CIESC Journal ›› 2021, Vol. 72 ›› Issue (12): 6262-6273.DOI: 10.11949/0438-1157.20211258
• Catalysis, kinetics and reactors • Previous Articles Next Articles
Wenxuan LIU( ),Jiayi ZHANG,Qi LU,Haochen ZHANG(
),Jiayi ZHANG,Qi LU,Haochen ZHANG( )
)
Received:2021-08-31
Revised:2021-10-28
Online:2021-12-22
Published:2021-12-05
Contact:
Haochen ZHANG
通讯作者:
张皓晨
作者简介:刘文萱(1999—),女,博士研究生,基金资助:CLC Number:
Wenxuan LIU, Jiayi ZHANG, Qi LU, Haochen ZHANG. Investigation of electroreduction of carbon dioxide into formate based on machine learning[J]. CIESC Journal, 2021, 72(12): 6262-6273.
刘文萱, 张嘉毅, 陆奇, 张皓晨. 基于机器学习的二氧化碳电化学还原制备甲酸盐研究[J]. 化工学报, 2021, 72(12): 6262-6273.
Add to citation manager EndNote|Ris|BibTeX

Fig.1 The structure of the graphene-N6-M1-M2 model[where grey, light blue, and dark blue spheres represent C, N, and transition-metal atoms (M1 and M2), respectively]
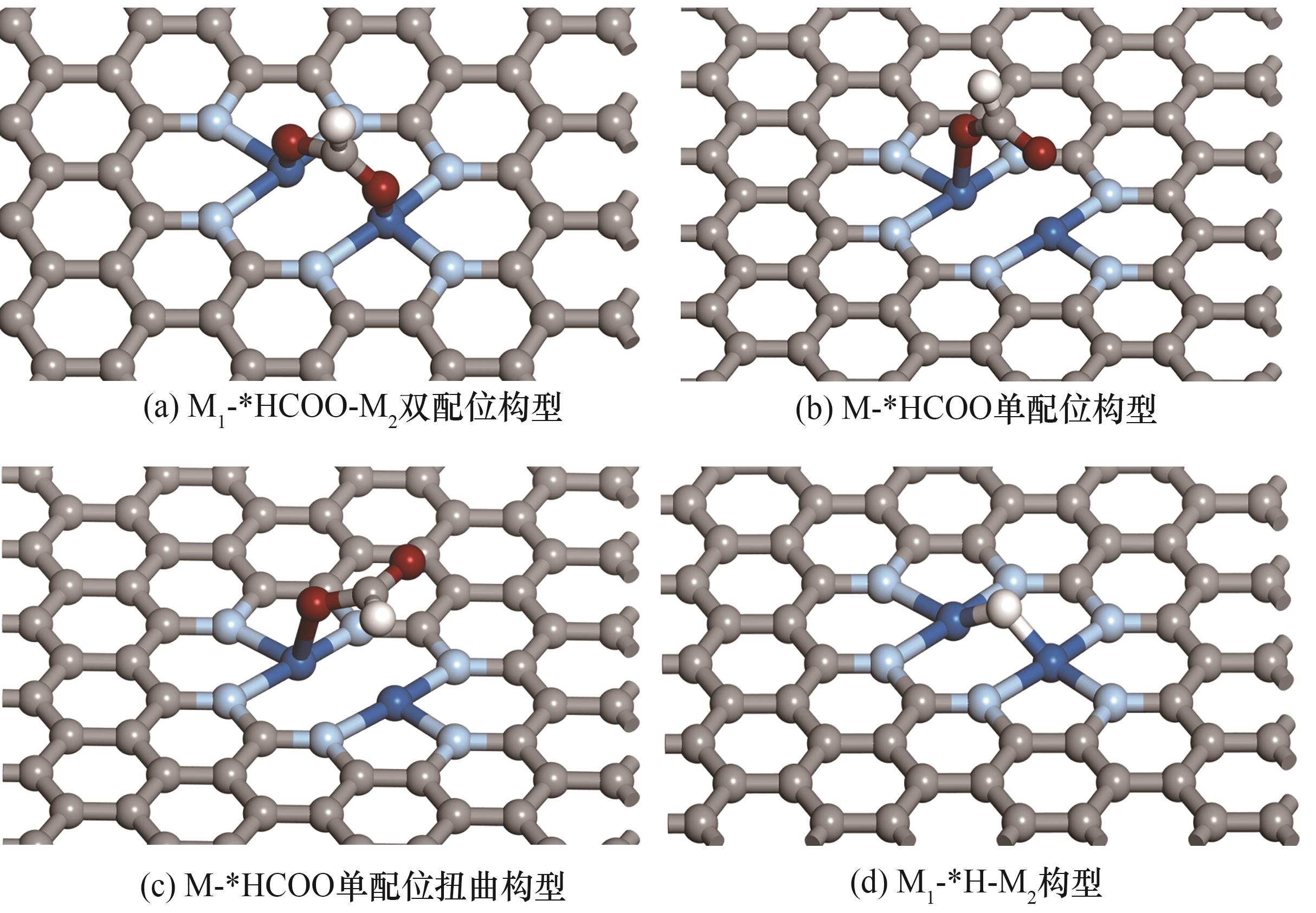
Fig.2 Different adsorption configurations of key intermediates of CO2ER and HER[where grey, light blue, white, red and dark blue spheres represent C, N, H, O and transition-metal atoms (M1 and M2), respectively]
| 序号 | M1 | M2 | *HCOO吸附构型 | ΔG*H/eV | ΔG*HCOO/eV |
|---|---|---|---|---|---|
| 1 | Cu | Ni | M1-*HCOO-M2双配位型 | 0.43 | 0.89 |
| 2 | Co | Pd | M-*HCOO单配位扭曲型 | -0.76 | 0.26 |
| 3 | Rh | Ir | M-*HCOO单配位扭曲型 | -0.50 | 0.51 |
| 4 | Rh | Pt | M-*HCOO单配位扭曲型 | -0.61 | 0.54 |
| 5 | Ir | Cr | M-*HCOO单配位型 | -0.32 | -0.43 |
| 6 | Ir | Mn | M-*HCOO单配位型 | -0.32 | 0.00 |
| 7 | Zn | Fe | M1-*HCOO-M2双配位型 | -0.45 | -0.85 |
| 8 | Ru | Fe | M1-*HCOO-M2双配位型 | -0.05 | 0.12 |
| 9 | Cr | Co | M1-*HCOO-M2双配位型 | -0.29 | -0.48 |
| 10 | Mn | Ag | M-*HCOO单配位型 | -0.66 | -0.62 |
| 11 | Ag | Co | M1-*HCOO-M2双配位型 | 0.08 | 0.27 |
| 12 | Cr | Pd | M-*HCOO单配位型 | -0.43 | -0.77 |
| 13 | Ni | Pt | M-*HCOO单配位扭曲型 | -0.34 | 1.31 |
| 14 | Ag | Au | M-*HCOO单配位型 | -0.24 | 0.92 |
| 15 | Mn | Pt | M-*HCOO单配位型 | -0.61 | -0.53 |
| 16 | Zn | Ni | M1-*HCOO-M2双配位型 | -0.31 | -0.25 |
| 17 | Ru | Cu | M1-*HCOO-M2双配位型 | -0.68 | -0.13 |
| 18 | Cu | Ag | M1-*HCOO-M2双配位型 | 0.13 | 0.13 |
| 19 | Ni | Co | M1-*HCOO-M2双配位型 | -0.59 | 0.53 |
| 20 | Ru | Co | M1-*HCOO-M2双配位型 | -0.24 | 0.34 |
| 21 | Cr | Mn | M1-*HCOO-M2双配位型 | -0.18 | -1.21 |
| 22 | Rh | Ag | M-*HCOO单配位型 | -0.02 | 0.96 |
| 23 | Pd | Zn | M-*HCOO单配位型 | -0.72 | -0.38 |
