化工学报 ›› 2025, Vol. 76 ›› Issue (6): 2701-2713.DOI: 10.11949/0438-1157.20241495
赵清萍1,2,3( ), 张敏1(
), 张敏1( ), 赵辉4(
), 赵辉4( ), 王刚3,4, 邱永福1
), 王刚3,4, 邱永福1
收稿日期:2024-12-24
修回日期:2025-01-13
出版日期:2025-06-25
发布日期:2025-07-09
通讯作者:
张敏,赵辉
作者简介:赵清萍(1997—),女,硕士研究生,2353836671@qq.com
基金资助:
Qingping ZHAO1,2,3( ), Min ZHANG1(
), Min ZHANG1( ), Hui ZHAO4(
), Hui ZHAO4( ), Gang WANG3,4, Yongfu QIU1
), Gang WANG3,4, Yongfu QIU1
Received:2024-12-24
Revised:2025-01-13
Online:2025-06-25
Published:2025-07-09
Contact:
Min ZHANG, Hui ZHAO
摘要:
丙酸甲酯(MP)是一种重要的有机合成原料,也是合成甲基丙烯酸甲酯的关键中间体,在航空航天、电子信息、新能源汽车等多个领域有着广泛应用。针对MP的合成过程,构建高效的催化剂体系对满足MP需求量的快速增长具有重要的现实意义。围绕乙烯氢甲酯化的钯膦酸均相催化剂体系探究了钯前体、酸促进剂、膦/钯摩尔比和酸/钯摩尔比对催化活性的影响。通过核磁共振氢谱表征手段揭示了不同酸根阴离子与甲醇间存在氢键作用,实现对甲醇的活化脱氢,阐明了催化剂转化频率(TOF)与氢键强度的正相关关系和反应活化能与氢键强度的负相关关系。同时优化了搅拌转速、反应压力、催化剂浓度、水含量等反应条件参数,并获得了连续反应过程中催化剂活性的变化规律,表明催化体系具有良好的稳定性。最后通过反应动力学研究得到甲醇、一氧化碳、乙烯的反应级数分别为1.25、0.58、0。
中图分类号:
赵清萍, 张敏, 赵辉, 王刚, 邱永福. 乙烯氢甲酯化合成丙酸甲酯的氢键作用机制及反应动力学研究[J]. 化工学报, 2025, 76(6): 2701-2713.
Qingping ZHAO, Min ZHANG, Hui ZHAO, Gang WANG, Yongfu QIU. Hydrogen bond effect and kinetic studies on hydroesterification of ethylene to methyl propionate[J]. CIESC Journal, 2025, 76(6): 2701-2713.
| 序号 | 催化剂组成 | 反应温度/℃ | 反应压力/MPa | 转化频率TOF/h-1 | 选择性/% | 产率/% |
|---|---|---|---|---|---|---|
| 1[ | Pd(OAc)2∶PPh3∶p-TsOH=1∶30∶20 | 115 | 4.5 | 5000 | 98 | — |
| 2[ | Pd(PPh3)2(TsO)2∶PPh3∶p-TsOH=1∶6∶8 | 120 | 4 | 5700 | — | — |
| 3[ | cis-[Pd(SO4)(PPh3)2] ∶PPh3∶H2SO4=1∶107∶8 | 100 | 0.6 | 2168 | — | 98 |
| 4[ | Pd(OAc)2∶TPP∶MSA∶SATA=1∶10∶5∶60 | 100 | 2 | — | 98 | — |
| 5[ | Pd(OAc)2∶dtbpp∶MSA =1∶1.2∶2.5 | 120 | 4 | 25000 | 97.4 | — |
| 6[ | Pd(acac)2∶pytbpx∶p-TsOH=1∶1.2∶2.5 | 120 | 4 | — | 99 | 97 |
| 7[ | Pd(acac)2∶pytbpf∶p-TsOH=1∶2∶16 | 100 | 3 | 46000 | 99 | — |
| 8[ | Pd(OAc)2∶dtbpx∶P-[VSpIm][CH3C6H4SO3]0.5=1∶5∶4 | 80 | — | — | 100 | 94.4 |
| 9[ | Pd(OAc)2∶dtbpx∶[SBMI][p-TsOH]=1∶5∶77.9 | 80 | 2.2 | — | — | 99 |
