化工学报 ›› 2024, Vol. 75 ›› Issue (6): 2262-2273.DOI: 10.11949/0438-1157.20240088
收稿日期:2024-01-18
修回日期:2024-02-29
出版日期:2024-06-25
发布日期:2024-07-03
通讯作者:
潘艳秋
作者简介:霍宗伟(1999—),男,硕士研究生,hzw_dut0402@163.com
基金资助:
Zongwei HUO( ), Yabin NIU, Yanqiu PAN(
), Yabin NIU, Yanqiu PAN( )
)
Received:2024-01-18
Revised:2024-02-29
Online:2024-06-25
Published:2024-07-03
Contact:
Yanqiu PAN
摘要:
膜法分离油田采出废水中的重油组分是膜污染的重要影响因素,且不同膜分离条件下膜面附近的复杂油滴行为直接影响膜分离效果,所以研究膜面附近的高黏度油滴行为十分必要。基于实际油田采出废水物性数据的实验测定结果,采用耗散粒子动力学(DPD)方法结合高黏度油滴轨迹和油滴间相互作用能结果对油滴行为进行细化分析。结果表明,双油滴呈现掠过和聚并两大类行为,又可各分为三个行为阶段:油滴间排液、油滴接触和油滴复稳;双油滴间距和油滴与膜面间距的减小有助于油滴聚并,从而有利于油水分离,且油滴与膜面间距的减小会导致油滴碰撞后的位置更远离膜面;表面活性剂的存在阻碍油滴聚并,不利于油水分离,且离子型表面活性剂因其亲水头间的强排斥力而阻碍作用更强;第3油滴的存在会促进双油滴聚并,有利于油水分离,其促聚并程度受油滴碰撞角的影响。研究结果可为后续的油滴与分离膜相互作用及油水分离效果的研究提供依据,也可为油水膜分离条件的设置和技术优化提供理论支持。
中图分类号:
霍宗伟, 牛亚宾, 潘艳秋. 油水膜分离中高黏度油滴行为研究和影响因素分析[J]. 化工学报, 2024, 75(6): 2262-2273.
Zongwei HUO, Yabin NIU, Yanqiu PAN. Behavior of high viscosity oil droplets in oil-water membrane separation and its influencing factors[J]. CIESC Journal, 2024, 75(6): 2262-2273.
| 体系分类 | 自扩散系数/(m2/s) | 动力黏度/(mPa·s) | 界面张力/(mN/m) |
|---|---|---|---|
| 水[ | 2.30×10-9 | 0.89 | — |
| 油 | 2.02×10-11 | 136.14 | — |
| 油-水 | — | — | 8.9654 |
| 油-水-CTAB | — | — | 0.2746 |
| 油-水-SDS | — | — | 0.3261 |
| 油-水-SLS | — | — | 1.7496 |
| 油-水-Tween-80 | — | — | 7.6272 |
表1 物性参数实验测定结果
Table 1 Experimental results of physical property parameters
| 体系分类 | 自扩散系数/(m2/s) | 动力黏度/(mPa·s) | 界面张力/(mN/m) |
|---|---|---|---|
| 水[ | 2.30×10-9 | 0.89 | — |
| 油 | 2.02×10-11 | 136.14 | — |
| 油-水 | — | — | 8.9654 |
| 油-水-CTAB | — | — | 0.2746 |
| 油-水-SDS | — | — | 0.3261 |
| 油-水-SLS | — | — | 1.7496 |
| 油-水-Tween-80 | — | — | 7.6272 |
| 名称 | DPD无量纲符号 | DPD无量纲单位 | 实际值 |
|---|---|---|---|
| 质量 | m | 1 | 1.14×10-24 kg |
| 长度 | rc、x、z、…… | 1 | 1.5066×10-9 m |
| 温度 | Θ | 1 (kBT) | 298 K |
| 能量 | E | 1 (kBT) | 4.1164×10-21 J |
| 时间 | t | 1 (rc(m/E)1/2) | 25.0266 ps |
| 速度 | v | 1 (1/(m/E)1/2) | 60.1999 m/s |
| 剪切速率 | G | 1 (1/t) | 0.03996 ps-1 |
表2 DPD无量纲单位及对应实际值
Table 2 DPD dimensionless units and corresponding actual values