Table 1 ΔG*H, ΔG*HCOO and *HCOO configurations of 23 randomly selected DMSCs
| 序号 | M1 | M2 | *HCOO吸附构型 | ΔG*H/eV | ΔG*HCOO/eV |
|---|---|---|---|---|---|
| 1 | Cu | Ni | M1-*HCOO-M2双配位型 | 0.43 | 0.89 |
| 2 | Co | Pd | M-*HCOO单配位扭曲型 | -0.76 | 0.26 |
| 3 | Rh | Ir | M-*HCOO单配位扭曲型 | -0.50 | 0.51 |
| 4 | Rh | Pt | M-*HCOO单配位扭曲型 | -0.61 | 0.54 |
| 5 | Ir | Cr | M-*HCOO单配位型 | -0.32 | -0.43 |
| 6 | Ir | Mn | M-*HCOO单配位型 | -0.32 | 0.00 |
| 7 | Zn | Fe | M1-*HCOO-M2双配位型 | -0.45 | -0.85 |
| 8 | Ru | Fe | M1-*HCOO-M2双配位型 | -0.05 | 0.12 |
| 9 | Cr | Co | M1-*HCOO-M2双配位型 | -0.29 | -0.48 |
| 10 | Mn | Ag | M-*HCOO单配位型 | -0.66 | -0.62 |
| 11 | Ag | Co | M1-*HCOO-M2双配位型 | 0.08 | 0.27 |
| 12 | Cr | Pd | M-*HCOO单配位型 | -0.43 | -0.77 |
| 13 | Ni | Pt | M-*HCOO单配位扭曲型 | -0.34 | 1.31 |
| 14 | Ag | Au | M-*HCOO单配位型 | -0.24 | 0.92 |
| 15 | Mn | Pt | M-*HCOO单配位型 | -0.61 | -0.53 |
| 16 | Zn | Ni | M1-*HCOO-M2双配位型 | -0.31 | -0.25 |
| 17 | Ru | Cu | M1-*HCOO-M2双配位型 | -0.68 | -0.13 |
| 18 | Cu | Ag | M1-*HCOO-M2双配位型 | 0.13 | 0.13 |
| 19 | Ni | Co | M1-*HCOO-M2双配位型 | -0.59 | 0.53 |
| 20 | Ru | Co | M1-*HCOO-M2双配位型 | -0.24 | 0.34 |
| 21 | Cr | Mn | M1-*HCOO-M2双配位型 | -0.18 | -1.21 |
| 22 | Rh | Ag | M-*HCOO单配位型 | -0.02 | 0.96 |
| 23 | Pd | Zn | M-*HCOO单配位型 | -0.72 | -0.38 |
| 特征 | 定义 | 特征 | 定义 |
|---|---|---|---|
| Z1 、Z2 | M1和M2的原子序数 | (Z1+Z2)/2 | 平均原子序数 |
| N1 、N2 | M1和M2的价电子数 | (N1+N2)/2 | 平均价电子数 |
| PE1 、PE2 | M1和M2的电负性[ | (PE1+PE2)/2 | 平均电负性 |
| IE1 、IE2 | M1和M2的第一电离能[ | (IE1+IE2)/2 | 平均第一电离能 |
| EA1 、EA2 | M1和M2的电子亲和能[ | (EA1+EA2)/2 | 平均电子亲和能 |
| Nd1 、Nd2 | M1和M2的d电子数 | (Nd1+Nd2)/2 | 平均d电子数 |
| WF1 、WF2 | M1和M2的功函数[ | (WF1+WF2)/2 | 平均功函数 |
| r1 、r2 | M1和M2的原子半径[ | (r1+r2)/2 | 平均原子半径 |
| R1 、R2 | M1和M2的范德华半径[ | (R1+R2)/2 | 平均范德华半径 |
| IE1/Nd1、IE2/Nd2 | 第一电离能除以d电子数 | [(Z1+Z2)/2]2 | 平均原子序数的平方值 |
| EA1/Nd1、EA2/Nd2 | 电子亲和能除以d电子数 | [(N1+N2)/2]2 | 平均价电子数的平方值 |
| PE1/Nd1、PE2/Nd2 | 电负性除以d电子数 | [(PE1+PE2)/2]2 | 平均电负性的平方值 |
| PE1×Nd1、PE2×Nd2 | 电负性与d电子数之积 | [(IE1+IE2)/2]2 | 平均第一电离能的平方值 |
| PE1+PE2、PE1-PE2 | 电负性之和、电负性之差 | [(EA1+EA2)/2]2 | 平均电子亲和能的平方值 |
| r1+r2 | 原子半径之和 | [(Nd1+Nd2)/2]2 | 平均d电子数的平方值 |
| R1+R2 | 范德华半径之和 | [(WF1+WF2)/2]2 | 平均功函数的平方值 |
| WF1/Nd1、WF2/Nd2 | 功函数除以d电子数 | [(r1+r2)/2]2 | 平均原子半径的平方值 |
| WF1+WF2、WF1-WF2 | 功函数之和、功函数之差 | [(R1+R2)/2]2 | 平均范德华半径的平方值 |