| 10[ | Pd(OAc)2∶dtbpx∶SiO2-[SBMI][p-TsOH] =1∶10∶25.6 | 85 | 0.5 | 650 | — | — |
| 本工作 | Pd(OAc)2∶dtbpx∶p-TsOH=1∶2∶5 | 60 | 2 | 9359 | 约100 | — |
表1 相关文献对比
Table 1 Comparison of relevant literatures
| 序号 | 催化剂组成 | 反应温度/℃ | 反应压力/MPa | 转化频率TOF/h-1 | 选择性/% | 产率/% |
|---|---|---|---|---|---|---|
| 1[ | Pd(OAc)2∶PPh3∶p-TsOH=1∶30∶20 | 115 | 4.5 | 5000 | 98 | — |
| 2[ | Pd(PPh3)2(TsO)2∶PPh3∶p-TsOH=1∶6∶8 | 120 | 4 | 5700 | — | — |
| 3[ | cis-[Pd(SO4)(PPh3)2] ∶PPh3∶H2SO4=1∶107∶8 | 100 | 0.6 | 2168 | — | 98 |
| 4[ | Pd(OAc)2∶TPP∶MSA∶SATA=1∶10∶5∶60 | 100 | 2 | — | 98 | — |
| 5[ | Pd(OAc)2∶dtbpp∶MSA =1∶1.2∶2.5 | 120 | 4 | 25000 | 97.4 | — |
| 6[ | Pd(acac)2∶pytbpx∶p-TsOH=1∶1.2∶2.5 | 120 | 4 | — | 99 | 97 |
| 7[ | Pd(acac)2∶pytbpf∶p-TsOH=1∶2∶16 | 100 | 3 | 46000 | 99 | — |
| 8[ | Pd(OAc)2∶dtbpx∶P-[VSpIm][CH3C6H4SO3]0.5=1∶5∶4 | 80 | — | — | 100 | 94.4 |
| 9[ | Pd(OAc)2∶dtbpx∶[SBMI][p-TsOH]=1∶5∶77.9 | 80 | 2.2 | — | — | 99 |
| 10[ | Pd(OAc)2∶dtbpx∶SiO2-[SBMI][p-TsOH] =1∶10∶25.6 | 85 | 0.5 | 650 | — | — |
| 本工作 | Pd(OAc)2∶dtbpx∶p-TsOH=1∶2∶5 | 60 | 2 | 9359 | 约100 | — |
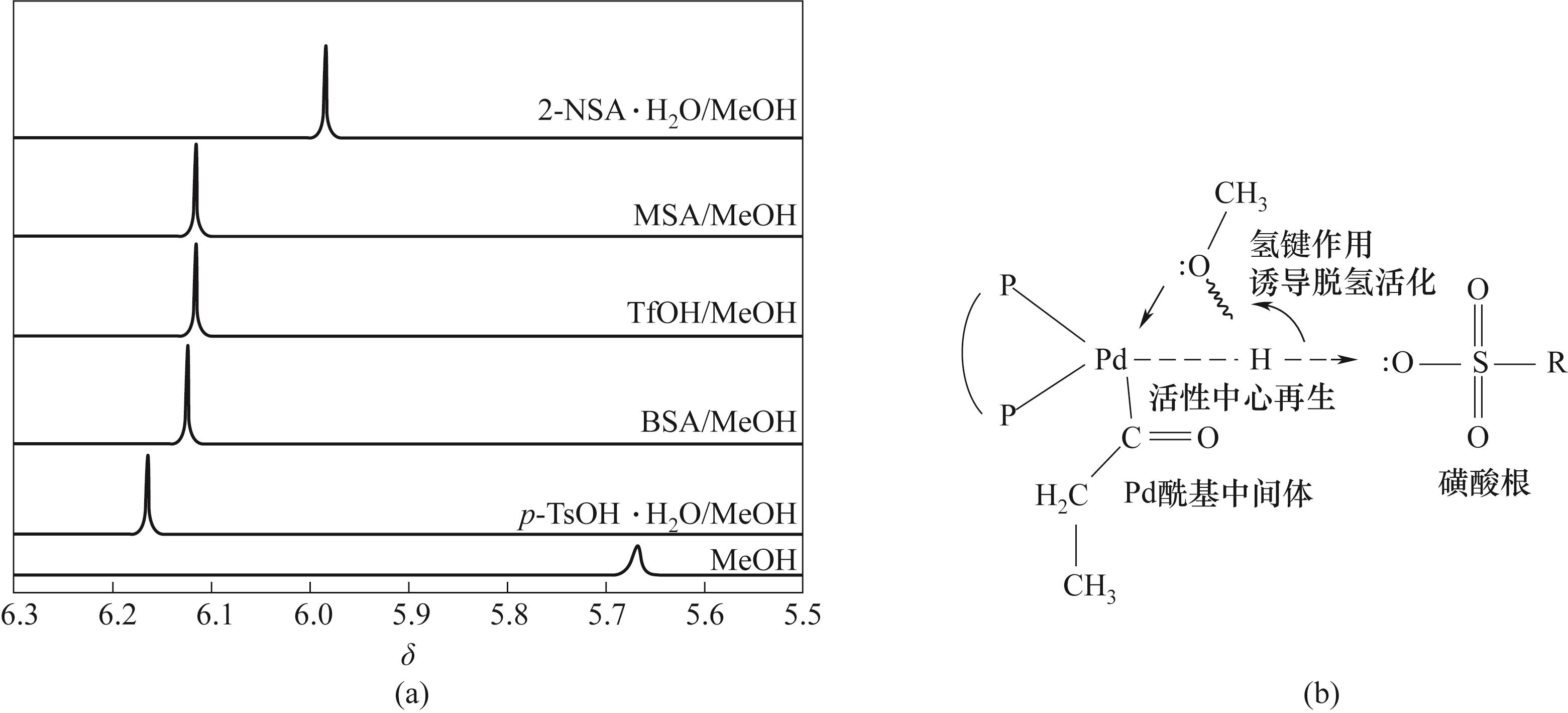
图4 (a) 甲醇以及酸促进剂作用下甲醇中羟基氢的1H NMR化学位移;(b) 酸与甲醇羟基氢通过氢键作用对甲醇的活化脱氢
Fig.4 (a) 1H NMR chemical shift of hydroxyl hydrogen in methanol under action of methanol and acid promoter; (b) Activated dehydrogenation of methanol by hydrogen bonding between acid and hydroxyl hydrogen of methanol
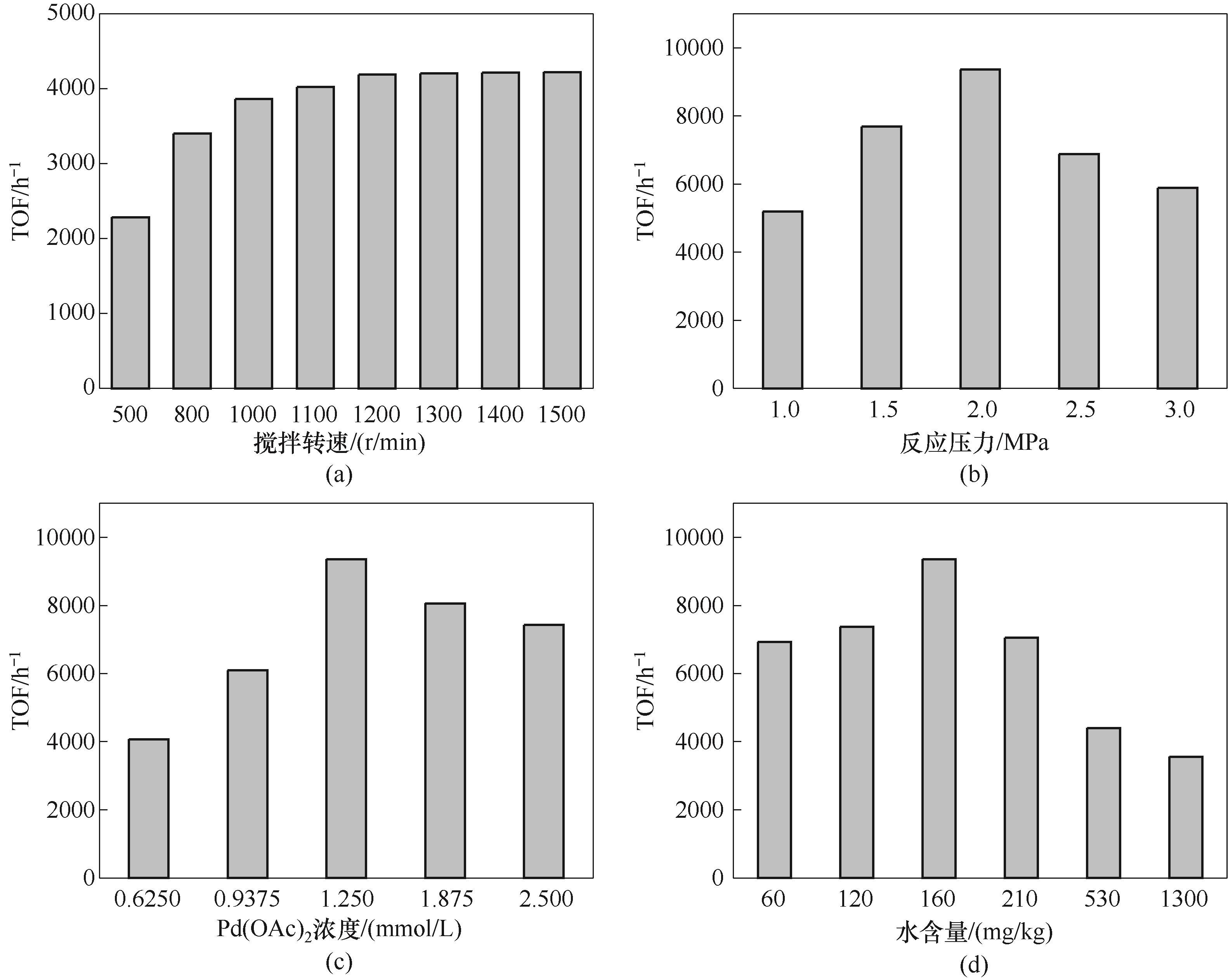
图10 搅拌转速(a)、反应压力(b)、Pd(OAc)2浓度(c)、水含量(d)对乙烯氢甲酯化反应的影响
Fig.10 Effect of reaction condition on hydroesterification of ethylene: (a) stirring speed; (b) reaction pressure; (c) Pd(OAc)2 concentration; (d) water content
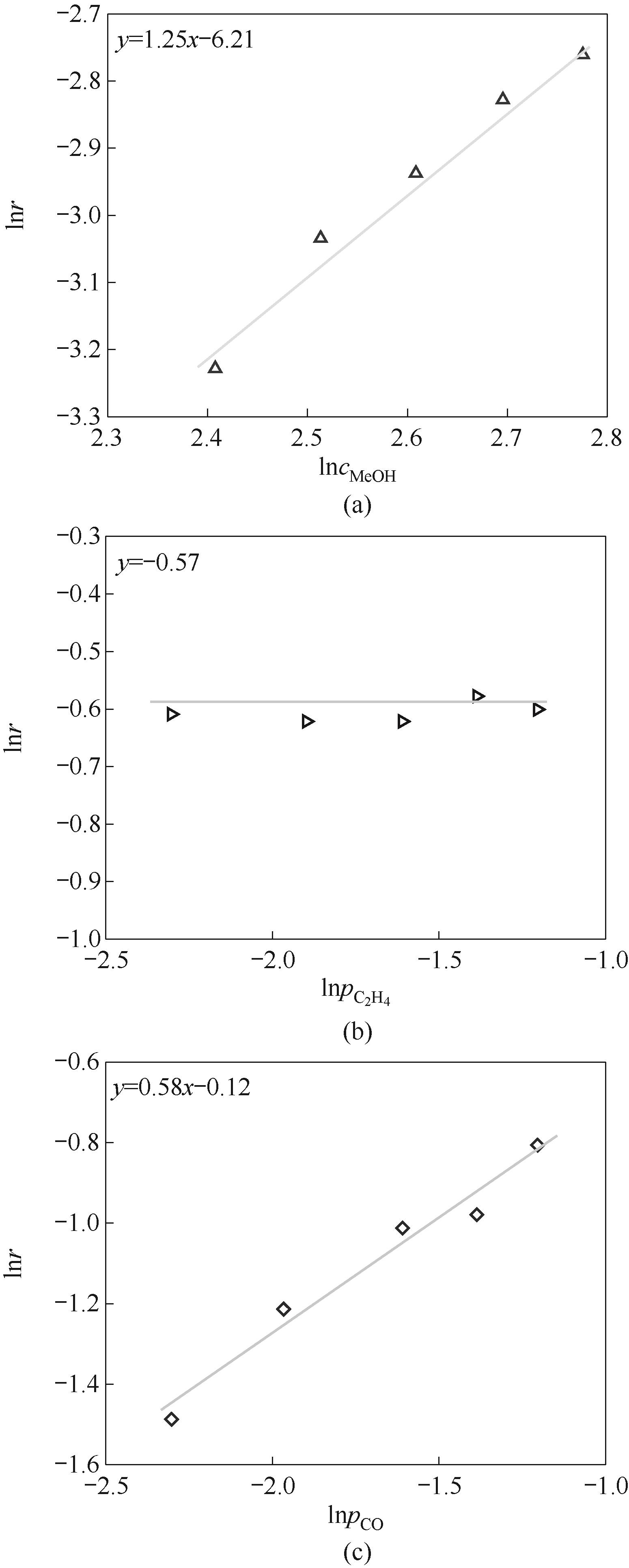
图11 甲醇浓度(a)、乙烯分压(b)、CO分压(c)对反应速率的影响
Fig.11 Effect of reaction conditions on reaction rate: (a) methanol concentration; (b) C2H4 partial pressure; (c) CO partial pressure

