CIESC Journal ›› 2025, Vol. 76 ›› Issue (11): 5923-5932.DOI: 10.11949/0438-1157.20250307
• Catalysis, kinetics and reactors • Previous Articles
Zhicheng TANG( ), Tianwei WANG, Rongwen LYU, Shufen ZHANG(
), Tianwei WANG, Rongwen LYU, Shufen ZHANG( )
)
Received:2025-03-25
Revised:2025-05-15
Online:2025-12-19
Published:2025-11-25
Contact:
Shufen ZHANG
通讯作者:
张淑芬
作者简介:唐志成(1995—),男,博士研究生,zhichengtang@mail.dlut.edu.cn
基金资助:CLC Number:
Zhicheng TANG, Tianwei WANG, Rongwen LYU, Shufen ZHANG. Amination of bromaminic acid catalyzed by Mn-containing basic copper carbonate electrocatalyst based on electrochemical reduction[J]. CIESC Journal, 2025, 76(11): 5923-5932.
唐志成, 王添巍, 吕荣文, 张淑芬. 含Mn碱式碳酸铜电催化剂催化溴氨酸芳胺化反应研究[J]. 化工学报, 2025, 76(11): 5923-5932.
Add to citation manager EndNote|Ris|BibTeX
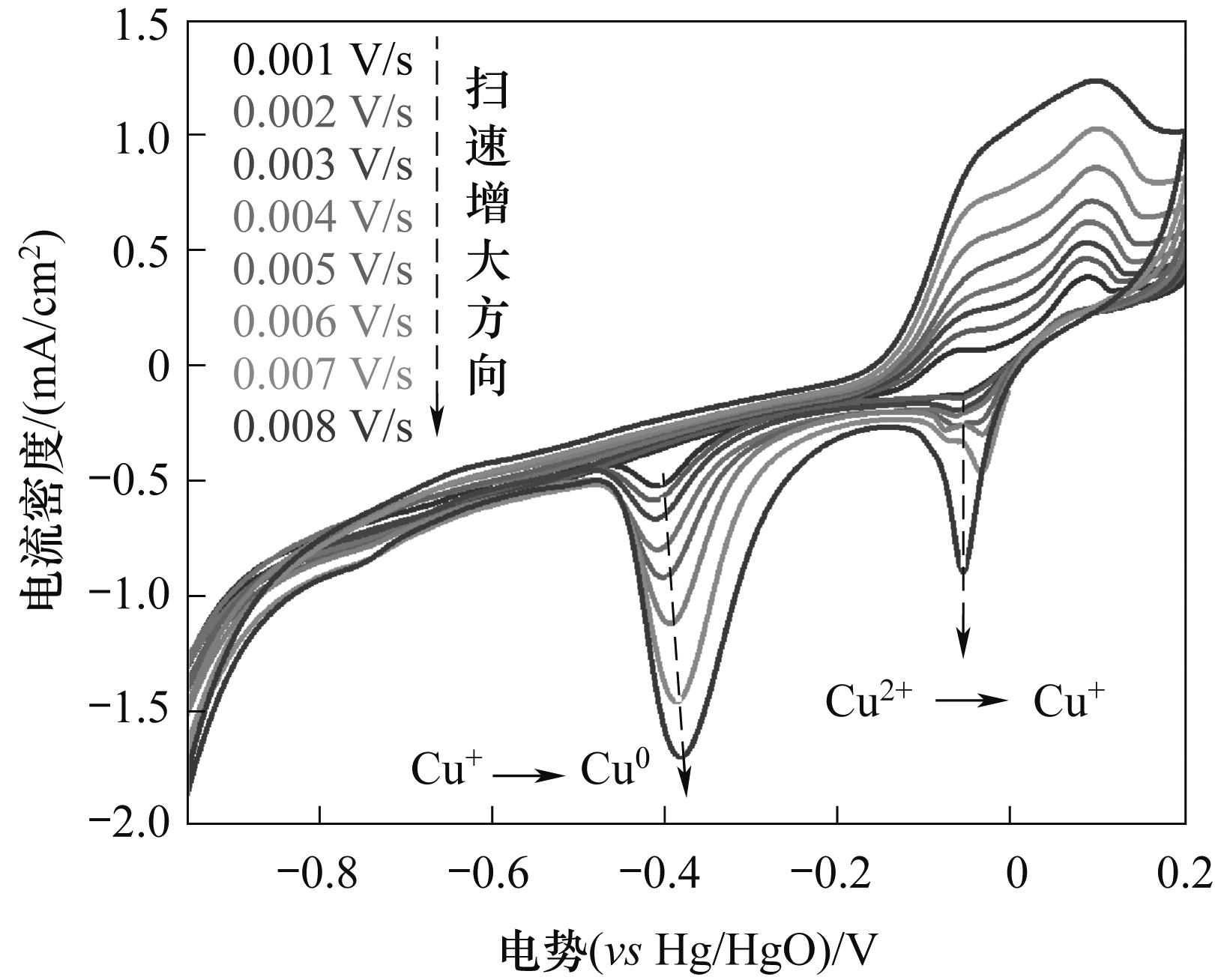
Fig.1 Cyclic voltammetry curves of graphite electrodes at different scanning speeds in a 0.1 mol/L sodium bicarbonate solution containing 0.1 g of basic copper carbonate
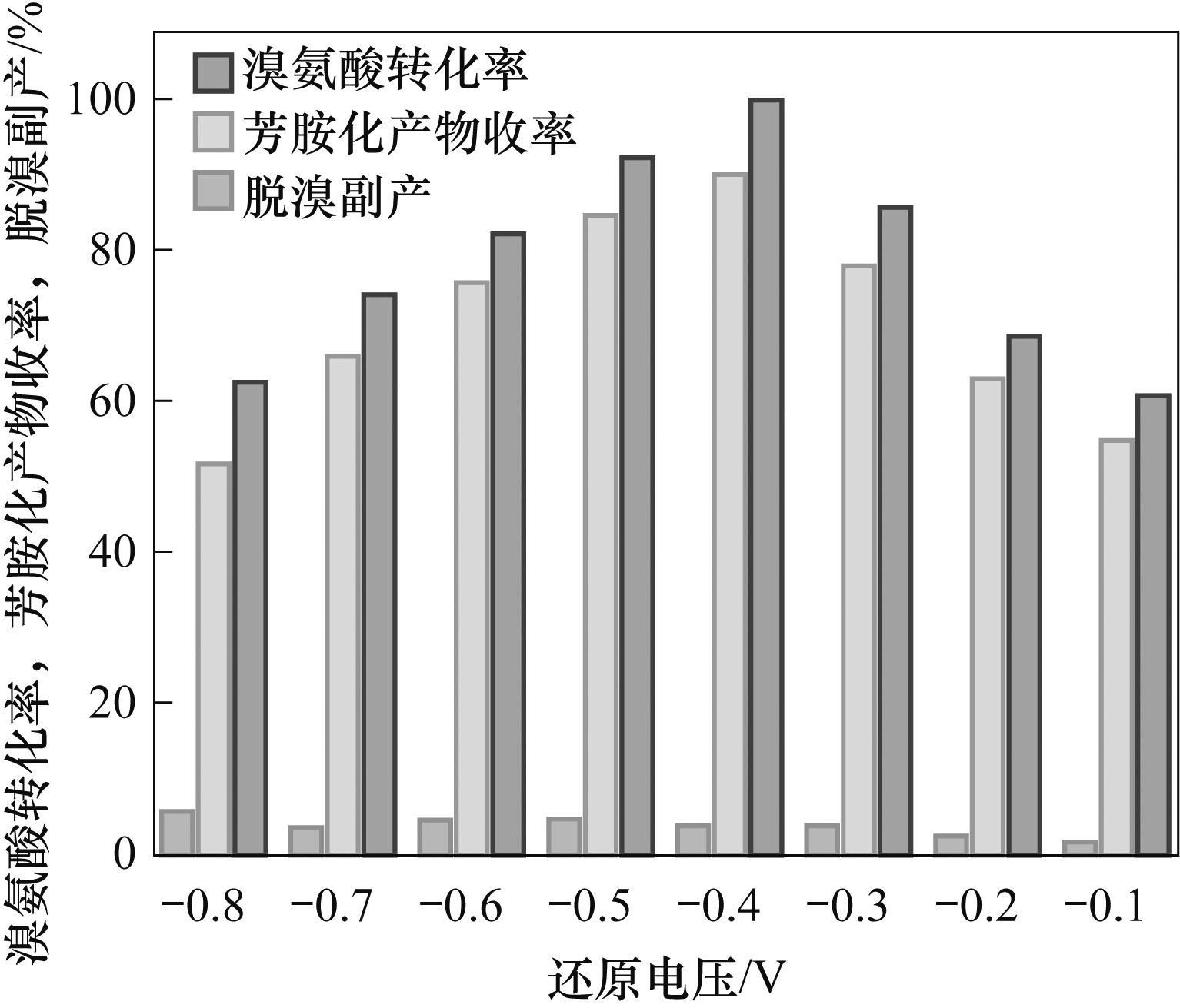
Fig.2 The relationship between the conversion rate of bromamic acid, the yields and the by-product of debromination with the reduction voltage in amination reaction of bromamic acid and M-acid
| 编号 | 还原电压/V | 溴氨酸转化率/% | 收率/% | 脱溴副产/% |
|---|---|---|---|---|