Table 2 Complete feature space of 52 features for ML
| 特征 | 定义 | 特征 | 定义 |
|---|---|---|---|
| Z1 、Z2 | M1和M2的原子序数 | (Z1+Z2)/2 | 平均原子序数 |
| N1 、N2 | M1和M2的价电子数 | (N1+N2)/2 | 平均价电子数 |
| PE1 、PE2 | M1和M2的电负性[ | (PE1+PE2)/2 | 平均电负性 |
| IE1 、IE2 | M1和M2的第一电离能[ | (IE1+IE2)/2 | 平均第一电离能 |
| EA1 、EA2 | M1和M2的电子亲和能[ | (EA1+EA2)/2 | 平均电子亲和能 |
| Nd1 、Nd2 | M1和M2的d电子数 | (Nd1+Nd2)/2 | 平均d电子数 |
| WF1 、WF2 | M1和M2的功函数[ | (WF1+WF2)/2 | 平均功函数 |
| r1 、r2 | M1和M2的原子半径[ | (r1+r2)/2 | 平均原子半径 |
| R1 、R2 | M1和M2的范德华半径[ | (R1+R2)/2 | 平均范德华半径 |
| IE1/Nd1、IE2/Nd2 | 第一电离能除以d电子数 | [(Z1+Z2)/2]2 | 平均原子序数的平方值 |
| EA1/Nd1、EA2/Nd2 | 电子亲和能除以d电子数 | [(N1+N2)/2]2 | 平均价电子数的平方值 |
| PE1/Nd1、PE2/Nd2 | 电负性除以d电子数 | [(PE1+PE2)/2]2 | 平均电负性的平方值 |
| PE1×Nd1、PE2×Nd2 | 电负性与d电子数之积 | [(IE1+IE2)/2]2 | 平均第一电离能的平方值 |
| PE1+PE2、PE1-PE2 | 电负性之和、电负性之差 | [(EA1+EA2)/2]2 | 平均电子亲和能的平方值 |
| r1+r2 | 原子半径之和 | [(Nd1+Nd2)/2]2 | 平均d电子数的平方值 |
| R1+R2 | 范德华半径之和 | [(WF1+WF2)/2]2 | 平均功函数的平方值 |
| WF1/Nd1、WF2/Nd2 | 功函数除以d电子数 | [(r1+r2)/2]2 | 平均原子半径的平方值 |
| WF1+WF2、WF1-WF2 | 功函数之和、功函数之差 | [(R1+R2)/2]2 | 平均范德华半径的平方值 |
| 1 | Herron J A, Kim J, Upadhye A A, et al. A general framework for the assessment of solar fuel technologies[J]. Energy & Environmental Science, 2015, 8(1): 126-157. |
| 2 | Davis S J, Caldeira K, Matthews H D. Future CO2 emissions and climate change from existing energy infrastructure[J]. Science, 2010, 329(5997): 1330-1333. |
| 3 | Wu J H, Huang Y, Ye W, et al. CO2 reduction: from the electrochemical to photochemical approach[J]. Advanced Science, 2017, 4(11): 1700194. |
| 4 | Bonetto R, Crisanti F, Sartorel A. Carbon dioxide reduction mediated by iron catalysts: mechanism and intermediates that guide selectivity[J]. ACS Omega, 2020, 5(34): 21309-21319. |
| 5 | Li F W, Chen L, Xue M Q, et al. Towards a better Sn: efficient electrocatalytic reduction of CO2 to formate by Sn/SnS2 derived from SnS2 nanosheets[J]. Nano Energy, 2017, 31: 270-277. |
| 6 | Chen C, Khosrowabadi Kotyk J F, Sheehan S W. Progress toward commercial application of electrochemical carbon dioxide reduction[J]. Chem, 2018, 4(11): 2571-2586. |
| 7 | Lu Q, Jiao F. Electrochemical CO2 reduction: electrocatalyst, reaction mechanism, and process engineering[J]. Nano Energy, 2016, 29: 439-456. |