图12 (a) TOF随反应时间的变化趋势;(b) MP含量对乙烯氢甲酯化反应催化活性的影响
Fig.12 (a) Trend of TOF with reaction time; (b) Effect of MP concentration on catalytic activity of ethylene hydroesterification reaction
| [1] | Shariati A, Florusse L J, Peters C J. Solubility of ethylene in methyl propionate[J]. Fluid Phase Equilibria, 2015, 387: 143-145. |
| [2] | Cavinato G, Toniolo L. Carbonylation of ethene catalysed by Pd(Ⅱ)-phosphine complexes[J]. Molecules, 2014, 19(9): 15116-15161. |
| [3] | Fan H, Qi L, Wang H Y. Hexafluorophosphate anion intercalation into graphite electrode from methyl propionate[J]. Solid State Ionics, 2017, 300: 169-174. |
| [4] | 赵俊丽, 李保鹏, 蔡洪波. 材料对锂离子电池低温性能影响研究[J]. 电源技术, 2023, 47(8): 1028-1032. |
| Zhao J L, Li B P, Cai H B. Influence of materials on low-temperature performance of lithium-ion batteries[J]. Chinese Journal of Power Sources, 2023, 47(8): 1028-1032. | |
| [5] | Guo Z J, Zhang G L, Wang L, et al. Fe-modified Cs–P/γ-Al2O3 catalyst for synthesis of methyl methacrylate from methyl propionate and formaldehyde[J]. Industrial & Engineering Chemistry Research, 2020, 59(8): 3334-3341. |
| [6] | Liu J Y, Li Z X, Bian Y H, et al. Promotional effect of Ti on catalytic performance of Cs/Ti-SiO2 for conversion of methyl propionate and formaldehyde to methyl methacrylate[J]. Chemical Engineering Science, 2024, 283: 119441. |
| [7] | Feng C X, Liu J Y, Zhao K, et al. Influence of pore structure on catalytic performance of Cs-Zr/SiO2 catalyst for methyl methacrylate synthesis from methyl propionate and formaldehyde[J]. Chemical Engineering Science, 2025, 301: 120760. |
| [8] | Wang Y N, Yan R Y, Lv Z P, et al. Lanthanum and cesium-loaded SBA-15 catalysts for MMA synthesis by aldol condensation of methyl propionate and formaldehyde[J]. Catalysis Letters, 2016, 146(9): 1808-1818. |
| [9] | 李斌, 解铭, 齐翔, 等. 乙烯路线制备甲基丙烯酸甲酯研究进展[J]. 化工进展, 2019, 38(4): 1739-1745. |