| 1 | -0.1 | 61 | 55 | 2 |
| 2 | -0.2 | 69 | 63 | 3 |
| 3 | -0.3 | 86 | 78 | 4 |
| 4 | -0.4 | 100 | 90 | 4 |
| 5 | -0.5 | 93 | 85 | 5 |
| 6 | -0.6 | 82 | 76 | 5 |
| 7 | -0.7 | 74 | 66 | 4 |
| 8 | -0.8 | 63 | 52 | 6 |
Table 1 Effect of reduction voltage on amination of bromaminic acid with M acid
| 编号 | 还原电压/V | 溴氨酸转化率/% | 收率/% | 脱溴副产/% |
|---|---|---|---|---|
| 1 | -0.1 | 61 | 55 | 2 |
| 2 | -0.2 | 69 | 63 | 3 |
| 3 | -0.3 | 86 | 78 | 4 |
| 4 | -0.4 | 100 | 90 | 4 |
| 5 | -0.5 | 93 | 85 | 5 |
| 6 | -0.6 | 82 | 76 | 5 |
| 7 | -0.7 | 74 | 66 | 4 |
| 8 | -0.8 | 63 | 52 | 6 |
| 编号 | 催化方式 | 溴氨酸转化率/% | 收率/% | 脱溴副产/% |
|---|---|---|---|---|