| 8 | Hui S R, Shaigan N M, Neburchilov V, et al. Three-dimensional cathodes for electrochemical reduction of CO2: from macro-to nano-engineering[J]. Nanomaterials, 2020, 10(9): 1884. |
| 9 | Gao W L, Liang S Y, Wang R J, et al. Industrial carbon dioxide capture and utilization: state of the art and future challenges[J]. Chemical Society Reviews, 2020, 49(23): 8584-8686. |
| 10 | Yang M R, Li H J, Luo N D, et al. Electro-chemical reduction of carbon dioxide into ethylene: catalyst, conditions and mechanism[J]. Progress in Chemistry, 2019, 31(2/3): 245-257. |
| 11 | Chen S H, Su Y Q, Deng P L, et al. Highly selective carbon dioxide electroreduction on structure-evolved copper perovskite oxide toward methane production[J]. ACS Catalysis, 2020, 10(8): 4640-4646. |
| 12 | Yu J L, Liu H Y, Song S Q, et al. Electrochemical reduction of carbon dioxide at nanostructured SnO2/carbon aerogels: the effect of tin oxide content on the catalytic activity and formate selectivity[J]. Applied Catalysis A: General, 2017, 545: 159-166. |
| 13 | Agarwal A S, Zhai Y M, Hill D, et al. The electrochemical reduction of carbon dioxide to formate/formic acid: engineering and economic feasibility[J]. ChemSusChem, 2011, 4(9): 1301-1310. |
| 14 | Loges B, Boddien A, Gärtner F, et al. Catalytic generation of hydrogen from formic acid and its derivatives: useful hydrogen storage materials[J]. Topics in Catalysis, 2010, 53(13/14): 902-914. |
| 15 | Li J, Jiao J Q, Zhang H C, et al. Two-dimensional SnO2 nanosheets for efficient carbon dioxide electroreduction to formate[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(12): 4975-4982. |
| 16 | Mou K W, Chen Z P, Yao S Y, et al. Enhanced electrochemical reduction of carbon dioxide to formate with in situ grown indium-based catalysts in an aqueous electrolyte[J]. Electrochimica Acta, 2018, 289: 65-71. |
| 17 | Fu Y S, Li Y N, Zhang X, et al. Novel hierarchical SnO2 microsphere catalyst coated on gas diffusion electrode for enhancing energy efficiency of CO2 reduction to formate fuel[J]. Applied Energy, 2016, 175: 536-544. |
| 18 | Chen Y H, Kanan M W. Tin oxide dependence of the CO2 reduction efficiency on tin electrodes and enhanced activity for tin/tin oxide thin-film catalysts[J]. Journal of the American Chemical Society, 2012, 134(4): 1986-1989. |
