化工学报 ›› 2025, Vol. 76 ›› Issue (5): 2198-2208.DOI: 10.11949/0438-1157.20241238
徐智超1( ), 俞镇东1, 吴昊峰1, 吴沛文1,2(
), 俞镇东1, 吴昊峰1, 吴沛文1,2( ), 武洪翔3, 巢艳红3, 朱文帅1,2(
), 武洪翔3, 巢艳红3, 朱文帅1,2( ), 刘植昌1, 徐春明1
), 刘植昌1, 徐春明1
收稿日期:2024-11-01
修回日期:2025-02-24
出版日期:2025-05-25
发布日期:2025-06-13
通讯作者:
吴沛文,朱文帅
作者简介:徐智超(2000—),男,硕士研究生,xzhichao2022@163.com
基金资助:
Zhichao XU1( ), Zhendong YU1, Haofeng WU1, Peiwen WU1,2(
), Zhendong YU1, Haofeng WU1, Peiwen WU1,2( ), Hongxiang WU3, Yanhong CHAO3, Wenshuai ZHU1,2(
), Hongxiang WU3, Yanhong CHAO3, Wenshuai ZHU1,2( ), Zhichang LIU1, Chunming XU1
), Zhichang LIU1, Chunming XU1
Received:2024-11-01
Revised:2025-02-24
Online:2025-05-25
Published:2025-06-13
Contact:
Peiwen WU, Wenshuai ZHU
摘要:
针对生物柴油中微量硫化物难脱除的问题,采用离子交换法构筑了富酸位Ce-13X分子筛,将其用于生物柴油吸附脱硫体系。通过一系列表征对其物相组成、结构形貌、酸强度、元素价态和酸性等性质进行分析。结果表明,Ce的引入增加了13X分子筛的强B酸位点以及弱L酸位点浓度。在最优实验条件温度40℃,剂油比为1∶80下,经过30 min,生物柴油中的硫含量从8 μg·g-1降至目标浓度2 μg·g-1以下。吸附动力学表明该吸附过程符合准二级动力学模型且整个吸附过程主要由液膜扩散和颗粒内扩散控制,吸附热力学表明Ce-13X对硫醇的吸附为自发放热过程。该研究不仅提出了一种富酸位13X分子筛的构筑方法,而且能够构筑高效吸附脱硫体系,实现生物柴油中微量硫化物超深度脱除。
中图分类号:
徐智超, 俞镇东, 吴昊峰, 吴沛文, 武洪翔, 巢艳红, 朱文帅, 刘植昌, 徐春明. 富酸位13X分子筛的制备及其超深度吸附脱除生物柴油中硫醇[J]. 化工学报, 2025, 76(5): 2198-2208.
Zhichao XU, Zhendong YU, Haofeng WU, Peiwen WU, Hongxiang WU, Yanhong CHAO, Wenshuai ZHU, Zhichang LIU, Chunming XU. Preparation of acid-rich 13X molecular sieve and its ultra-deep adsorption removal of mercaptan in biodiesel[J]. CIESC Journal, 2025, 76(5): 2198-2208.