| Li B, Xie M, Qi X, et al. Progress in preparation of methyl methacrylate by ethylene route[J]. Chemical Industry and Engineering Progress, 2019, 38(4): 1739-1745. | |
| [10] | Moraru M D, Bildea C S, Kiss A A. Novel eco-efficient process for methyl methacrylate production[J]. Industrial & Engineering Chemistry Research, 2021, 60(3): 1290-1301. |
| [11] | 韩健, 徐玲玲. MMA基混凝土修补材料的研究进展[J]. 中国胶粘剂, 2021, 30(1): 68-72. |
| Han J, Xu L L. Research progress of MMA-based concrete repair material[J]. China Adhesives, 2021, 30(1): 68-72. | |
| [12] | Kara A, Erdem B. Synthesis, characterization and catalytic properties of sulfonic acid functionalized magnetic-poly(divinylbenzene-4-vinylpyridine) for esterification of propionic acid with methanol[J]. Journal of Molecular Catalysis A: Chemical, 2011, 349(1/2): 42-47. |
| [13] | 李柏春, 张静雅, 王凤竹, 等. 酯化法合成丙酸甲酯的动力学研究[J]. 石油化工, 2017, 46(12): 1468-1472. |
| Li B C, Zhang J Y, Wang F Z, et al. Reaction kinetics of synthesized methyl propionate by esterification[J]. Petrochemical Technology, 2017, 46(12): 1468-1472. | |
| [14] | 张勇, 吴玉塘, 贾朝霞. 甲酸甲酯与乙烯加氢酯化合成丙酸甲酯[J]. 天然气化工, 1996, 21(1): 5-8, 1. |
| Zhang Y, Wu Y T, Jia Z X. Preparation of methyl propionate from ethylene and methyl formate by hydroesterification[J]. Natural Gas Chemical Industry, 1996, 21(1): 5-8, 1. | |
| [15] | Jenner G, Ben Taleb A. Ruthenium catalysed ethylene: methyl formate reactions. Synthesis of propanol and ketones[J]. Journal of Molecular Catalysis, 1994, 91(1): 31-43. |
| [16] | García-Suárez E J, Khokarale S G, van Buu O N, et al. Pd-catalyzed ethylene methoxycarbonylation with Brønsted acid ionic liquids as promoter and phase-separable reaction media[J]. Green Chem, 2014, 16(1): 161-166. |
| [17] | Xu J X, Yuan Y, Wu X F. Ethylene as a synthon in carbonylative synthesis[J]. Coordination Chemistry Reviews, 2023, 477: 214947. |
