CIESC Journal ›› 2024, Vol. 75 ›› Issue (12): 4532-4546.DOI: 10.11949/0438-1157.20240633
• Catalysis, kinetics and reactors • Previous Articles Next Articles
Haotian MA1( ), Tirui JING1, Chengcheng LIU1, Turap YUSAN2, Zhe ZHANG2, Yidi WANG1(
), Tirui JING1, Chengcheng LIU1, Turap YUSAN2, Zhe ZHANG2, Yidi WANG1( ), Qinghong WANG1, Chunmao CHEN1, Chunming XU1
), Qinghong WANG1, Chunmao CHEN1, Chunming XU1
Received:2024-06-07
Revised:2024-07-29
Online:2025-01-03
Published:2024-12-25
Contact:
Yidi WANG
马浩天1( ), 荆体瑞1, 刘程程1, 玉散·吐拉甫2, 张喆2, 王一迪1(
), 荆体瑞1, 刘程程1, 玉散·吐拉甫2, 张喆2, 王一迪1( ), 王庆宏1, 陈春茂1, 徐春明1
), 王庆宏1, 陈春茂1, 徐春明1
通讯作者:
王一迪
作者简介:马浩天(2000—),男,硕士研究生,mht16735367888@163.com
基金资助:CLC Number:
Haotian MA, Tirui JING, Chengcheng LIU, Turap YUSAN, Zhe ZHANG, Yidi WANG, Qinghong WANG, Chunmao CHEN, Chunming XU. Study on reduction performance and kinetics of Sr-modified LaFeO3 for methane chemical looping reforming[J]. CIESC Journal, 2024, 75(12): 4532-4546.
马浩天, 荆体瑞, 刘程程, 玉散·吐拉甫, 张喆, 王一迪, 王庆宏, 陈春茂, 徐春明. Sr改性LaFeO3用于甲烷化学链重整的还原性能与动力学研究[J]. 化工学报, 2024, 75(12): 4532-4546.
Add to citation manager EndNote|Ris|BibTeX
| 函数名称 | 机理 | 函数编号 | g(α) | f(α) |
|---|---|---|---|---|
| Avrami-Erofeev方程 | 成核核增长 | A2 | [-ln(1-α)]1/2 | 2(1-α)[-ln(1-α)]1/2 |
| A3 | [-ln(1-α)]1/3 | 3(1-α)[-ln(1-α)]2/3 | ||
| A4 | [-ln(1-α)]1/4 | 4(1-α)[-ln(1-α)]3/4 | ||
| Prout-Tompkins方程 | 自催化模型 | B1 | ln[α/(1-α)] | α/(1-α) |
| Mampel Power法则 | 相界面反应(一维) | C1 | α | 1 |
| 收缩模型 | 收缩圆柱体(面积),相界面反应,圆柱形对称 | C2 | 1- (1-α)1/2 | 2(1-α)1/2 |
| 收缩球体(体积),相界面反应,球形对称 | C3 | 1- (1-α)1/3 | 3(1-α)2/3 | |
| Jander方程 | 一维扩散模型 | D1 | α2 | |
| 三维扩散,球形对称 | D3 | 1- | ||
| 反应级数模型 | 一级化学反应 | R1 | -ln(1-α) | 1-α |
| 二级化学反应 | R2 | [1/(1-α)]-1 | (1-α)2 | |
| 三级化学反应 | R3 | [1/(1-α)2]-1 | (1-α) 3 |
Table 1 Differential and integral expressions of mechanism function
| 函数名称 | 机理 | 函数编号 | g(α) | f(α) |
|---|---|---|---|---|
| Avrami-Erofeev方程 | 成核核增长 | A2 | [-ln(1-α)]1/2 | 2(1-α)[-ln(1-α)]1/2 |
| A3 | [-ln(1-α)]1/3 | 3(1-α)[-ln(1-α)]2/3 | ||
| A4 | [-ln(1-α)]1/4 | 4(1-α)[-ln(1-α)]3/4 | ||
| Prout-Tompkins方程 | 自催化模型 | B1 | ln[α/(1-α)] | α/(1-α) |
| Mampel Power法则 | 相界面反应(一维) | C1 | α | 1 |
| 收缩模型 | 收缩圆柱体(面积),相界面反应,圆柱形对称 | C2 | 1- (1-α)1/2 | 2(1-α)1/2 |
| 收缩球体(体积),相界面反应,球形对称 | C3 | 1- (1-α)1/3 | 3(1-α)2/3 | |
| Jander方程 | 一维扩散模型 | D1 | α2 | |
| 三维扩散,球形对称 | D3 | 1- | ||
| 反应级数模型 | 一级化学反应 | R1 | -ln(1-α) | 1-α |
| 二级化学反应 | R2 | [1/(1-α)]-1 | (1-α)2 | |
| 三级化学反应 | R3 | [1/(1-α)2]-1 | (1-α) 3 |
| 参数 | LaFeO3 | La0.8Sr0.2FeO3 | La0.6Sr0.4FeO3 | La0.4Sr0.6FeO3 | |
|---|---|---|---|---|---|
| 键长/nm | Fe—O(1) | 0.20073 | 0.204536 | 0.2116 | 0.1941 |