| 名称 | DPD无量纲符号 | DPD无量纲单位 | 实际值 |
|---|---|---|---|
| 质量 | m | 1 | 1.14×10-24 kg |
| 长度 | rc、x、z、…… | 1 | 1.5066×10-9 m |
| 温度 | Θ | 1 (kBT) | 298 K |
| 能量 | E | 1 (kBT) | 4.1164×10-21 J |
| 时间 | t | 1 (rc(m/E)1/2) | 25.0266 ps |
| 速度 | v | 1 (1/(m/E)1/2) | 60.1999 m/s |
| 剪切速率 | G | 1 (1/t) | 0.03996 ps-1 |
| 液相类型 | Nm_par | s | γ | rc |
|---|---|---|---|---|
| 水相 | 4 | 0.25 | 20 | 1.0 |
| 油相 | 4 | 0.25 | 2650 | 1.0 |
| 油水相间 | 4 | 0.25 | 39.7 | 1.0 |
表3 DPD模拟参数集结果
Table 3 Results of DPD simulation parameters
| 液相类型 | Nm_par | s | γ | rc |
|---|---|---|---|---|
| 水相 | 4 | 0.25 | 20 | 1.0 |
| 油相 | 4 | 0.25 | 2650 | 1.0 |
| 油水相间 | 4 | 0.25 | 39.7 | 1.0 |
| 珠子类型 | T-H | T | T-T | O | W | N | NA | S1 | S2 | BR | TIO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T-H | 98.85 | ||||||||||
| T | 123.97 | 132.10 | |||||||||
| T-T | 118.01 | 134.73 | 105.28 | ||||||||
| O | 125.59 | 86.69 | 95.60 | 86.20 | |||||||
| W | 136.98 | 153.27 | 145.08 | 197.24 | 104.00 | ||||||
| N | 49.48 | 130.75 | 29.33 | 126.02 | 8.13 | 265.32 | |||||
| NA | 33.78 | 160.55 | 35.40 | 131.10 | 55.69 | 261.00 | 276.54 | ||||
| S1 | 31.69 | 145.39 | 56.42 | 145.89 | 36.07 | 0 | 0 | 239.10 | |||
| S2 | 41.82 | 150.08 | 63.15 | 166.39 | 65.43 | 0 | 0 | 246.92 | 259.00 | ||
| BR | 63.96 | 165.04 | 83.17 | 241.39 | 101.53 | 0 | 0 | 251.53 | 259.31 | 351.76 | |
| TIO | 108.18 | 162.72 | 141.61 | 196.84 | 66.70 | 63.80 | 138.58 | 13.02 | 10.59 | 168.58 | 0 |
表4 包含珠子间静电相互作用的保守力参数
Table 4 Conservative force parameters including electrostatic interactions between beads
| 珠子类型 | T-H | T | T-T | O | W | N | NA | S1 | S2 | BR | TIO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T-H | 98.85 | ||||||||||
| T | 123.97 | 132.10 | |||||||||
| T-T | 118.01 | 134.73 | 105.28 | ||||||||
| O | 125.59 | 86.69 | 95.60 | 86.20 | |||||||
| W | 136.98 | 153.27 | 145.08 | 197.24 | 104.00 | ||||||
| N | 49.48 | 130.75 | 29.33 | 126.02 | 8.13 | 265.32 | |||||
| NA | 33.78 | 160.55 | 35.40 | 131.10 | 55.69 | 261.00 | 276.54 | ||||
| S1 | 31.69 | 145.39 | 56.42 | 145.89 | 36.07 | 0 | 0 | 239.10 | |||