| [18] | Clegg W, Eastham G R, Elsegood M R J, et al. Synthesis and reactivity of palladium hydrido-solvento complexes, including a key intermediate in the catalytic methoxycarbonylation of ethene to methyl propanoate[J]. Journal of the Chemical Society, Dalton Transactions, 2002(17): 3300-3308. |
| [19] | 王鲁明, 李增喜. 丙酸甲酯催化合成过程研究进展[J]. 工程研究——跨学科视野中的工程, 2024, 16(5): 481-499. |
| Wang L M, Li Z X. Research progress on the catalytic synthesis process of methyl propionate[J]. Journal of Engineering Studies, 2024, 16(5): 481-499. | |
| [20] | Yang J, Yuan Y Z. Promoting effect of lewis acid on the olefin hydroesterification catalyzed by triphenylphosphine-palladium complex[J]. Catalysis Letters, 2009, 131(3): 643-648. |
| [21] | Dong K W, Sang R, Fang X J, et al. Efficient palladium-catalyzed alkoxycarbonylation of bulk industrial olefins using ferrocenyl phosphine ligands[J]. Angewandte Chemie International Edition, 2017, 56(19): 5267-5271. |
| [22] | Drent E. Process for the preparation of polyketones: DE3566549A[P]. 1985-09-10. |
| [23] | Pugh R I, Drent E, Pringle P G. Tandem isomerisation-carbonylation catalysis: highly active p a l l a d i u m ( Ⅱ ) catalysts for the selective methoxycarbonylation of internal alkenes to linear esters[J]. Chemical Communications, 2001(16): 1476-1477. |
| [24] | Clegg W, Elsegood M R J, Eastham G R, et al. Highly active and selective catalysts for the production of methyl propanoate via the methoxycarbonylation of ethene[J]. Chemical Communications, 1999(18): 1877-1878. |
| [25] | Vondran J, Furst M R L, Eastham G R, et al. Magic of alpha: the chemistry of a remarkable bidentate phosphine, 1,2-bis(di-tert-butylphosphinomethyl)benzene[J]. Chemical Reviews, 2021, 121(11): 6610-6653. |
| [26] | Seayad A, Kelkar A A, Toniolo L, et al. Hydroesterification of styrene using an in situ formed Pd(OTs)2(PPh3)2 complex catalyst[J]. Journal of Molecular Catalysis A: Chemical, 2000, 151(1/2): 47-59. |