| Fe—O(2) | 0.20099 | 0.212309 | 0.1960 | 0.1920 | |
| Fe—O(2) | 0.20020 | 0.180842 | 0.1960 | 0.1970 | |
| 键角/(°) | Fe—O(1)—Fe | 155.90 | 145.35 | 134.80 | 170.50 |
| Fe—O(2)—Fe | 157.01 | 169.06 | 166.20 | 177.00 | |
| 键长方差 | 0.0027 | 4.50 | 1.30 | 0.11 | |
Table 2 Crystal structure parameters of La1-x Sr x FeO3 oxygen carrier
| 参数 | LaFeO3 | La0.8Sr0.2FeO3 | La0.6Sr0.4FeO3 | La0.4Sr0.6FeO3 | |
|---|---|---|---|---|---|
| 键长/nm | Fe—O(1) | 0.20073 | 0.204536 | 0.2116 | 0.1941 |
| Fe—O(2) | 0.20099 | 0.212309 | 0.1960 | 0.1920 | |
| Fe—O(2) | 0.20020 | 0.180842 | 0.1960 | 0.1970 | |
| 键角/(°) | Fe—O(1)—Fe | 155.90 | 145.35 | 134.80 | 170.50 |
| Fe—O(2)—Fe | 157.01 | 169.06 | 166.20 | 177.00 | |
| 键长方差 | 0.0027 | 4.50 | 1.30 | 0.11 | |
| 载氧体 | Fe2O3/% (质量分数) | La2O3/% (质量分数) | SrO/% (质量分数) | La/% (质量分数) | Sr/% (质量分数) | La/Sr 摩尔比 | 摩尔比 理论值 |
|---|---|---|---|---|---|---|---|
| LaFeO3 | 34.32 | 64.76 | — | 55.23 | — | — | — |
| La0.8Sr0.2FeO3 | 35.12 | 53.72 | 10.22 | 45.81 | 8.64 | 0.78/0.22 | 0.8/0.2 |
| La0.6Sr0.4FeO3 | 35.71 | 42.32 | 21.01 | 36.09 | 17.76 | 0.57/0.43 | 0.6/0.4 |
| La0.4Sr0.6FeO3 | 34.36 | 27.11 | 36.57 | 28.12 | 27.92 | 0.39/0.61 | 0.4/0.6 |
Table 3 XRF compositional analysis of La1-x Sr x FeO3 oxygen carrier
| 载氧体 | Fe2O3/% (质量分数) | La2O3/% (质量分数) | SrO/% (质量分数) | La/% (质量分数) | Sr/% (质量分数) | La/Sr 摩尔比 | 摩尔比 理论值 |
|---|---|---|---|---|---|---|---|
| LaFeO3 | 34.32 | 64.76 | — | 55.23 | — | — | — |
| La0.8Sr0.2FeO3 | 35.12 | 53.72 | 10.22 | 45.81 | 8.64 | 0.78/0.22 | 0.8/0.2 |
| La0.6Sr0.4FeO3 | 35.71 | 42.32 | 21.01 | 36.09 | 17.76 | 0.57/0.43 | 0.6/0.4 |
| La0.4Sr0.6FeO3 | 34.36 | 27.11 | 36.57 | 28.12 | 27.92 | 0.39/0.61 | 0.4/0.6 |
| 载氧体 | 不同氧元素百分比/% | ||||
|---|---|---|---|---|---|
| O1(O2-) | O2 ( | O3 (—OH, | O4 (H2O) | Oads/Olatt | |
| LaFeO3 | 50.53 | 15.32 | 18.06 | 16.09 | 0.30 |
| La0.8Sr0.2FeO3 | 37.18 | 18.82 | 28.27 | 15.73 | 0.51 |
| La0.6Sr0.4FeO3 | 33.39 | 15.84 | 33.82 | 16.96 | 0.47 |
| La0.4Sr0.6FeO3 | 42.68 | 16.55 | 29.06 | 11.72 | 0.39 |
Table 4 XPS results of oxygen element O 1s in La1-x Sr x FeO3 oxygen carrier
| 载氧体 | 不同氧元素百分比/% | ||||
|---|---|---|---|---|---|
| O1(O2-) | O2 ( | O3 (—OH, | O4 (H2O) | Oads/Olatt | |
| LaFeO3 | 50.53 | 15.32 | 18.06 | 16.09 | 0.30 |
| La0.8Sr0.2FeO3 | 37.18 | 18.82 | 28.27 | 15.73 | 0.51 |
| La0.6Sr0.4FeO3 | 33.39 | 15.84 | 33.82 | 16.96 | 0.47 |
| La0.4Sr0.6FeO3 | 42.68 | 16.55 | 29.06 | 11.72 | 0.39 |
| 载氧体 | 比表面积/ (m2/g) | 孔体积/ (10-3 cm3/g) | 平均孔径/nm |
|---|---|---|---|
| LaFeO3 | 15.27 | 2.4 | 6.41 |
| La0.8Sr0.2FeO3 | 19.35 | 3.7 | 10.99 |
| La0.6Sr0.4FeO3 | 17.91 | 3.2 | 5.88 |
| La0.4Sr0.6FeO3 | 12.11 | 1.7 | 4.51 |