| S2 | 41.82 | 150.08 | 63.15 | 166.39 | 65.43 | 0 | 0 | 246.92 | 259.00 | ||
| BR | 63.96 | 165.04 | 83.17 | 241.39 | 101.53 | 0 | 0 | 251.53 | 259.31 | 351.76 | |
| TIO | 108.18 | 162.72 | 141.61 | 196.84 | 66.70 | 63.80 | 138.58 | 13.02 | 10.59 | 168.58 | 0 |
| 体系分类 | 实验值/ (mN/m) | 模拟值/ (mN/m) | 相对 误差/% |
|---|---|---|---|
| 油-水 | 8.9654 | 9.6743 | 7.91 |
| 油-水-CTAB | 0.2746 | 0.2539 | 7.54 |
| 油-水-SDS | 0.3261 | 0.3815 | 16.99 |
| 油-水-SLS | 1.7496 | 1.6540 | 5.46 |
| 油-水-Tween-80 | 7.6272 | 6.7254 | 11.82 |
表5 界面张力实验值及模拟值
Table 5 Experimental and simulated results of interfacial tension
| 体系分类 | 实验值/ (mN/m) | 模拟值/ (mN/m) | 相对 误差/% |
|---|---|---|---|
| 油-水 | 8.9654 | 9.6743 | 7.91 |
| 油-水-CTAB | 0.2746 | 0.2539 | 7.54 |
| 油-水-SDS | 0.3261 | 0.3815 | 16.99 |
| 油-水-SLS | 1.7496 | 1.6540 | 5.46 |
| 油-水-Tween-80 | 7.6272 | 6.7254 | 11.82 |
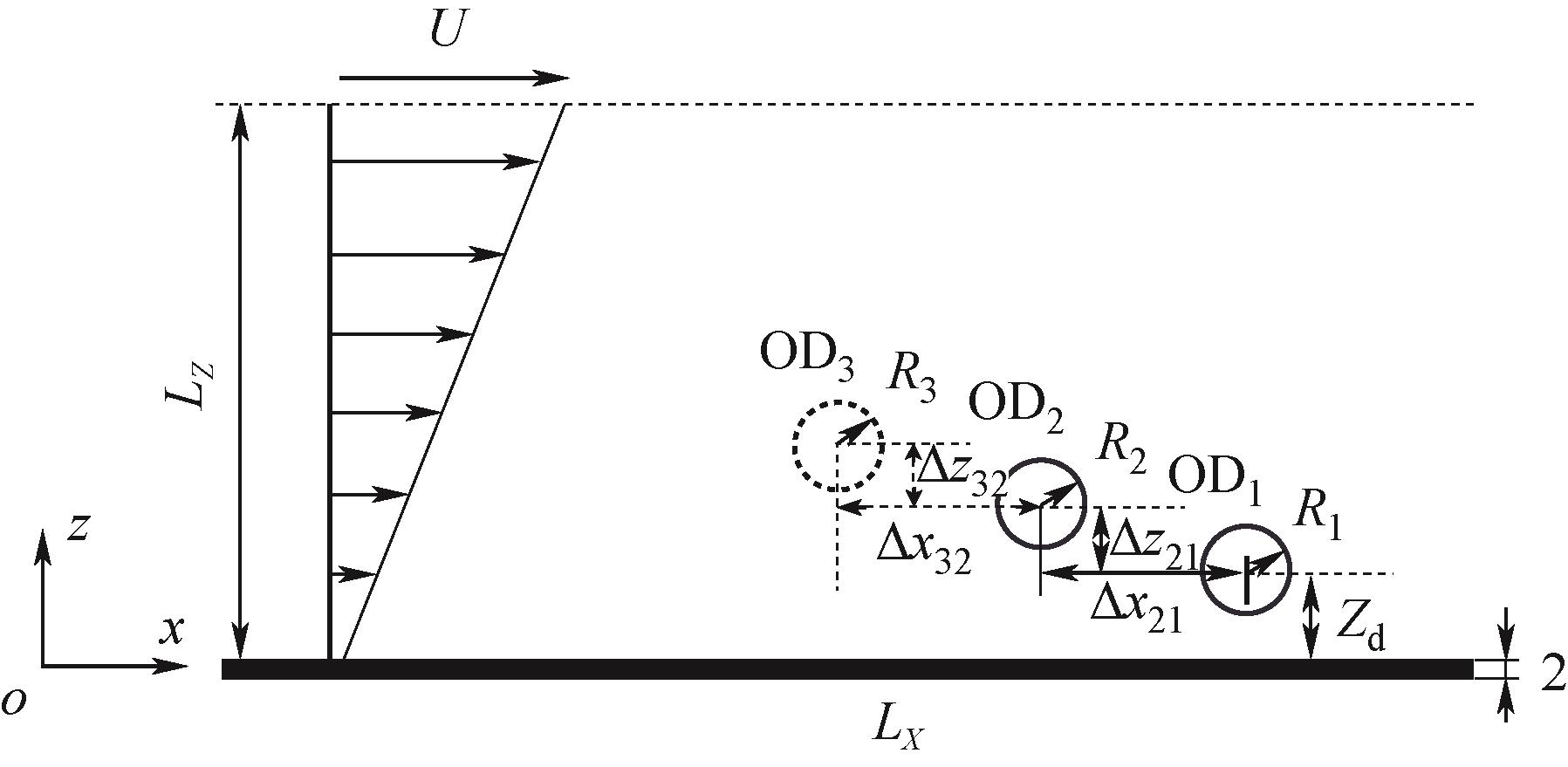
图3 膜面附近剪切流下双、三油滴体系物理模型示意图
Fig.3 Schematic diagram of the physical model of double and triple oil droplet system under shear flow near the membrane surface
| OD3位置 | θ12/(°) | θ23/(°) | 中间行为 | 影响阶段 | 区域和易聚并程度① | |
|---|---|---|---|---|---|---|
| 三油滴对照组 | 25.36 | 30.28 | OD2-OD3先排液、先碰撞 | 油滴间排液阶段(Ⅰ) | A(++) | |
| Δz32_0改变 | 0.9R1 | 30.34 | 27.74 | OD1-OD2先排液,OD2-OD3先碰撞 | A(+++) | |
| 0.8R1 | 30.68 | 20.97 | B(+) | |||
| 0.7R1 | 39.28 | 19.29 | OD1-OD2碰撞前中程时OD2-OD3碰撞 | 油滴碰撞阶段(Ⅱ-1) | A(++) | |
| Δx32_0改变 | -5.25R1 | 39.01 | 28.59 | A(++++) | ||
| -5.50R1 | 55.31 | 20.84 | A(+++) | |||
| -5.75R1 | 81.14 | 18.87 | OD1-OD2碰撞后程时OD2-OD3碰撞 | 油滴逃离阶段(Ⅱ-2) | B(+) | |
| -6.00R1 | 93.82 | 18.35 | B(+) | |||
表6 Δz32_0和Δx32_0改变时的油滴行为结果
Table 6 Oil droplet behavior results when Δz32_0 and Δx32_0 change
| OD3位置 | θ12/(°) | θ23/(°) | 中间行为 | 影响阶段 | 区域和易聚并程度① | |
|---|---|---|---|---|---|---|
| 三油滴对照组 | 25.36 | 30.28 | OD2-OD3先排液、先碰撞 | 油滴间排液阶段(Ⅰ) | A(++) | |
| Δz32_0改变 | 0.9R1 | 30.34 | 27.74 | OD1-OD2先排液,OD2-OD3先碰撞 | A(+++) | |
| 0.8R1 | 30.68 | 20.97 | B(+) | |||
| 0.7R1 | 39.28 | 19.29 | OD1-OD2碰撞前中程时OD2-OD3碰撞 | 油滴碰撞阶段(Ⅱ-1) | A(++) | |