| 19 | Pander J E III, Baruch M F, Bocarsly A B. Probing the mechanism of aqueous CO2 reduction on post-transition-metal electrodes using ATR-IR spectroelectrochemistry[J]. ACS Catalysis, 2016, 6(11): 7824-7833. |
| 20 | Zhu X R, Yan J X, Gu M, et al. Activity origin and design principles for oxygen reduction on dual-metal-site catalysts: a combined density functional theory and machine learning study[J]. The Journal of Physical Chemistry Letters, 2019, 10(24): 7760-7766. |
| 21 | He Q, Yu B, Li Z H, et al. Density functional theory for battery materials[J]. Energy & Environmental Materials, 2019, 2(4): 264-279. |
| 22 | Li M R, Garg S, Chang X X, et al. Toward excellence of transition metal-based catalysts for CO2 electrochemical reduction: an overview of strategies and rationales[J]. Small Methods, 2020, 4(7): 2000033. |
| 23 | Yang Z, Gao W, Jiang Q. A machine learning scheme for the catalytic activity of alloys with intrinsic descriptors[J]. Journal of Materials Chemistry A, 2020, 8(34): 17507-17515. |
| 24 | Chen Y, Huang Y, Cheng T, et al. Identifying active sites for CO2 reduction on dealloyed gold surfaces by combining machine learning with multiscale simulations[J]. Journal of the American Chemical Society, 2019, 141(29): 11651-11657. |
| 25 | Zhong M, Tran K, Min Y, et al. Accelerated discovery of CO2 electrocatalysts using active machine learning[J]. Nature, 2020, 581(7807): 178-183. |
| 26 | Ma X, Li Z, Achenie L E, et al. Machine-learning-augmented chemisorption model for CO2 electroreduction catalyst screening[J]. The Journal of Physical Chemistry Letters, 2015, 6(18): 3528-3533. |
| 27 | Mayer F D, Hosseini-Benhangi P, Sánchez-Sánchez C M, et al. Scanning electrochemical microscopy screening of CO2 electroreduction activities and product selectivities of catalyst arrays[J]. Communications Chemistry, 2020, 3: 155. |
| 28 | Wu D H, Zhang J Y, Cheng M J, et al. Machine learning investigation of supplementary adsorbate influence on copper for enhanced electrochemical CO2 reduction performance[J]. The Journal of Physical Chemistry C, 2021, 125(28): 15363-15372. |
| 29 | Zhang H C, Goddard W A, Lu Q, et al. The importance of grand-canonical quantum mechanical methods to describe the effect of electrode potential on the stability of intermediates involved in both electrochemical CO2 reduction and hydrogen evolution[J]. Physical Chemistry Chemical Physics, 2018, 20(4): 2549-2557. |