| [27] | Wang H, Zhao Y F, Zhang F T, et al. Hydrogen-bond donor and acceptor cooperative catalysis strategy for cyclic dehydration of diols to access O-heterocycles[J]. Science Advances, 2021, 7(22): eabg0396. |
| [28] | Dong K W, Sang R, Wei Z H, et al. Cooperative catalytic methoxycarbonylation of alkenes: uncovering the role of palladium complexes with hemilabile ligands[J]. Chemical Science, 2018, 9(9): 2510-2516. |
| [29] | Drent D, Budzelaar P H M, Jager W W, et al. Carbonylation catalyst process: EP044144TA1[P]. 1991-02-05. |
| [30] | Vavasori A, Cavinato G, Toniolo L. Effect of a hydride source (water, hydrogen, p-toluenesulfonic acid) on the hydroesterification of ethylene to methyl propionate using a Pd(PPh3)2(TsO)2 (TsO = p-toluenesulfonate anion) catalyst precursor[J]. Journal of Molecular Catalysis A: Chemical, 2001, 176(1/2): 11-18. |
| [31] | Cavinato G, Facchetti S, Toniolo L. Ethene hydromethoxycarbonylation catalyzed by cis-[Pd(SO4)(PPh3)2]/H2SO4/PPh3 [J]. Journal of Molecular Catalysis A: Chemical, 2010, 333(1/2): 180-185. |
| [32] | Khokarale S G, García-Suárez E J, Xiong J, et al. Zwitterion enhanced performance in palladium-phosphine catalyzed ethylene methoxycarbonylation[J]. Catalysis Communications, 2014, 44: 73-75. |
| [33] | Pugh R, Drent E. Methoxycarbonylation versus hydroacylation of ethene; dramatic influence of the ligand in cationic palladium catalysis [J]. Advanced Synthesis & Catalysis, 2002, 344(8): 837-840. |
| [34] | Dong K, Fang X, Gülak S, et al. Highly active and efficient catalysts for alkoxycarbonylation of alkenes [J]. Nat. Commun., 2017, 8(1): 14117. |
| [35] | Wang L M, Bian Y H, Wu Z Y, et al. Revealing the role of hydrogen bond, mechanism and kinetic for hydroesterification of ethylene to methyl propionate[J]. Chemical Engineering Journal, 2023, 470: 144331. |