| 1 | 含Mn碱式碳酸铜,电化学还原,-0.4 V | 100 | 93 | 3 |
| 2 | 硫酸铜,电化学还原,-0.4 V | 97 | 88 | 3 |
| 3 | 无外加铜源,电化学还原,-0.4 V | 0 | 0 | 0 |
| 4 | 硫酸铜 | 69 | 63 | 3 |
| 5 | 硫酸铜+抗坏血酸 | 98 | 87 | 8 |
| 6 | 硫酸铜+葡萄糖 | 99 | 90 | 6 |
Table 2 Comparison of results of amination of bromaminic acid catalyzed by electroreduction and chemical reduction
| 编号 | 催化方式 | 溴氨酸转化率/% | 收率/% | 脱溴副产/% |
|---|---|---|---|---|
| 1 | 含Mn碱式碳酸铜,电化学还原,-0.4 V | 100 | 93 | 3 |
| 2 | 硫酸铜,电化学还原,-0.4 V | 97 | 88 | 3 |
| 3 | 无外加铜源,电化学还原,-0.4 V | 0 | 0 | 0 |
| 4 | 硫酸铜 | 69 | 63 | 3 |
| 5 | 硫酸铜+抗坏血酸 | 98 | 87 | 8 |
| 6 | 硫酸铜+葡萄糖 | 99 | 90 | 6 |
| 物种 | 条纹编号 | 晶格尺寸/nm | 晶面 |
|---|---|---|---|