Table 5 Specific surface area and porosity data of La1-x Sr x FeO3 oxygen carriers
| 载氧体 | 比表面积/ (m2/g) | 孔体积/ (10-3 cm3/g) | 平均孔径/nm |
|---|---|---|---|
| LaFeO3 | 15.27 | 2.4 | 6.41 |
| La0.8Sr0.2FeO3 | 19.35 | 3.7 | 10.99 |
| La0.6Sr0.4FeO3 | 17.91 | 3.2 | 5.88 |
| La0.4Sr0.6FeO3 | 12.11 | 1.7 | 4.51 |
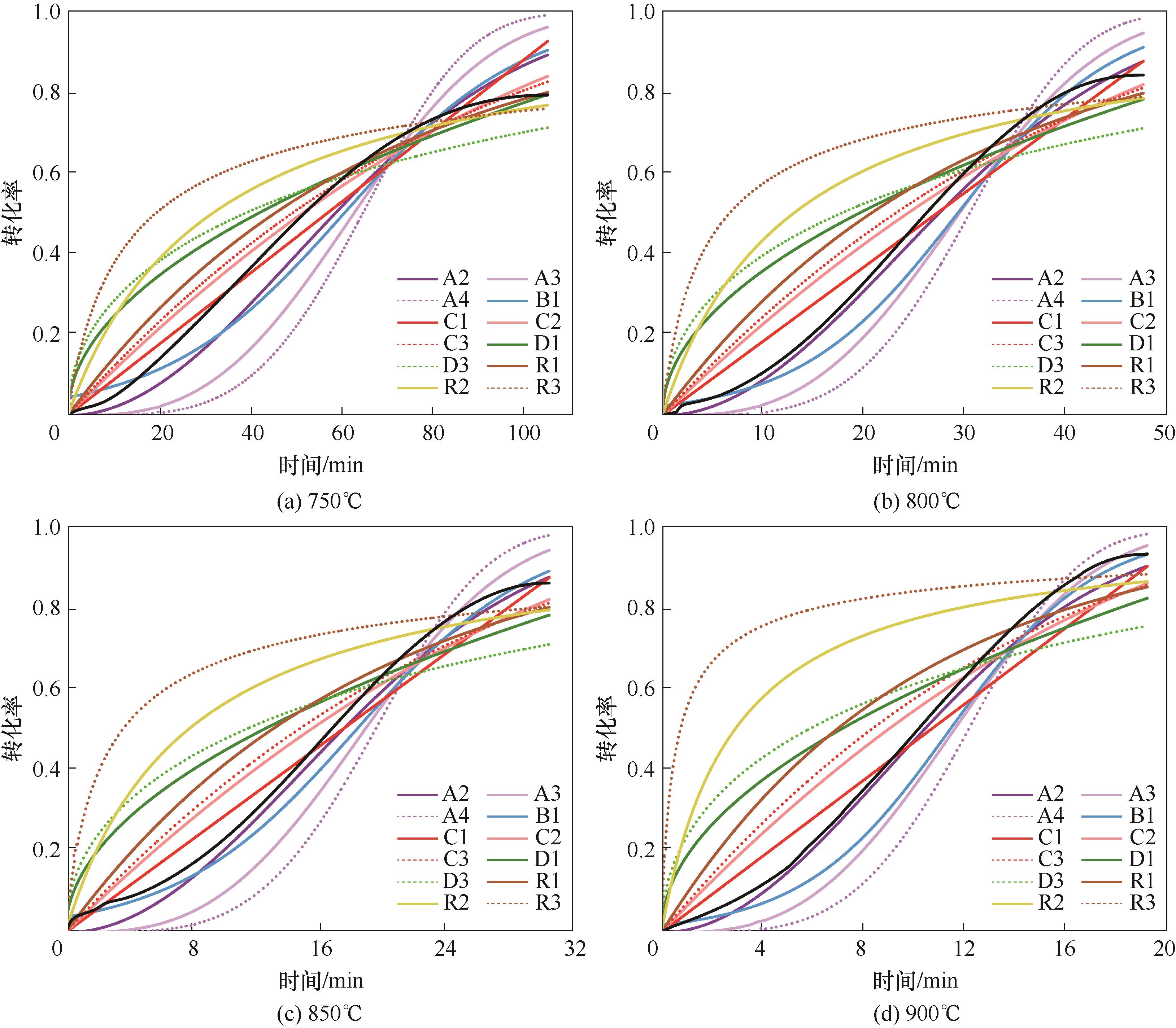
Fig.7 Fitting curves of common kinetic equations for reaction of La0.8Sr0.2FeO3 with CH4 at different temperatures (colored lines represent fitting results of different mechanism functions, black lines represent experimental results)
| 载氧体 | 成核核增长模型 | 自催化枝状模型 | ||||
|---|---|---|---|---|---|---|
| 活化能/(kJ/mol) | 指前因子/s-1 | 相关系数 | 活化能/(kJ/mol) | 指前因子/s-1 | 相关系数 | |
| LaFeO3 | 159.454 | 1.337907×106 | 0.9217 | 164.87 | 1.35×107 | 0.9922 |
| La0.8Sr0.2FeO3 | 112.798 | 8.586×103 | 0.9933 | 135.93 | 4.024×105 | 0.9595 |
| La0.6Sr0.4FeO3 | 163.684 | 2×106 | 0.9927 | 167.27 | 1.09×107 | 0.9912 |
| La0.4Sr0.6FeO3 | 167.350 | 1.988×106 | 0.9961 | 144.91 | 8.4×105 | 0.9990 |
Table 6 Main kinetic parameters of La1-x Sr x FeO3 oxygen carriers in different kinetic models
| 载氧体 | 成核核增长模型 | 自催化枝状模型 | ||||
|---|---|---|---|---|---|---|
| 活化能/(kJ/mol) | 指前因子/s-1 | 相关系数 | 活化能/(kJ/mol) | 指前因子/s-1 | 相关系数 | |
| LaFeO3 | 159.454 | 1.337907×106 | 0.9217 | 164.87 | 1.35×107 | 0.9922 |
| La0.8Sr0.2FeO3 | 112.798 | 8.586×103 | 0.9933 | 135.93 | 4.024×105 | 0.9595 |
| La0.6Sr0.4FeO3 | 163.684 | 2×106 | 0.9927 | 167.27 | 1.09×107 | 0.9912 |
| La0.4Sr0.6FeO3 | 167.350 | 1.988×106 | 0.9961 | 144.91 | 8.4×105 | 0.9990 |
| 载氧体 | 温度区间/K | 实验方法 | 模型 | Ea/(kJ/mol) | 文献 |
|---|---|---|---|---|---|
| LaFeO3 | 1023~1173 | 等温TGA | 成核核增长/自催化 | 159/165 | 本研究 |
| LaFeO3 | 1073~1223 | 等温质谱 | 成核核增长 | 151 | [ |
| MnFe2O4 | 1073~1173 | 等温TGA | 扩散控制 | 139 | [ |
| CeO2 | 873~1123 | 等温TCD | — | 137 | [ |
| CoWO4 | 1123~1223 | 等温TGA | 一级化学反应 | 221 | [ |
Table 7 Reduction kinetic models and activation energies of oxygen carriers reported in literature
| 载氧体 | 温度区间/K | 实验方法 | 模型 | Ea/(kJ/mol) | 文献 |
|---|---|---|---|---|---|
| LaFeO3 | 1023~1173 | 等温TGA | 成核核增长/自催化 | 159/165 | 本研究 |
| LaFeO3 | 1073~1223 | 等温质谱 | 成核核增长 | 151 | [ |
| MnFe2O4 | 1073~1173 | 等温TGA | 扩散控制 | 139 | [ |
| CeO2 | 873~1123 | 等温TCD | — | 137 | [ |
| CoWO4 | 1123~1223 | 等温TGA | 一级化学反应 | 221 | [ |
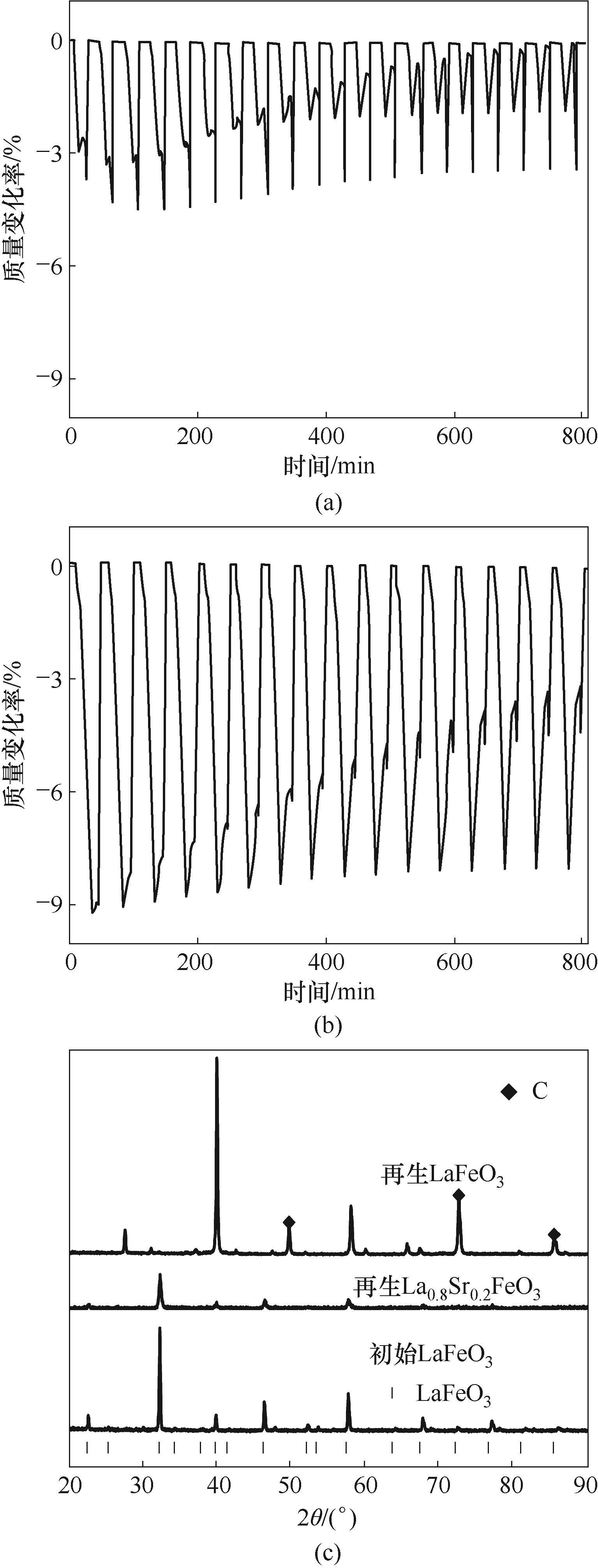
Fig.11 (a) Mass change of LaFeO3 during CH4 reduction-air oxidation process;(b) Mass change of La0.8Sr0.2FeO3 during CH4 reduction-air oxidation process; (c) XRD patterns comparison of regenerated and fresh oxygen carrier
| 1 | 符冠云. 氢能在我国能源转型中的地位和作用[J]. 中国煤炭, 2019, 45(10): 15-21. |