| [36] | García-Suárez E J, Khokarale S G, van Buu O N, et al. Pd-catalyzed ethylene methoxycarbonylation with Brønsted acid ionic liquids as promoter and phase-separable reaction media[J]. Green Chemistry, 2014, 16(1): 161-166. |
| [37] | Khokarale S G, García-Suárez E J, Fehrmann R, et al. Highly selective continuous gas-phase methoxycarbonylation of ethylene with supported ionic liquid phase (SILP) catalysts[J]. ChemCatChem, 2017, 9(10): 1824-1829. |
| [38] | 谭平华, 肖春妹, 熊国炎, 等. 乙烯羰基化合成研究进展[J]. 现代化工, 2011, 31(9): 28-31. |
| Tan P H, Xiao C M, Xiong G Y, et al. Progress in carbonylation synthesis of ethylene[J]. Modern Chemical Industry, 2011, 31(9): 28-31. | |
| [39] | 刘梦力, 曾波, 胡波, 等. 膦配体电子和空间效应对钯催化羰化酯化反应的影响[J]. 分子催化, 2022, 36(3): 253-273. |
| Liu M L, Zeng B, Hu B, et al. Influence of electronic and steric factors of phosphine ligands upon palladium-catalyzed alkoxycarbonylation[J]. Journal of Molecular Catalysis (China), 2022, 36(3): 253-273. | |
| [40] | de la Fuente V, Waugh M, Eastham G R, et al. Phosphine ligands in the palladium-catalysed methoxycarbonylation of ethene: insights into the catalytic cycle through an HP NMR spectroscopic study[J]. Chemistry, 2010, 16(23): 6919-6932. |
| [41] | Vavasori A, Toniolo L. Carbon monoxide-ethylene copolymerization catalyzed by a Pd(AcO)2/dppp/TsOH system: the promoting effect of water and of the acid[J]. Journal of Molecular Catalysis A: Chemical, 1996, 110(1): 13-23. |
| [42] | Tooze R P, Whiston K, Malyan A P, et al. Evidence for the hydride mechanism in the methoxycarbonylation of ethene catalysed by palladium-triphenylphosphine complexes[J]. Dalton Transactions, 2000(19): 3441-3444. |
| [43] | Vavasori A, Toniolo L, Cavinato G. Hydroesterification of cyclohexene using the complex Pd(PPh3)2(TsO)2 as catalyst precursor: effect of a hydrogen source (TsOH, H2O) on the TOF and a kinetic study (TsOH: p-toluenesulfonic acid)[J]. Journal of Molecular Catalysis A: Chemical, 2003, 191(1): 9-21. |