| 吸附剂 | 比表面积/ (m2·g-1) | 孔径/nm | 孔体积/ (cm3·g-1) | 元素含量/%(质量分数) | n(SiO2)/ n(Al2O3) | |||
|---|---|---|---|---|---|---|---|---|
| Si | Al | Na | Ce | |||||
| 13X | 702 | 0.95 | 0.27 | 18.92 | 17.72 | 18.65 | — | 2.05 |
| Ce-13X | 695 | 1.03 | 0.28 | 18.99 | 17.24 | 9.38 | 10.85 | 2.12 |
表1 吸附剂的孔结构参数及元素分析
Table 1 Pore structure parameters and elemental analysis of adsorbents
| 吸附剂 | 比表面积/ (m2·g-1) | 孔径/nm | 孔体积/ (cm3·g-1) | 元素含量/%(质量分数) | n(SiO2)/ n(Al2O3) | |||
|---|---|---|---|---|---|---|---|---|
| Si | Al | Na | Ce | |||||
| 13X | 702 | 0.95 | 0.27 | 18.92 | 17.72 | 18.65 | — | 2.05 |
| Ce-13X | 695 | 1.03 | 0.28 | 18.99 | 17.24 | 9.38 | 10.85 | 2.12 |
| 吸附剂 | 脱附温度/℃ | 酸量/(μmol·g-1) | |
|---|---|---|---|
| B酸 | L酸 | ||
| 13X | 200 | — | 196.4 |
| 350 | — | 153.1 | |
| Ce-13X | 200 | 318.5 | 151.2 |
| 350 | 324.1 | 54.5 | |
表2 吸附剂中Brønsted酸和Lewis酸的分布
Table 2 Distribution of Brønsted and Lewis acidity in the adsorbents
| 吸附剂 | 脱附温度/℃ | 酸量/(μmol·g-1) | |
|---|---|---|---|
| B酸 | L酸 | ||
| 13X | 200 | — | 196.4 |
| 350 | — | 153.1 | |
| Ce-13X | 200 | 318.5 | 151.2 |
| 350 | 324.1 | 54.5 | |
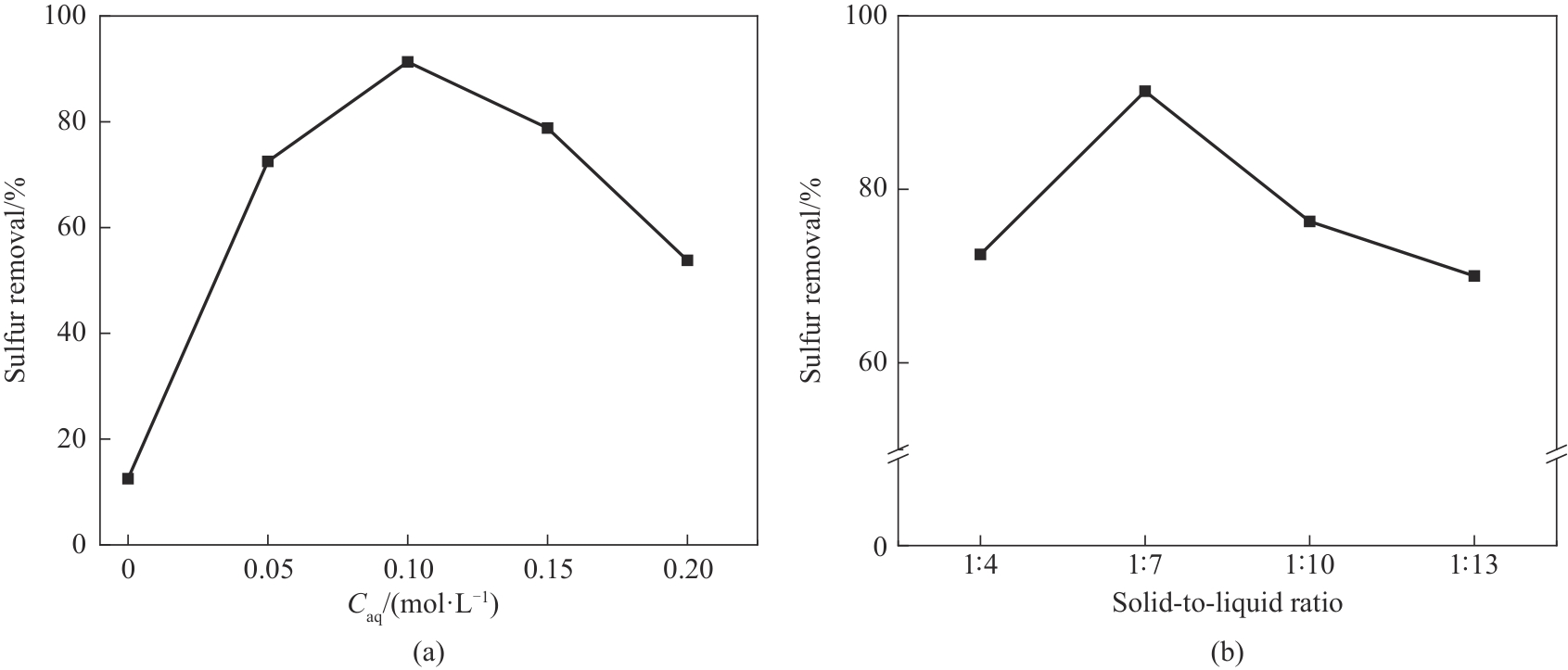
图9 离子交换溶液浓度(a)和固液比(b)对吸附脱硫性能的影响
Fig.9 Effects of ion exchange solution concentration (a) and solid-liquid ratio (b) on adsorption desulfurization performance
| 温度/℃ | qe,exp/(mg·g-1) | 准一级动力学 | 准二级动力学 | ||||
|---|---|---|---|---|---|---|---|
| k1/min-1 | qe1,cal/(mg·g-1) | R2 | k2/(g·mg-1·min-1) | qe2,cal/(mg·g-1) | R2 | ||
| 30 | 0.69 | 0.0213 | 0.49 | 0.9483 | 0.0657 | 0.76 | 0.9980 |
| 40 | 0.70 | 0.0324 | 0.19 | 0.9780 | 0.3961 | 0.71 | 0.9999 |
表3 Ce-13X吸附动力学拟合参数
Table 3 Fitting parameters of Ce-13X adsorption kinetics
| 温度/℃ | qe,exp/(mg·g-1) | 准一级动力学 | 准二级动力学 | ||||
|---|---|---|---|---|---|---|---|
| k1/min-1 | qe1,cal/(mg·g-1) | R2 | k2/(g·mg-1·min-1) | qe2,cal/(mg·g-1) | R2 | ||
| 30 | 0.69 | 0.0213 | 0.49 | 0.9483 | 0.0657 | 0.76 | 0.9980 |
| 40 | 0.70 | 0.0324 | 0.19 | 0.9780 | 0.3961 | 0.71 | 0.9999 |
| 阶段 | k/(mg·g-1·min-0.5) | C/(mg·g-1) | R2 |
|---|---|---|---|
| 第一阶段 | 0.0721 | 0.0399 | 0.9592 |
| 第二阶段 | 0.0139 | 0.4794 | 0.9633 |
| 第三阶段 | — | 0.6936 | — |
表4 颗粒内扩散模型的拟合参数
Table 4 Fitting parameters of the intraparticle diffusion model
| 阶段 | k/(mg·g-1·min-0.5) | C/(mg·g-1) | R2 |
|---|---|---|---|
| 第一阶段 | 0.0721 | 0.0399 | 0.9592 |
| 第二阶段 | 0.0139 | 0.4794 | 0.9633 |
| 第三阶段 | — | 0.6936 | — |
| 吸附剂 | qm/(mg·g-1) | Langmuir吸附等温线 | Freundlich吸附等温线 | |||