| Fu G Y. The status and role of hydrogen energy in China's energy transformation[J]. China Coal, 2019, 45(10): 15-21. | |
| 2 | Agency I E. Global Hydrogen Review 2022[M]. Paris: OECD, 2022. |
| 3 | 聂家波, 邓建悦. 燃料电池用氢气的制备工艺探讨[J]. 化工技术与开发, 2021, 50(8): 46-50. |
| Nie J B, Deng J Y. Discussion on preparation technology of hydrogen for fuel cell[J]. Technology & Development of Chemical Industry, 2021, 50(8): 46-50. | |
| 4 | Zhang H T, Sun Z X, Hu Y H. Steam reforming of methane: current states of catalyst design and process upgrading[J]. Renewable and Sustainable Energy Reviews, 2021, 149: 111330. |
| 5 | Luo M, Yi Y, Wang S Z, et al. Review of hydrogen production using chemical-looping technology[J]. Renewable and Sustainable Energy Reviews, 2018, 81: 3186-3214. |
| 6 | Liu R, Zhang X H, Liu T, et al. Dynamic oxygen migration and reaction over ceria-supported nickel oxides in chemical looping partial oxidation of methane[J]. Applied Catalysis B: Environmental, 2023, 328: 122478. |
| 7 | 付甜甜, 吐拉甫·玉散, 王邑维, 等. 镍修饰的铁基载氧体甲烷化学链制氢实验[J]. 燃烧科学与技术, 2020, 26(2): 125-132. |
| Fu T T, Turap Y S, Wang Y W, et al. Performance of iron-based oxygen carriers modified by NiO in chemical looping hydrogen generation process [J]. Journal of Combustion Science and Technology, 2020, 26(2): 125-132. | |
| 8 | Zhang X R, Wang J, Song Z L, et al. Co3O4-CeO2 for enhanced syngas by low-temperature methane conversion with CO2 utilization via a catalytic chemical looping process[J]. Fuel Processing Technology, 2023, 245: 107741. |
| 9 | Li F, Kim H R, Sridhar D, et al. Syngas chemical looping gasification process: oxygen carrier particle selection and performance[J]. Energy & Fuels, 2009, 23(8): 4182-4189. |
| 10 | Keller M, Fung J, Leion H, et al. Cu-impregnated alumina/silica bed materials for chemical looping reforming of biomass gasification gas[J]. Fuel, 2016, 180: 448-456. |
| 11 | Abad A, Mattisson T, Lyngfelt A, et al. Chemical-looping combustion in a 300 W continuously operating reactor system using a manganese-based oxygen carrier[J]. Fuel, 2006, 85(9): 1174-1185. |
| 12 | Dharanipragada N V R A, Buelens L C, Poelman H, et al. Mg-Fe-Al-O for advanced CO2 to CO conversion: carbon monoxide yield vs. oxygen storage capacity[J]. Journal of Materials Chemistry A, 2015, 3(31): 16251-16262. |
| 13 | Wang B W, Yan R, Zhao H B, et al. Investigation of chemical looping combustion of coal with CuFe2O4 oxygen carrier[J]. Energy & Fuels, 2011, 25(7): 3344-3354. |
| 14 | Ismail M, Liu W, Scott S A. The performance of Fe2O3-CaO oxygen carriers and the interaction of iron oxides with CaO during chemical looping combustion and H2 production[J]. Energy Procedia, 2014, 63: 87-97. |