| CuCO3 | Ⅲ | 0.286 | (20-1) |
Cu2(OH)2CO3 | Ⅱ | 0.218 | (041) |
| Ⅳ | 0.278 | (21-1) | |
| Ⅷ | 0.267 | (122) | |
| Na2Cu(CO3)2·3H2O | Ⅳ | 0.278 | (311) |
| Ⅶ | 0.284 | (114) | |
| Na3Cu2(CO3)3(OH)4·H2O | Ⅴ, Ⅵ | 0.267 | — |
| MnCO3 | Ⅰ | 0.239 | (110) |
Table 3 Lattice fringe information of Mn containing basic copper carbonate electrocatalysts
| 物种 | 条纹编号 | 晶格尺寸/nm | 晶面 |
|---|---|---|---|
| CuCO3 | Ⅲ | 0.286 | (20-1) |
Cu2(OH)2CO3 | Ⅱ | 0.218 | (041) |
| Ⅳ | 0.278 | (21-1) | |
| Ⅷ | 0.267 | (122) | |
| Na2Cu(CO3)2·3H2O | Ⅳ | 0.278 | (311) |
| Ⅶ | 0.284 | (114) | |
| Na3Cu2(CO3)3(OH)4·H2O | Ⅴ, Ⅵ | 0.267 | — |
| MnCO3 | Ⅰ | 0.239 | (110) |