| Δx32_0改变 | -5.25R1 | 39.01 | 28.59 | A(++++) | ||
| -5.50R1 | 55.31 | 20.84 | A(+++) | |||
| -5.75R1 | 81.14 | 18.87 | OD1-OD2碰撞后程时OD2-OD3碰撞 | 油滴逃离阶段(Ⅱ-2) | B(+) | |
| -6.00R1 | 93.82 | 18.35 | B(+) | |||
| 1 | Liang B, He X, Hou J J, et al. Membrane separation in organic liquid: technologies, achievements, and opportunities[J]. Advanced Materials, 2019, 31(45): e1806090. |
| 2 | Zhao G J, Han W J, Dong L L, et al. Sprayed separation membranes: a systematic review and prospective opportunities[J]. Green Energy & Environment, 2022, 7(6): 1143-1160. |
| 3 | Guiga W F, Lameloise M L. Membrane separation in food processing[M]//Green Food Processing Techniques. Amsterdam: Elsevier, 2019: 245-287. |
| 4 | Sirkar K K. Application of membrane technologies in the pharmaceutical industry[J]. Current Opinion in Drug Discovery & Development, 2000, 3(6): 714-722. |
| 5 | Jawad J, Hawari A H, Javaid Zaidi S. Artificial neural network modeling of wastewater treatment and desalination using membrane processes: a review[J]. Chemical Engineering Journal, 2021, 419: 129540. |
| 6 | Sutrisna P D, Kurnia K A, Siagian U W R, et al. Membrane fouling and fouling mitigation in oil-water separation: a review[J]. Journal of Environmental Chemical Engineering, 2022, 10(3): 107532. |
| 7 | Megias-Alguacil D, Feigl K, Dressler M, et al. Droplet deformation under simple shear investigated by experiment, numerical simulation and modeling[J]. Journal of Non-Newtonian Fluid Mechanics, 2005, 126(2/3): 153-161. |
| 8 | Vananroye A, van Puyvelde P, Moldenaers P. Effect of confinement on the steady-state behavior of single droplets during shear flow[J]. Journal of Rheology, 2007, 51(1): 139-153. |
| 9 | de Bruyn P, Chen D J, Moldenaers P, et al. The effects of geometrical confinement and viscosity ratio on the coalescence of droplet pairs in shear flow[J]. Journal of Rheology, 2014, 58(6): 1955-1980. |
| 10 | 桑义敏, 陈家庆, 易国庆, 等. 水包油型乳化液油滴的管内节流破碎行为与机理[J]. 过程工程学报, 2015, 15(6): 940-944. |
| Sang Y M, Chen J Q, Yi G Q, et al. Break-up behaviors and mechanism of oil-in-water emulsion droplets through a pipe restriction[J]. The Chinese Journal of Process Engineering, 2015, 15(6): 940-944. | |