|---|---|---|---|---|---|---|
| KL/(L·mg-1) | R2 | KF/(mg·g-1)(L·mg-1)1/n | 1/n | R2 | ||
| Ce-13X | 7.73 | 0.0026 | 0.9669 | 0.1057 | 0.5995 | 0.9904 |
表5 Ce-13X的Langmuir和Freundlich吸附等温线参数
Table 5 Langmuir and Freundlich adsorption isotherm parameters of Ce-13X
| 吸附剂 | qm/(mg·g-1) | Langmuir吸附等温线 | Freundlich吸附等温线 | |||
|---|---|---|---|---|---|---|
| KL/(L·mg-1) | R2 | KF/(mg·g-1)(L·mg-1)1/n | 1/n | R2 | ||
| Ce-13X | 7.73 | 0.0026 | 0.9669 | 0.1057 | 0.5995 | 0.9904 |
| ΔG/(kJ·mol-1) | ΔH/(kJ·mol-1) | ΔS/(J·mol-1·K-1) | R2 | |||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | |||
| -9.9114 | -9.0445 | -7.8336 | -7.3374 | -36.9286 | -89.2170 | 0.9859 |
表6 不同温度下Ce-13X的吸附热力学参数
Table 6 Adsorption thermodynamic parameters of Ce-13X at different temperatures
| ΔG/(kJ·mol-1) | ΔH/(kJ·mol-1) | ΔS/(J·mol-1·K-1) | R2 | |||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | |||
| -9.9114 | -9.0445 | -7.8336 | -7.3374 | -36.9286 | -89.2170 | 0.9859 |
| 1 | Jiang H B, Shao Z Y, Chen W B, et al. Study on diesel hydrotreating kinetics and the synergistic effect of CoMo and NiMo catalysts[J]. Energy & Fuels, 2024, 38(7): 6314-6324. |
| 2 | Oh P P, Lau H L N, Chen J, et al. A review on conventional technologies and emerging process intensification (PI) methods for biodiesel production[J]. Renewable and Sustainable Energy Reviews, 2012, 16(7): 5131-5145. |
| 3 | 黄宽, 马永德, 蔡镇平, 等. 油脂催化加氢转化制备第二代生物柴油研究进展[J]. 化工学报, 2023, 74(1): 380-396. |
| Huang K, Ma Y D, Cai Z P, et al. Research progress in catalytic hydroconversion of lipid to second-generation biodiesel[J]. CIESC Journal, 2023, 74(1): 380-396. | |
| 4 | Li Y M, Xu H, Li Z F, et al. Catalytic methanotreating of vegetable oil: a pathway to second-generation biodiesel[J]. Fuel, 2022, 311: 122504. |
| 5 | Haruna A, Merican Aljunid Merican Z, Gani Musa S, et al. Sulfur removal technologies from fuel oil for safe and sustainable environment[J]. Fuel, 2022, 329: 125370. |
| 6 | Xiong J, Luo J, Di J, et al. Macroscopic 3D boron nitride monolith for efficient adsorptive desulfurization[J]. Fuel, 2020, 261: 116448. |
| 7 | Wang Q, Zhang T, Zhang S L, et al. Extractive desulfurization of fuels using trialkylamine-based protic ionic liquids[J]. Separation and Purification Technology, 2020, 231: 115923. |
| 8 | Lin Y H, Feng L, Li X H, et al. Study on ultrasound-assisted oxidative desulfurization for crude oil[J]. Ultrasonics Sonochemistry, 2020, 63: 104946. |