| 15 | Hirabayashi D, Sakai Y, Yoshikawa T, et al. Mössbauer characterization of calcium–ferrite oxides prepared by calcining Fe2O3 and CaO[J]. Hyperfine Interactions, 2006, 167(1): 809-813. |
| 16 | Evdou A, Zaspalis V, Nalbandian L. Ferrites as redox catalysts for chemical looping processes[J]. Fuel, 2016, 165: 367-378. |
| 17 | Tang M C, Xu L, Fan M H. Progress in oxygen carrier development of methane-based chemical-looping reforming: a review[J]. Applied Energy, 2015, 151: 143-156. |
| 18 | Li Y L, Chen M Y, Jiang L, et al. Perovskites as oxygen storage materials for chemical looping partial oxidation and reforming of methane[J]. Physical Chemistry Chemical Physics, 2024, 26(3): 1516-1540. |
| 19 | Dai X P, Li R J, Yu C C, et al. Unsteady-state direct partial oxidation of methane to synthesis gas in a fixed-bed reactor using AFeO3 (A = La, Nd, Eu) perovskite-type oxides as oxygen storage[J]. The Journal of Physical Chemistry B, 2006, 110(45): 22525-22531. |
| 20 | Dai X P, Cheng J, Li Z Z, et al. Reduction kinetics of lanthanum ferrite perovskite for the production of synthesis gas by chemical-looping methane reforming[J]. Chemical Engineering Science, 2016, 153: 236-245. |
| 21 | Taylor F H, Buckeridge J, Catlow C R A. Screening divalent metals for A- and B-site dopants in LaFeO3 [J]. Chemistry of Materials, 2017, 29(19): 8147-8157. |
| 22 | He F, Li X N, Zhao K, et al. The use of La1- x Sr x FeO3 perovskite-type oxides as oxygen carriers in chemical-looping reforming of methane[J]. Fuel, 2013, 108: 465-473. |
| 23 | He F, Chen J, Liu S, et al. La1- x Sr x FeO3 perovskite-type oxides for chemical-looping steam methane reforming: identification of the surface elements and redox cyclic performance[J]. International Journal of Hydrogen Energy, 2019, 44(21): 10265-10276. |
| 24 | Ma Z, Lu Y G, Liu G F. Enhanced cyclic redox reactivity of Fe2O3/Al2O3 by Sr doping for chemical-looping combustion of solid fuels[J]. Fuel, 2022, 324: 124625. |
| 25 | Galwey A K. Meltingand thermal decompositions of solids[J]. Journal of Thermal Analysis and Calorimetry, 2007, 87(2): 601-615. |
| 26 | Feng Y C, Wang N N, Guo X, et al. Characteristics of dopant distribution and surface oxygen vacancy formation for modified Fe2O3 in chemical looping combustion[J]. Fuel, 2020, 276: 117942. |
| 27 | Zhang Y K, Zhao Y J, Yi Q, et al. NiO/κ-CeZrO4 functional oxygen carriers with Ni δ + and oxygen vacancy synergy for chemical looping partial oxidation reforming of methane[J]. Fuel Processing Technology, 2021, 219: 106875. |