| 11 | Li X Q, Fan Y Z, Liu R Q, et al. Numerical investigation of oil droplets motion in water using LBM[J]. Process Safety and Environmental Protection, 2021, 147: 965-971. |
| 12 | Hafsi Z, Elaoud S, Mishra M, et al. Numerical study of droplets coalescence in an oil-water separator[C]//Advances in Mechanical Engineering, Materials and Mechanics: Selected contributions from the 7th International Conference on Advances in Mechanical Engineering and Mechanics. Hammamet, Tunisia: Springer International Publishing, 2021: 449-454. |
| 13 | Zhang Y Z, Xu J B, He X F. Effect of surfactants on the deformation of single droplet in shear flow studied by dissipative particle dynamics[J]. Molecular Physics, 2018, 116(14): 1851-1861. |
| 14 | Pan D Y, Lin Y Q, Zhang L X, et al. Motion and deformation of immiscible droplet in plane Poiseuille flow at low Reynolds number[J]. Journal of Hydrodynamics, Ser. B, 2016, 28(4): 702-708. |
| 15 | Gao S J, Sun J C, Liu P P, et al. A robust polyionized hydrogel with an unprecedented underwater anti-crude-oil-adhesion property[J]. Advanced Materials, 2016, 28(26): 5307-5314. |
| 16 | Hoogerbrugge P J, Koelman J M V A. Simulating microscopic hydrodynamic phenomena with dissipative particle dynamics[J]. Europhysics Letters, 1992, 19: 155. |
| 17 | Español P, Warren P. Statistical mechanics of dissipative particle dynamics[J]. Europhysics Letters, 1995, 30(4): 191-196. |
| 18 | 张雪芳, 潘艳秋, 陈鹏鹏, 等. 乳化剂对动态膜分离油水乳化液过程的影响[J]. 化工进展, 2019, 38(2): 790-797. |
| Zhang X F, Pan Y Q, Chen P P, et al. Impact of emulsifier on separation of oil-in-water emulsion by dynamic membrane[J]. Chemical Industry and Engineering Progress, 2019, 38(2): 790-797. | |
| 19 | 王世荣, 李祥高, 刘东志, 等. 表面活性剂化学[M]. 2版. 北京: 化学工业出版社, 2010. |
| Wang S R, Li X G, Liu D Z. Surfactant Chemistry[M]. 2nd ed. Beijing: Chemical Industry Press, 2010. | |
| 20 | Harris K R, Woolf L A. Pressure and temperature dependence of the self diffusion coefficient of water and oxygen-18 water[J]. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, 1980, 76: 377. |
