with dual-sized pores[J]. Chemical Engineering Journal, 2012, 193/194: 96-101. |
| 56 | Song J, Sheng L, Cui Y J, et al. Liquid-liquid colliding micro-dispersion and general scaling laws in novel T-junction microdevices[J]. Chemical Engineering Science, 2022, 258: 117746. |
| 57 | Sheng L, Ma L, Chen Y C, et al. A comprehensive study of droplet formation in a capillary embedded step T-junction: from squeezing to jetting[J]. Chemical Engineering Journal, 2022, 427: 132067. |
| 58 | Plouffe P, Roberge D M, Sieber J, et al. Liquid-liquid mass transfer in a serpentine micro-reactor using various solvents[J]. Chemical Engineering Journal, 2016, 285: 605-615. |
| 59 | Kurt S K, Vural Gürsel I, Hessel V, et al. Liquid-liquid extraction system with microstructured coiled flow inverter and other capillary setups for single-stage extraction applications[J]. Chemical Engineering Journal, 2016, 284: 764-777. |
| 60 | Plouffe P, Roberge D M, Macchi A. Liquid-liquid flow regimes and mass transfer in various micro-reactors[J]. Chemical Engineering Journal, 2016, 300: 9-19. |
| 61 | Zhang J S, Wang K, Lin X Y, et al. Intensification of fast exothermic reaction by gas agitation in a microchemical system[J]. AIChE Journal, 2014, 60(7): 2724-2730. |
| 62 | Lin X Y, Wang K, Zhang J S, et al. Liquid-liquid mixing enhancement rules by microbubbles in three typical micro-mixers[J]. Chemical Engineering Science, 2015, 127: 60-71. |
| 63 | Chen Q C, Deng J, Luo G S. Micromixing performance and residence time distribution in a miniaturized magnetic reactor: experimental investigation and machine learning modeling[J]. Industrial & Engineering Chemistry Research, 2023, 62(8): 3577-3591. |
| 64 | Wang Y B, Tang T Y, Yan Z F, et al. Liquid-liquid dispersion and flow characteristics in a miniaturized annular rotating device[J]. Chemical Engineering Journal, 2023, 454: 140374. |
| 65 | Wang Y B, Du C C, Yan Z F, et al. Liquid-liquid flow and mass transfer characteristics in a miniaturized annular centrifugal device[J]. Chemical Engineering Journal, 2022, 431: 134264. |
| 66 | 李光晓, 刘塞尔, 苏远海. 微尺度内液-液传质及反应过程强化的研究进展[J]. 化工学报, 2021, 72(1): 452-467. |
| Li G X, Liu S E, Su Y H. Research progress on micro-scale internal liquid-liquid mass transfer and reaction process enhancement[J]. CIESC Journal, 2021, 72(1): 452-467. | |
| 67 | Knauf T, Racoes A, Dohmen W, et al. Method for producing nitrobenzene by adiabatic nitriding: US20150175521[P]. 2015-06-25. |
| 68 | 邓建, 骆广生. 一种连续绝热高温硝化甲苯制备单硝基甲苯的方法: 111004124A[P]. 2020-04-14. |