| 30 | Ma W C, Xie S J, Zhang X G, et al. Promoting electrocatalytic CO2 reduction to formate via sulfur-boosting water activation on indium surfaces[J]. Nature Communications, 2019, 10: 892. |
| 31 | Hastie T, Tibshirani R, Friedman J H, et al. The elements of statistical learning: data mining, inference, and prediction[J]. The Mathematical Intelligencer, 2004, 27(2): 83-85. |
| 32 | Friedman J H. Greedy function approximation: a gradient boosting machine[J]. The Annals of Statistics, 2001, 29(5): 1189-1232. |
| 33 | Kim J, Jung H, Jung S M, et al. Tailoring binding abilities by incorporating oxophilic transition metals on 3D nanostructured Ni arrays for accelerated alkaline hydrogen evolution reaction[J]. Journal of the American Chemical Society, 2021, 143(3): 1399-1408. |
| 34 | Speight J G. Lange's Handbook of Chemistry[M]. New York: McGraw-Hill, 2005: 1.132-1.156. |
| 35 | 喻典, 梁国明. 元素电子亲和势的密度泛函理论计算[J]. 重庆师范大学学报(自然科学版), 2005, 22(1): 39-42. |
| Yu D, Liang G M. A study of electron affinities of the elements by density functional theory[J]. Journal of Chongqing Teachers College (Natural Science Edition), 2005, 22(1): 39-42. | |
| 36 | Alvarez S. A cartography of the van der Waals territories[J]. Dalton Transactions, 2013, 42(24): 8617. |
| [1] | Yifei ZHANG, Fangchen LIU, Shuangxing ZHANG, Wenjing DU. Performance analysis of printed circuit heat exchanger for supercritical carbon dioxide [J]. CIESC Journal, 2023, 74(S1): 183-190. |
| [2] | Ruitao SONG, Pai WANG, Yunpeng WANG, Minxia LI, Chaobin DANG, Zhenguo CHEN, Huan TONG, Jiaqi ZHOU. Numerical simulation of flow boiling heat transfer in pipe arrays of carbon dioxide direct evaporation ice field [J]. CIESC Journal, 2023, 74(S1): 96-103. |
| [3] | Yitong LI, Hang GUO, Hao CHEN, Fang YE. Study on operating conditions of proton exchange membrane fuel cells with non-uniform catalyst distributions [J]. CIESC Journal, 2023, 74(9): 3831-3840. |
| [4] | Yepin CHENG, Daqing HU, Yisha XU, Huayan LIU, Hanfeng LU, Guokai CUI. Application of ionic liquid-based deep eutectic solvents for CO2 conversion [J]. CIESC Journal, 2023, 74(9): 3640-3653. |