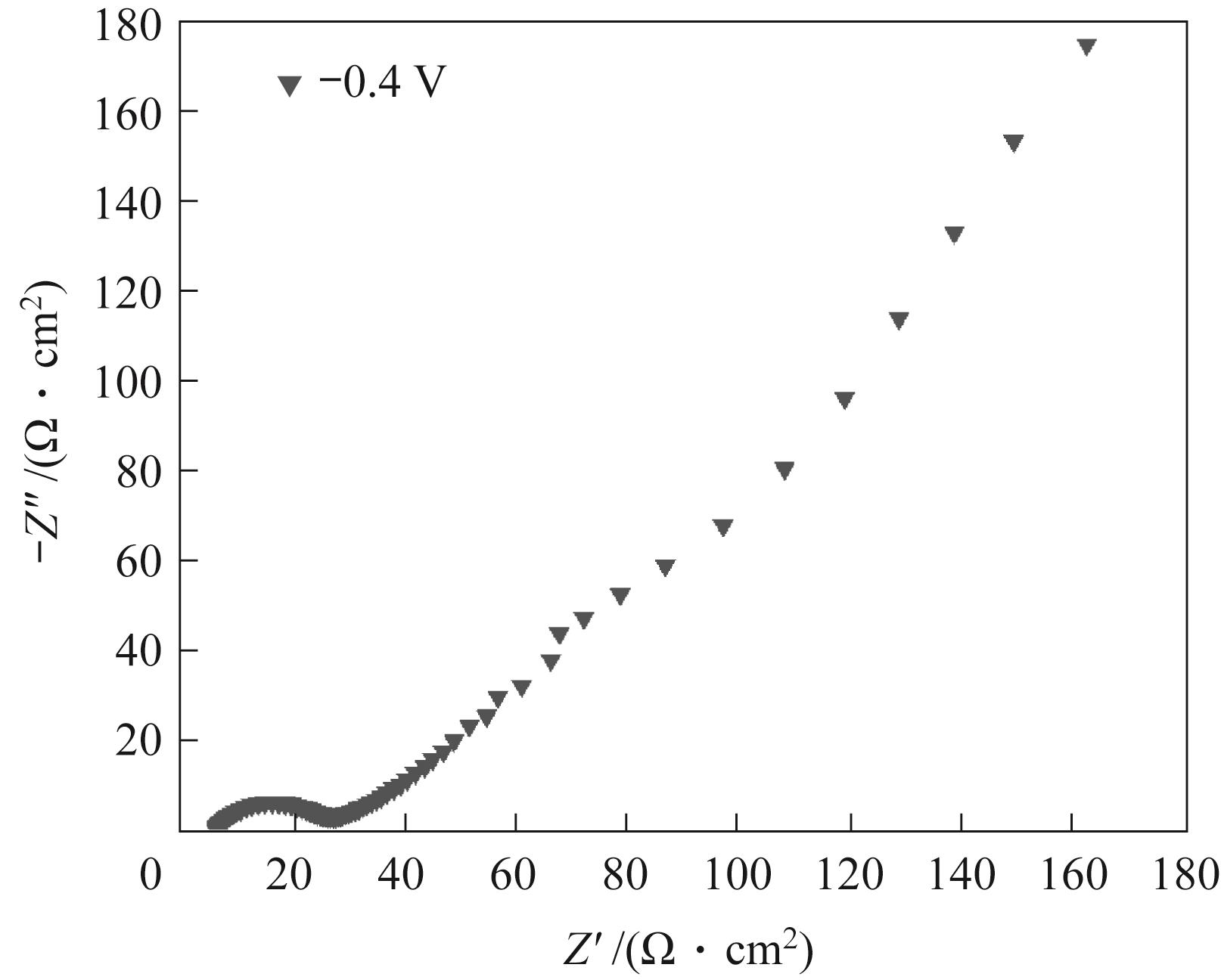
Fig.7 Nyquist diagram of graphite electrode after electrolysis at -0.4 V for 3 h in 10 ml 0.1 mol/L sodium bicarbonate solution containing 0.1 g electrocatalyst
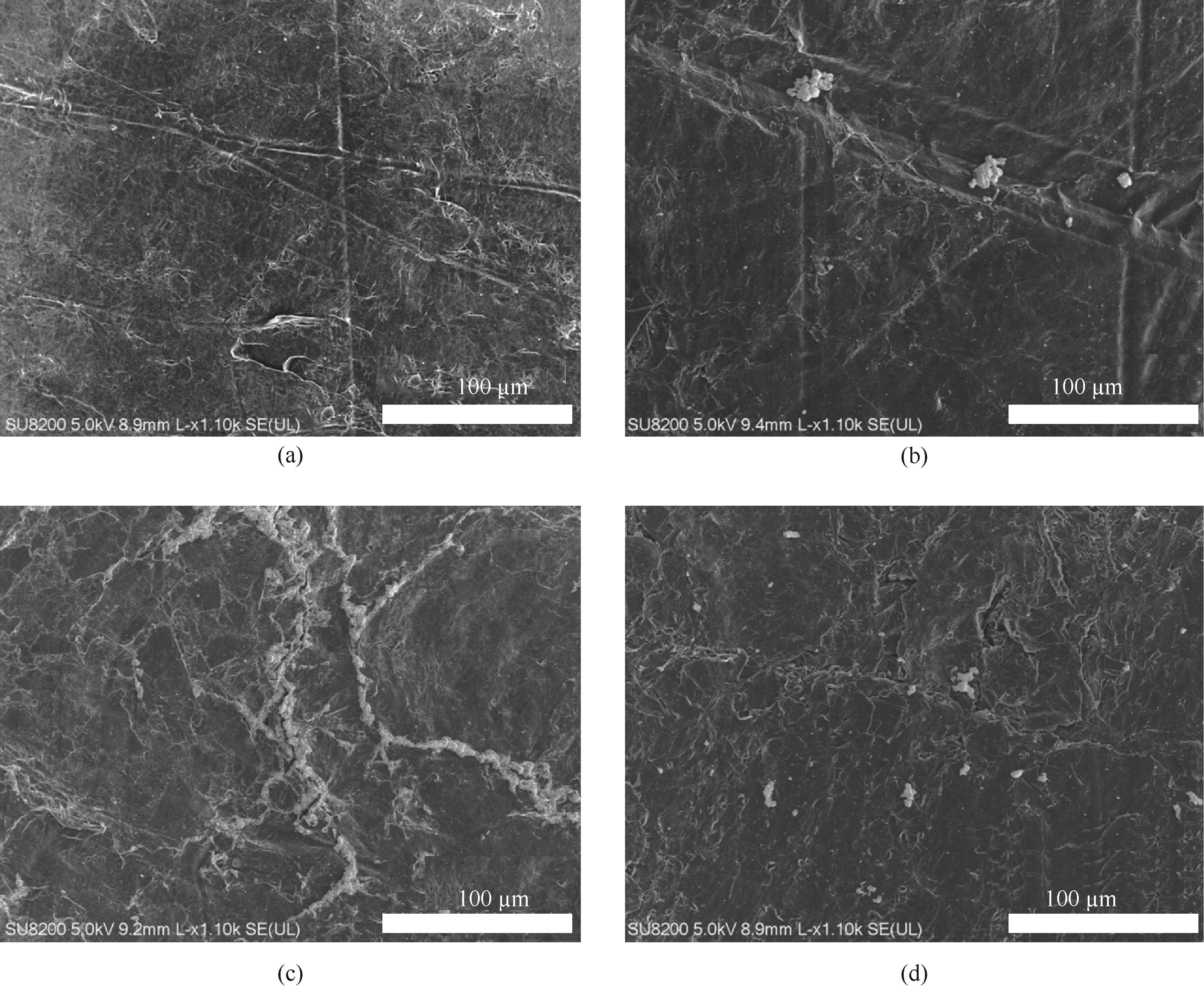
Fig.8 TEM images of graphite electrodes after electrolysis for 3 h in 10 ml 0.1 mol/L sodium bicarbonate solution containing 0.1 g of electrocatalyst at 0 V (a), -0.1 V (b), -0.4 V (c), -1 V (d)
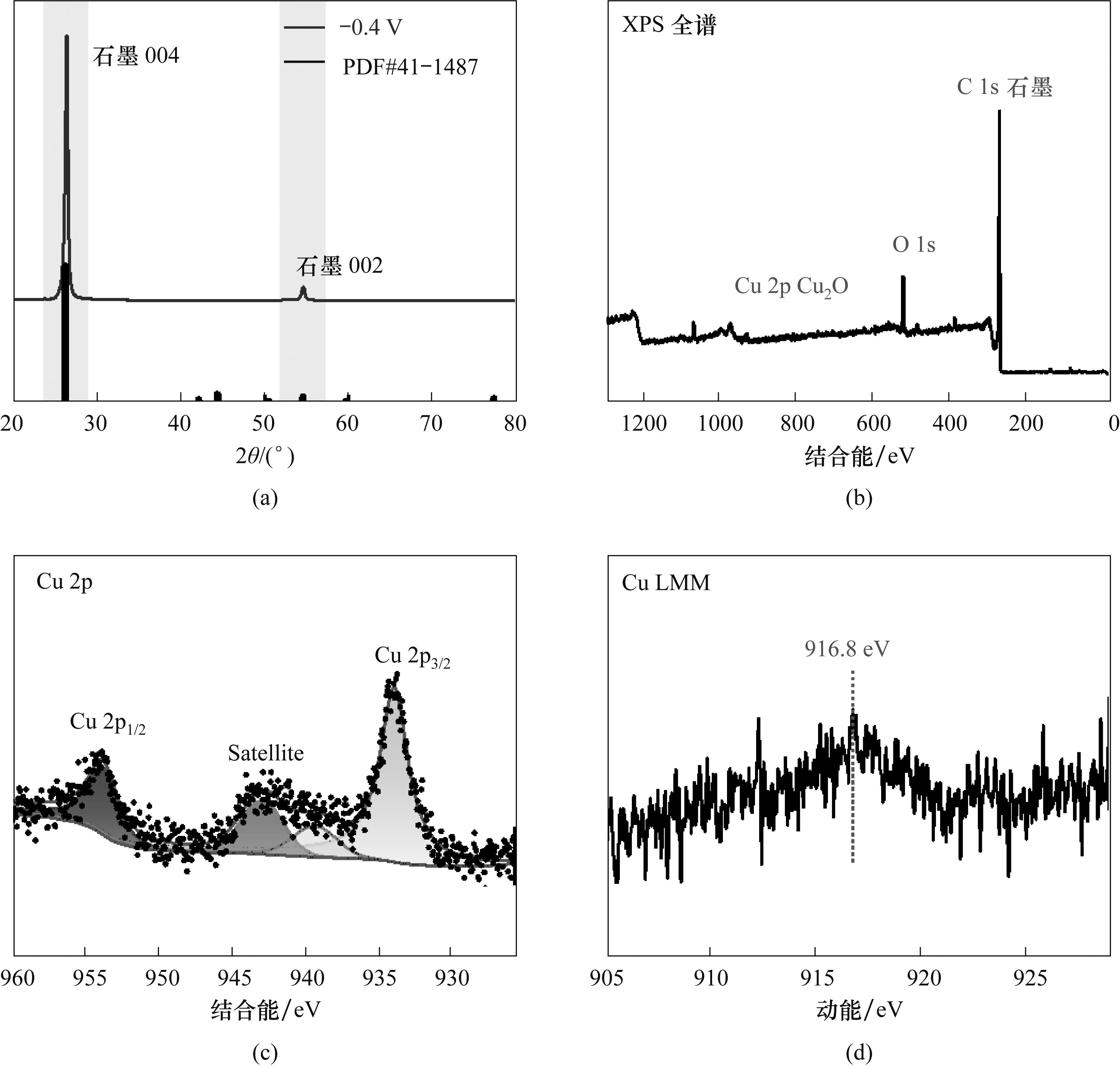
Fig.9 XRD pattern (a) and XPS survey (b), Cu 2p (c), Cu LMM(d) patterns of the graphite electrode after electrolysis in 10 ml 0.1 mol/L NaHCO3 solution containing 0.1 g of electrocatalyst at -0.4 V for 3 h