| 28 | Wang Y J, Zheng Y E, Wang Y H, et al. Syngas production modified by oxygen vacancies over CeO2-ZrO2-CuO oxygen carrier via chemical looping reforming of methane[J]. Applied Surface Science, 2019, 481: 151-160. |
| 29 | 吕熠. 基于铁酸镧化学链转化焦油模型化合物制备合成气的研究[D]. 北京: 清华大学, 2022. |
| Lv Y. Study on syngas production from tar model compounds via chemical looping using lanthanum ferrite[D]. Beijing: Tsinghua University, 2022. | |
| 30 | Pecchi G, Reyes P, Zamora R, et al. Effect of the preparation method on the catalytic activity of La1- x Ca x FeO3 perovskite-type oxides[J]. Catalysis Today, 2008, 133: 420-427. |
| 31 | Shannon R D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides[J]. Acta Crystallographica Section A, 1976, 32(5): 751-767. |
| 32 | Hirata T. Oxygen position, octahedral distortion, and bond-valence parameter from bond lengths in Ti1- x Sn x O2 (0≤x≤1)[J]. Journal of the American Ceramic Society, 2000, 83(12): 3205-3207. |
| 33 | Zhu Y L, Zhou W, Yu J, et al. Enhancing electrocatalytic activity of perovskite oxides by tuning cation deficiency for oxygen reduction and evolution reactions[J]. Chemistry of Materials, 2016, 28(6): 1691-1697. |
| 34 | Gosavi P V, Biniwale R B. Pure phase LaFeO3 perovskite with improved surface area synthesized using different routes and its characterization[J]. Materials Chemistry and Physics, 2010, 119(1/2): 324-329. |
| 35 | Margellou A, Manos D, Petrakis D, et al. Activation of persulfate by LaFe1- x Co x O3 perovskite catalysts for the degradation of phenolics: effect of synthetic method and metal substitution[J]. Science of the Total Environment, 2022, 832: 155063. |
| 36 | Li P, Chen X Y, Li Y D, et al. A review on oxygen storage capacity of CeO2-based materials: influence factors, measurement techniques, and applications in reactions related to catalytic automotive emissions control[J]. Catalysis Today, 2019, 327: 90-115. |
| 37 | Rossetti I, Forni L. Catalytic flameless combustion of methane over perovskites prepared by flame-hydrolysis[J]. Applied Catalysis B: Environmental, 2001, 33(4): 345-352. |
| 38 | Zhang X H, Liu R, Liu T, et al. Redox catalysts for chemical looping methane conversion[J]. Trends in Chemistry, 2023, 5(7): 512-525. |
| 39 | Wei H J, Cao Y, Ji W J, et al. Lattice oxygen of La1- x Sr x MO3 (M=Mn, Ni) and LaMnO3- α F β perovskite oxides for the partial oxidation of methane to synthesis gas[J]. Catalysis Communications, 2008, 9(15): 2509-2514. |
| 40 | Rydén M, Lyngfelt A, Mattisson T, et al. Novel oxygen-carrier materials for chemical-looping combustion and chemical-looping reforming; La x Sr1- x Fe y Co1- y O3- δ perovskites and mixed-metal oxides of NiO, Fe2O3 and Mn3O4 [J]. International Journal of Greenhouse Gas Control, 2008, 2(1): 21-36. |