| [44] | Yang D, Liu L, Wang D L, et al. Novel multi-dentate phosphines for Pd-catalyzed alkoxycarbonylation of alkynes promoted by H2O additive[J]. Journal of Catalysis, 2019, 371: 236-244. |
| [45] | Cavinato G, Toniolo L, Vavasori A. Characterization and catalytic activity of trans-[Pd(COCH2CH3)(TsO)(PPh3)2], isolated from the hydro-methoxycarbonylation of ethene catalyzed by [Pd(TsO)2(PPh3)2] [J]. Journal of Molecular Catalysis A: Chemical, 2004, 219(2): 233-240. |
| [1] | 麦棹铭, 武颖韬, 王维, 穆海宝, 黄佐华, 汤成龙. 正十二烷-甲烷双燃料非线性着火特性及稀释气体效应研究[J]. 化工学报, 2025, 76(6): 3115-3124. |
| [2] | 杨猛, 丁晓倩, 余涛, 刘畅, 汤成龙, 黄佐华. 甲烷/氧化亚氮绿色推进剂自着火特性实验及动力学[J]. 化工学报, 2025, 76(3): 1221-1229. |
| [3] | 郭珊, 田雨, 徐永滨, 王朋, 刘治明. 废旧电池再资源化制备高性能中熵合金催化剂及其性能研究[J]. 化工学报, 2025, 76(1): 231-240. |
| [4] | 赵焕娟, 包颖昕, 于康, 刘婧, 钱新明. 多元组分爆轰不稳定性定量实验研究[J]. 化工学报, 2024, 75(S1): 339-348. |
| [5] | 张兆想, 蔡茂坤, 任志英, 贾晓红, 郭飞. 温度及其波动对橡胶密封硫化过程影响的仿真分析[J]. 化工学报, 2024, 75(2): 715-726. |
| [6] | 王学云, 郁肖兵, 彭万旺, 沈岩松. 熔渣气化炉喷嘴燃烧区行为的数值模拟研究[J]. 化工学报, 2024, 75(2): 659-674. |
| [7] | 卓红英, 赵忠正, 沈铮, 杨小峰, 黄延强. 正-仲氢催化转化研究进展[J]. 化工学报, 2024, 75(11): 3883-3895. |
| [8] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [9] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [10] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [11] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [12] | 禹进, 余彬彬, 蒋新生. 一种基于虚拟组分的燃烧调控化学作用量化及分析方法研究[J]. 化工学报, 2023, 74(3): 1303-1312. |
| [13] | 刘世君, 郑安庆, 陈晓丽, 付娟, 苏秋成. 纤维素增强环氧树脂复合材料热解特性研究[J]. 化工学报, 2023, 74(12): 4968-4978. |
| [14] | 刘宗鹏, 胡少剑, 张宇宁, 马玲, 李磊, 武本成, 朱建华. 复合型多元醇酯合成反应的热力学分析及动力学研究[J]. 化工学报, 2023, 74(11): 4475-4486. |
| [15] | 章蕾, 宋孝辉, 张建庭, 屠美玲, 杨阿三. 氨甲环酸异构化过程的反应动力学研究[J]. 化工学报, 2023, 74(10): 4173-4181. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号