| Deng J, Luo G S. A method for preparing mononitrotoluene by continuous adiabatic high-temperature nitration of toluene: 111004124A[P]. 2020-04-14. | |
| 69 | 邓建, 王凯, 骆广生. 面向硝基化学品安全生产的绝热连续微反应技术发展及思考[J]. 化工进展, 2023, 42(8): 3923-3925. |
| Deng J, Wang K, Luo G S. Development and consideration of adiabatic continuous microreaction technology for safe production of nitro compounds[J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3923-3925. | |
| 70 | Liu Y C, Jiang J C, Huang A C, et al. Hazard assessment of the thermal stability of nitrification by-products by using an advanced kinetic model[J]. Process Safety and Environmental Protection, 2022, 160: 91-101. |
| 71 | Qian Y N, Chen M M, Zhan J H, et al. Thermal runaway mechanisms and kinetics of benzene nitrification system based on microcalorimetry experiments and ReaxFF molecular dynamics simulation[J]. Thermochimica Acta, 2023, 725: 179522. |
| 72 | Yao H, Ni L, Liu Y S, et al. Process hazard and thermal risk evaluation of m-xylene nitration with mixed acid[J]. Process Safety and Environmental Protection, 2023, 175: 238-250. |
| [1] | 徐英宇, 杨国强, 彭璟, 孙海宁, 张志炳. 微界面高级氧化处理煤化工废水的研究[J]. 化工学报, 2024, 75(S1): 283-291. |
| [2] | 赵焕娟, 包颖昕, 于康, 刘婧, 钱新明. 多元组分爆轰不稳定性定量实验研究[J]. 化工学报, 2024, 75(S1): 339-348. |
| [3] | 杨子驰, 谢冰琪, 石瑞莘, 雷虹, 陈晨, 周才金, 张吉松. 套管膜式微反应器内高效安全的气液传质-反应过程研究进展[J]. 化工学报, 2024, 75(9): 3011-3027. |
| [4] | 徐宏标, 杨亮, 李子栋, 刘道平. 盐水微滴/泡沫铜复合体系中甲烷水合物生成动力学研究[J]. 化工学报, 2024, 75(9): 3287-3296. |
| [5] | 祝赫, 张仪, 齐娜娜, 张锴. 欧拉-欧拉双流体模型中颗粒黏性对液固散式流态化的影响[J]. 化工学报, 2024, 75(9): 3103-3112. |
| [6] | 丁湧, 李文建, 陈昭宇, 曹立辉, 刘轩铭, 任强强, 胡松, 向军. 废旧晶体硅光伏组件EVA有氧热解动力学与产物特性[J]. 化工学报, 2024, 75(9): 3310-3319. |
| [7] | 唐昊, 胡定华, 李强, 张轩畅, 韩俊杰. 抗加速度双切线弧流道内气泡动力学行为数值与可视化研究[J]. 化工学报, 2024, 75(9): 3074-3082. |
| [8] | 罗正航, 李敬宇, 陈伟雄, 种道彤, 严俊杰. 摇摆运动下低流率蒸汽冷凝换热特性和气泡受力数值模拟[J]. 化工学报, 2024, 75(8): 2800-2811. |
| [9] | 曾港, 陈林, 杨董, 袁海专, 黄彦平. 矩形通道内超临界CO2局部热流场可视化实验[J]. 化工学报, 2024, 75(8): 2831-2839. |
| [10] | 罗莉, 陈文尧, 张晶, 钱刚, 周兴贵, 段学志. 氧化铝结构与表面性质调控及其催化甲醇脱水制二甲醚性能研究[J]. 化工学报, 2024, 75(7): 2522-2532. |
| [11] | 董可豪, 周敬之, 周峰, 陈海家, 淮秀兰, 李栋. 超薄空间复杂边界条件下气体流动压降实验[J]. 化工学报, 2024, 75(7): 2505-2521. |
| [12] | 吴邦汉, 林定标, 陆海峰, 郭晓镭, 刘海峰. 竖直管气动物流传输系统管道压降和传送瓶输送特性[J]. 化工学报, 2024, 75(7): 2465-2473. |
| [13] | 马君霞, 李林涛, 熊伟丽. 基于Tri-training GPR的半监督软测量建模方法[J]. 化工学报, 2024, 75(7): 2613-2623. |
| [14] | 杨艳, 郭亚丽, 于硕文, 潘泊年, 沈胜强. 液氨喷射泵热力性能的计算分析[J]. 化工学报, 2024, 75(6): 2134-2142. |
| [15] | 徐嘉宇, 陈飞国, 徐骥, 葛蔚. 颗粒体系的多尺度混合指数[J]. 化工学报, 2024, 75(6): 2214-2221. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号