| 9 | Umar Hussain M, Kainat K, Nawaz H, et al. SERS characterization of biochemical changes associated with biodesulfurization of dibenzothiophene using Gordonia sp. HS126-4N[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2024, 320: 124534. |
| 10 | Lee K X, Valla J A. Adsorptive desulfurization of liquid hydrocarbons using zeolite-based sorbents: a comprehensive review[J]. Reaction Chemistry & Engineering, 2019, 4(8): 1357-1386. |
| 11 | Danmaliki G I, Saleh T A, Shamsuddeen A A. Response surface methodology optimization of adsorptive desulfurization on nickel/activated carbon[J]. Chemical Engineering Journal, 2017, 313: 993-1003. |
| 12 | Yoosuk B, Silajan A, Prasassarakich P. Deep adsorptive desulfurization over ion-exchanged zeolites: individual and simultaneous effect of aromatic and nitrogen compounds[J]. Journal of Cleaner Production, 2020, 248: 119291. |
| 13 | Hamad K I, Humadi J I, Abdulkareem H A, et al. Efficient immobilization of acids into activated carbon for high durability and continuous desulfurization of diesel fuel[J]. Energy Science & Engineering, 2023, 11(10): 3662-3679. |
| 14 | Zhang C Y, Zhang X W, Tao Z P, et al. Defect engineering and post-synthetic reduction of Cu based metal-organic frameworks towards efficient adsorption desulfurization[J]. Chemical Engineering Journal, 2023, 455: 140487. |
| 15 | Neubauer R, Husmann M, Weinlaender C, et al. Acid base interaction and its influence on the adsorption kinetics and selectivity order of aromatic sulfur heterocycles adsorbing on Ag-Al2O3 [J]. Chemical Engineering Journal, 2017, 309: 840-849. |
| 16 | Liu X J, Yi D Z, Cui Y Y, et al. Adsorption desulfurization and weak competitive behavior from 1-hexene over cesium-exchanged Y zeolites (CsY)[J]. Journal of Energy Chemistry, 2018, 27(1): 271-277. |
| 17 | Yang R T, Hernández-Maldonado A J, Yang F H. Desulfurization of transportation fuels with zeolites under ambient conditions[J]. Science, 2003, 301(5629): 79-81. |
| 18 | Cha Y H, Lee K B. Examining the impact of different anions in Cu precursors on sulfur adsorption through zeolites with Cu ion-exchange[J]. Chemical Engineering Journal, 2023, 468: 143461. |
| 19 | Zu Y, Guan L J, Guo Z S, et al. Deep removal of thiophene and benzothiophene in low-sulfur fuels over the efficient CeAlSBA-15 adsorbent synthesized by sequential alumination and cerium incorporation[J]. Chemical Engineering Journal, 2021, 416: 127984. |