| 41 | Long Y H, Yang K, Gu Z H, et al. Hydrogen generation from water splitting over polyfunctional perovskite oxygen carriers by using coke oven gas as reducing agent[J]. Applied Catalysis B: Environmental, 2022, 301: 120778. |
| 42 | Huang Z, Gao N, Lin Y, et al. Exploring the migration and transformation of lattice oxygen during chemical looping with NiFe2O4 oxygen carrier[J]. Chemical Engineering Journal, 2022, 429: 132064. |
| 43 | Zhu K Y, Shi F, Zhu X F, et al. The roles of oxygen vacancies in electrocatalytic oxygen evolution reaction[J]. Nano Energy, 2020, 73: 104761. |
| 44 | Jaber J O, Probert S D. Pyrolysis and gasification kinetics of Jordanian oil-shales[J]. Applied Energy, 1999, 63(4): 269-286. |
| 45 | Zhou L P, Zhao H, Tong Y N, et al. Evolution of coke formation and its effect on β-zeolite in catalytic cracking[J]. Industrial & Engineering Chemistry Research, 2022, 61(48): 17440-17456. |
| 46 | Go K S, Son S R, Kim S D. Reaction kinetics of reduction and oxidation of metal oxides for hydrogen production[J]. International Journal of Hydrogen Energy, 2008, 33(21): 5986-5995. |
| 47 | Otsuka K, Wang Y, Nakamura M. Direct conversion of methane to synthesis gas through gas-solid reaction using CeO2-ZrO2 solid solution at moderate temperature[J]. Applied Catalysis A: General, 1999, 183(2): 317-324. |
| 48 | de los Ríos Castillo T, Salinas Gutiérrez J, López Ortiz A, et al. Global kinetic evaluation during the reduction of CoWO4 with methane for the production of hydrogen[J]. International Journal of Hydrogen Energy, 2013, 38(28): 12519-12526. |
| 49 | Chen L Y, Liu D C, Xue J, et al. Mechanism of phase segregation in ilmenite oxygen carriers for chemical-looping combustion[J]. Chemical Engineering Journal, 2024, 479: 147921. |
| [1] | Meilin SHI, Lianda ZHAO, Xingjian DENG, Jingsong WANG, Haibin ZUO, Qingguo XUE. Research progress on catalytic methane reforming process [J]. CIESC Journal, 2024, 75(S1): 25-39. |
| [2] | Huanjuan ZHAO, Yingxin BAO, Kang YU, Jing LIU, Xinming QIAN. Quantitative experimental study on detonation instability of multi-component [J]. CIESC Journal, 2024, 75(S1): 339-348. |
| [3] | Hongbiao XU, Liang YANG, Zidong LI, Daoping LIU. Kinetics of methane hydrate formation in saline droplets/copper foam composite system [J]. CIESC Journal, 2024, 75(9): 3287-3296. |
| [4] | Yong DING, Wenjian LI, Zhaoyu CHEN, Lihui CAO, Xuanming LIU, Qiangqiang REN, Song HU, Jun XIANG. Aerobic pyrolysis kinetic and product characteristics of waste crystalline silicon photovo ltaic modules’ EVA [J]. CIESC Journal, 2024, 75(9): 3310-3319. |