| 21 | Huber M L, Perkins R A, Laesecke A, et al. New international formulation for the viscosity of H2O[J]. Journal of Physical and Chemical Reference Data, 2009, 38(2): 101-125. |
| 22 | Groot R D, Rabone K L. Mesoscopic simulation of cell membrane damage, morphology change and rupture by nonionic surfactants[J]. Biophysical Journal, 2001, 81(2): 725-736. |
| 23 | Liu J, Zheng Z J, Li F Z, et al. Nanoparticle chemically end-linking elastomer network with super-low hysteresis loss for fuel-saving automobile[J]. Nano Energy, 2016, 28: 87-96. |
| 24 | Goodarzi F, Zendehboudi S. Effects of salt and surfactant on interfacial characteristics of water/oil systems: molecular dynamic simulations and dissipative particle dynamics[J]. Industrial & Engineering Chemistry Research, 2019, 58(20): 8817-8834. |
| 25 | Groot R D, Warren P B. Dissipative particle dynamics: bridging the gap between atomistic and mesoscopic simulation[J]. The Journal of Chemical Physics, 1997, 107(11): 4423-4435. |
| 26 | Gidituri H, Akella V S, Vedantam S, et al. Phase separation in binary fluid mixtures with symmetric and asymmetric Schmidt numbers: a DPD study[J]. The Journal of Chemical Physics, 2019, 150(23): 234903. |
| 27 | Krafnick R C, García A E. Efficient Schmidt number scaling in dissipative particle dynamics[J]. The Journal of Chemical Physics, 2015, 143(24): 243106. |
| 28 | Boromand A, Jamali S, Maia J M. Viscosity measurement techniques in dissipative particle dynamics[J]. Computer Physics Communications, 2015, 196: 149-160. |
| 29 | Thompson A P, Aktulga H M, Berger R, et al. LAMMPS — a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales[J]. Computer Physics Communications, 2022, 271: 108171. |
| 30 | Lauriello N, Kondracki J, Buffo A, et al. Simulation of high Schmidt number fluids with dissipative particle dynamics: parameter identification and robust viscosity evaluation[J]. Physics of Fluids, 2021, 33(7): 073106. |