| 20 | Lee K X, Tsilomelekis G, Valla J A. Removal of benzothiophene and dibenzothiophene from hydrocarbon fuels using CuCe mesoporous Y zeolites in the presence of aromatics[J]. Applied Catalysis B: Environmental, 2018, 234: 130-142. |
| 21 | Lv J M, Ma Y L, Chang X, et al. Removal and removing mechanism of tetracycline residue from aqueous solution by using Cu-13X[J]. Chemical Engineering Journal, 2015, 273: 247-253. |
| 22 | Ren X Y, Cao J P, Zhao X Y, et al. Enhancement of aromatic products from catalytic fast pyrolysis of lignite over hierarchical HZSM-5 by piperidine-assisted desilication[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(2): 1792-1802. |
| 23 | Zeng Y B, Walker H, Zhu Q Z. Reduction of nitrate by NaY zeolite supported Fe, Cu/Fe and Mn/Fe nanoparticles[J]. Journal of Hazardous Materials, 2017, 324: 605-616. |
| 24 | Vinodh R, Deviprasath C, Muralee Gopi C V V, et al. Novel 13X zeolite/PANI electrocatalyst for hydrogen and oxygen evolution reaction[J]. International Journal of Hydrogen Energy, 2020, 45(53): 28337-28349. |
| 25 | Xiang X J, Guo T, Yin Y M, et al. High adsorption capacity Fe@13X zeolite for direct air CO2 capture[J]. Industrial & Engineering Chemistry Research, 2023, 62(12): 5420-5429. |
| 26 | Dashtpeyma G, Shabanian S R, Ahmadpour J, et al. The investigation of adsorption desulphurization performance using bimetallic CuCe and NiCe mesoporous Y zeolites: modification of Y zeolite by H4EDTA-NaOH sequential treatment[J]. Fuel Processing Technology, 2022, 235: 107379. |
| 27 | Zhu J C, Hu L F, He J, et al. Removal of ethyl mercaptan over Fe and Ce doped hexaniobate nanotubes[J]. Ceramics International, 2022, 48(17): 25267-25276. |
| 28 | Ma Y G, Zhang D Y, Sun H M, et al. Fe-Ce mixed oxides supported on carbon nanotubes for simultaneous removal of NO and Hg0 in flue gas[J]. Industrial & Engineering Chemistry Research, 2018, 57(9): 3187-3194. |
| 29 | Kumar K M, Mahendhiran M, Diaz M C, et al. Green synthesis of Ce3+ rich CeO2 nanoparticles and its antimicrobial studies[J]. Materials Letters, 2018, 214: 15-19. |
| 30 | Wei F J, Guo X Q, Liao J J, et al. Ultra-deep removal of thiophene in coke oven gas over Y zeolite: effect of acid modification on adsorption desulfurization[J]. Fuel Processing Technology, 2021, 213: 106632. |
| 31 | Luo J, Chao Y H, Tang Z Y, et al. Design of Lewis acid centers in bundlelike boron nitride for boosting adsorptive desulfurization performance[J]. Industrial & Engineering Chemistry Research, 2019, 58(29): 13303-13312. |