| [5] | Jie CHEN, Yongsheng LIN, Kai XIAO, Chen YANG, Ting QIU. Study on catalytic synthesis of sec-butanol by tunable choline-based basic ionic liquids [J]. CIESC Journal, 2023, 74(9): 3716-3730. |
| [6] | Xuejin YANG, Jintao YANG, Ping NING, Fang WANG, Xiaoshuang SONG, Lijuan JIA, Jiayu FENG. Research progress in dry purification technology of highly toxic gas PH3 [J]. CIESC Journal, 2023, 74(9): 3742-3755. |
| [7] | Yali HU, Junyong HU, Suxia MA, Yukun SUN, Xueyi TAN, Jiaxin HUANG, Fengyuan YANG. Development of novel working fluid and study on electrochemical characteristics of reverse electrodialysis heat engine [J]. CIESC Journal, 2023, 74(8): 3513-3521. |
| [8] | Xin YANG, Xiao PENG, Kairu XUE, Mengwei SU, Yan WU. Preparation of molecularly imprinted-TiO2 and its properties of photoelectrocatalytic degradation of solubilized PHE [J]. CIESC Journal, 2023, 74(8): 3564-3571. |
| [9] | Feifei YANG, Shixi ZHAO, Wei ZHOU, Zhonghai NI. Sn doped In2O3 catalyst for selective hydrogenation of CO2 to methanol [J]. CIESC Journal, 2023, 74(8): 3366-3374. |
| [10] | Rui HONG, Baoqiang YUAN, Wenjing DU. Analysis on mechanism of heat transfer deterioration of supercritical carbon dioxide in vertical upward tube [J]. CIESC Journal, 2023, 74(8): 3309-3319. |
| [11] | Kaixuan LI, Wei TAN, Manyu ZHANG, Zhihao XU, Xuyu WANG, Hongbing JI. Design of cobalt-nitrogen-carbon/activated carbon rich in zero valent cobalt active site and application of catalytic oxidation of formaldehyde [J]. CIESC Journal, 2023, 74(8): 3342-3352. |
| [12] | Jiali GE, Tuxiang GUAN, Xinmin QIU, Jian WU, Liming SHEN, Ningzhong BAO. Synthesis of FeF3 nanoparticles covered by vertical porous carbon for high performance Li-ion battery cathode [J]. CIESC Journal, 2023, 74(7): 3058-3067. |
| [13] | Yuanhao QU, Wenyi DENG, Xiaodan XIE, Yaxin SU. Study on electro-osmotic dewatering of sludge assisted by activated carbon/graphite [J]. CIESC Journal, 2023, 74(7): 3038-3050. |
| [14] | Pan LI, Junyang MA, Zhihao CHEN, Li WANG, Yun GUO. Effect of the morphology of Ru/α-MnO2 on NH3-SCO performance [J]. CIESC Journal, 2023, 74(7): 2908-2918. |
| [15] | Mengmeng ZHANG, Dong YAN, Yongfeng SHEN, Wencui LI. Effect of electrolyte types on the storage behaviors of anions and cations for dual-ion batteries [J]. CIESC Journal, 2023, 74(7): 3116-3126. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||