| 31 | Pivkin I V, Karniadakis G E. Coarse-graining limits in open and wall-bounded dissipative particle dynamics systems[J]. The Journal of Chemical Physics, 2006, 124(18): 184101. |
| 32 | Vishnyakov A, Neimark A V. Self-assembly in Nafion membranes upon hydration: water mobility and adsorption isotherms[J]. The Journal of Physical Chemistry B, 2014, 118(38): 11353-11364. |
| 33 | Visser D C, Hoefsloot H C J, Iedema P D. Modelling multi-viscosity systems with dissipative particle dynamics[J]. Journal of Computational Physics, 2006, 214(2): 491-504. |
| 34 | Sepehr F, Paddison S J. Dissipative particle dynamics interaction parameters from ab initio calculations[J]. Chemical Physics Letters, 2016, 645: 20-26. |
| [1] | 卢飞, 鲁波娜, 许光文. 气固微型流化床反应分析仪的理想流型判据分析[J]. 化工学报, 2024, 75(6): 2201-2213. |
| [2] | 黄斌, 丰生杰, 傅程, 张威. 液滴撞击单丝铺展特性的数值研究[J]. 化工学报, 2024, 75(6): 2233-2242. |
| [3] | 李娟, 曹耀文, 朱章钰, 石雷, 李佳. 仿生正形尾鳍结构微通道流动与传热特性数值研究及结构优化[J]. 化工学报, 2024, 75(5): 1802-1815. |
| [4] | 赵光耀, 杨明磊, 钱锋. 基于降方差采样策略的随机重构法[J]. 化工学报, 2024, 75(5): 1939-1950. |
| [5] | 王金山, 王世学, 朱禹. 冷却表面温差对高温质子交换膜燃料电池性能的影响[J]. 化工学报, 2024, 75(5): 2026-2035. |
| [6] | 李怡菲, 董新宇, 王为术, 刘璐, 赵一璠. 微肋板表面干冰升华喷雾冷却传热数值模拟[J]. 化工学报, 2024, 75(5): 1830-1842. |
| [7] | 刘帆, 张芫通, 陶成, 胡成玉, 杨小平, 魏进家. 歧管式射流微通道液冷散热性能[J]. 化工学报, 2024, 75(5): 1777-1786. |
| [8] | 秦晗淞, 李国梁, 闫昊, 冯翔, 刘熠斌, 陈小博, 杨朝合. 多级孔ZSM-5分子筛中油酸甲酯催化裂解吸附和扩散行为模拟研究[J]. 化工学报, 2024, 75(5): 1870-1881. |
| [9] | 李静, 张方芳, 王帅帅, 徐建华, 张朋远. 凹腔结构对正丁烷部分预混火焰可燃极限的影响[J]. 化工学报, 2024, 75(5): 2081-2090. |
| [10] | 谢磊, 徐永生, 林梅. 不同截面肋柱-软尾结构单相流动传热比较[J]. 化工学报, 2024, 75(5): 1787-1801. |
| [11] | 王文雅, 张玮, 楼小玲, 钟若菲, 陈冰冰, 贠军贤. 纳米纤维素嵌合型晶胶微球的多微管成形与模拟[J]. 化工学报, 2024, 75(5): 2060-2071. |
| [12] | 周康, 王建新, 于海, 魏朝良, 范丰奇, 车昕昊, 张磊. 基于分子动力学模拟的矿物基础油泡沫破裂性能研究[J]. 化工学报, 2024, 75(4): 1668-1678. |
| [13] | 张子佳, 仇昕月, 孙翔, 罗志斌, 罗海中, 贺高红, 阮雪华. 聚酰亚胺膜材料分子结构设计强化CO2渗透性研究进展[J]. 化工学报, 2024, 75(4): 1137-1152. |
| [14] | 刘东飞, 张帆, 刘铮, 卢滇楠. 机器学习势及其在分子模拟中的应用综述[J]. 化工学报, 2024, 75(4): 1241-1255. |
| [15] | 张政, 汪妩琼, 张雅静, 王康军, 吉远辉. 理论计算在药物制剂设计中的研究进展[J]. 化工学报, 2024, 75(4): 1429-1438. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号