| 32 | Wang J J, Li L H, Wen Z H, et al. Insight into the relationship between effective active sites and ultra-deep adsorption desulfurization performance of CuCeY with different Cu precursors[J]. Fuel Processing Technology, 2023, 250: 107930. |
| 33 | 张畅, 秦玉才, 高雄厚, 等. Ce改性对Y型分子筛酸性及其催化转化性能的调变机制[J]. 物理化学学报, 2015, 31(2): 344-352. |
| Zhang C, Qin Y C, Gao X H, et al. Modulation of the acidity and catalytic conversion properties of Y zeolites modified by cerium cations[J]. Acta Physico-Chimica Sinica, 2015, 31(2): 344-352. | |
| 34 | Wang J J, Zhao J C, Wen Z H, et al. Regulating cerium location in Y zeolite and its influence on adsorptive desulfurization for thiophene in benzene[J]. Separation and Purification Technology, 2025, 353: 128517. |
| 35 | Luo J, Yan M, Ji H Y, et al. Three-dimensional Ce-MOFs-derived Ce@C-BN nanobundles for adsorptive desulfurization[J]. Applied Surface Science, 2022, 590: 152926. |
| [1] | 马瑞洁, 黄子轩, 关雪倩, 陈光进, 刘蓓. ZIF-8/DMPU浆液分离C2H6/ CH4混合气研究[J]. 化工学报, 2025, 76(5): 2262-2269. |
| [2] | 胡嘉朗, 姜明源, 金律铭, 张永刚, 胡鹏, 纪红兵. 机器学习辅助MOFs高通量计算筛选及气体分离研究进展[J]. 化工学报, 2025, 76(5): 1973-1996. |
| [3] | 齐昊, 王玉杰, 李申辉, 邹琦, 刘轶群, 赵之平. 双金属Co/Zn-ZIFs中C3H6和C3H8吸附和扩散行为分子模拟研究[J]. 化工学报, 2025, 76(5): 2313-2326. |
| [4] | 陶春珲, 李印辉, 傅钰, 段然, 赵泽一, 唐羽丰, 张罡, 马和平. 不同吸附剂对低浓度Kr气的选择性吸附与纯化[J]. 化工学报, 2025, 76(5): 2358-2366. |
| [5] | 张越, 刘佳鑫, 马敬, 刘毅. 金属有机骨架膜应用于海水提铀研究进展[J]. 化工学报, 2025, 76(5): 2087-2100. |
| [6] | 郭彭涛, 王婷, 薛波, 应允攀, 刘大欢. 用于CH4/N2分离的多吸附位点超微孔MOF[J]. 化工学报, 2025, 76(5): 2304-2312. |
| [7] | 唐磊, 王振菲, 李聪利, 杨佳辉, 郑浩, 石琪, 董晋湘. Co-MOF-74和Mg-MOF-74的CO工作吸附容量及操作条件[J]. 化工学报, 2025, 76(5): 2279-2293. |
| [8] | 李艳, 雷美丽, 李鑫钢. 基于分离性能的顺序式模拟移动床结构调控策略[J]. 化工学报, 2025, 76(5): 2219-2229. |
| [9] | 巴雅琪, 吴涛, 邸安頔, 陆安慧. 多孔炭材料用于低碳烃分离的研究进展[J]. 化工学报, 2025, 76(5): 2136-2157. |
| [10] | 谈朋, 李雪梅, 刘晓勤, 孙林兵. 基于柔性MOFs的磁响应复合材料及其丙烯吸附性能研究[J]. 化工学报, 2025, 76(5): 2230-2240. |
| [11] | 李征, 庄铠泽, 赵东杰, 宋燕星, 王功明. 事件驱动的深度信念网络软测量模型设计方法[J]. 化工学报, 2025, 76(4): 1693-1701. |
| [12] | 晋伊浩, 罗俊欣, 胡章茂, 王唯, 殷谦. 亲水改性硫酸镁/膨胀蛭石复合材料的吸附储热性能[J]. 化工学报, 2025, 76(4): 1852-1862. |
| [13] | 蔡天姿, 张海丰, 林海丹, 张子龙, 周鹏宇, 王柏林, 李小年. 硼掺杂氮基石墨烯检测变压器油中溶解气体CO和CO2的密度泛函理论研究[J]. 化工学报, 2025, 76(4): 1841-1851. |
| [14] | 陶智能, 邱彤, 王保国. 阴离子交换膜电解水制氢稳态建模[J]. 化工学报, 2025, 76(4): 1711-1721. |
| [15] | 朱峰, 赵跃, 马凤翔, 刘伟. 改性UIO-66对SF6/N2混合气体及其分解产物的吸附特性[J]. 化工学报, 2025, 76(4): 1604-1616. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号