| [5] | Xinyi LUO, Qiang XU, Yonglu SHE, Tengfei NIE, Liejin GUO. Study on bubble dynamic characteristics and mass transfer mechanism in photoelectrochemical water splitting for hydrogen production [J]. CIESC Journal, 2024, 75(9): 3083-3093. |
| [6] | Junfeng WANG, Junjie ZHANG, Wei ZHANG, Jiale WANG, Shuyan SHUANG, Yadong ZHANG. Liquid-phase discharge plasma decomposition of methanol for hydrogen production: optimization of electrode configuration [J]. CIESC Journal, 2024, 75(9): 3277-3286. |
| [7] | Jialei CAO, Liyan SUN, Dewang ZENG, Fan YIN, Zixiang GAO, Rui XIAO. Numerical simulation of chemical looping hydrogen generation with dual fluidized bed reactors [J]. CIESC Journal, 2024, 75(8): 2865-2874. |
| [8] | Jiaqi DING, Haitao LIU, Pu ZHAO, Xiangning ZHU, Xiaofang WANG, Rong XIE. Study on intelligent rolling prediction of the multiphase flows in coal-supercritical water fluidized bed reactor for hydrogen production [J]. CIESC Journal, 2024, 75(8): 2886-2896. |
| [9] | Yongqi TONG, Jie CHENG, Hai LIN, Xi CHEN, Haibo ZHAO. CPFD simulation of a 10 MWth chemical looping combustion reactor [J]. CIESC Journal, 2024, 75(8): 2949-2959. |
| [10] | Li LUO, Wenyao CHEN, Jing ZHANG, Gang QIAN, Xinggui ZHOU, Xuezhi DUAN. Alumina structure and surface property regulation for catalyzing methanol dehydration to dimethyl ether [J]. CIESC Journal, 2024, 75(7): 2522-2532. |
| [11] | Lihao LIU, Ting HUANG, Yu YONG, Xinhao LUO, Zeming ZHAO, Shangfei SONG, Bohui SHI, Guangjin CHEN, Jing GONG. CH4-hydrate formation and solid-phase deposition in salt-sand coexisting flow systems [J]. CIESC Journal, 2024, 75(5): 1987-2000. |
| [12] | Wenya WANG, Wei ZHANG, Xiaoling LOU, Ruofei ZHONG, Bingbing CHEN, Junxian YUN. Multi-microtubes formation and simulation of nanocellulose-embedded cryogel microspheres [J]. CIESC Journal, 2024, 75(5): 2060-2071. |
| [13] | Xiao XUE, Minjing SHANG, Yuanhai SU. Advances on continuous-flow synthesis of drugs in microreactors [J]. CIESC Journal, 2024, 75(4): 1439-1454. |
| [14] | Yiwei FAN, Wei LIU, Yingying LI, Peixia WANG, Jisong ZHANG. Research progress on catalytic dehydrogenation of dodecahydro-N-ethylcarbazole as liquid organic hydrogen carrier [J]. CIESC Journal, 2024, 75(4): 1198-1208. |
| [15] | Anran XU, Kai LIU, Na WANG, Zhenyu ZHAO, Hong LI, Xin GAO. Strong wave-absorbing catalyst cooperates with microwave energy to enhance fructose dehydration to produce 5-hydroxymethylfurfural [J]. CIESC Journal, 2024, 75(4): 1565-1577. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||