| 底物 | 还原 电压/V | 溴氨酸 转化率/% | 收率/% | 脱溴 副产/% |
|---|---|---|---|---|
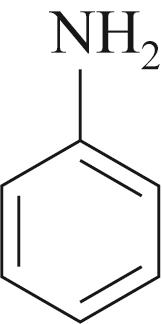 | -0.2 | 95 | 89 | 3 |
| -0.3 | 100 | 90 | 3 | |
| -0.4 | 94 | 89 | 4 | |
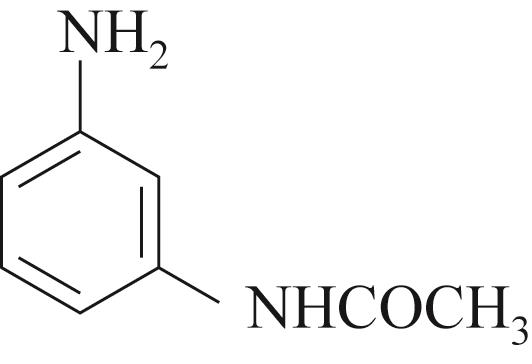 | -0.3 | 91 | 80 | 3 |
| -0.4 | 100 | 93 | 3 | |
| -0.5 | 95 | 89 | 4 | |
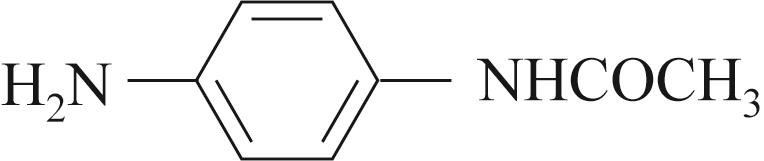 | -0.4 | 90 | 82 | 5 |
| -0.5 | 100 | 91 | 5 | |
| -0.6 | 94 | 85 | 6 |
Table 4 Substrate scope for Mn containing basic copper carbonate and electrochemical reduction catalyzed amination of bromaminic acid
| 底物 | 还原 电压/V | 溴氨酸 转化率/% | 收率/% | 脱溴 副产/% |
|---|---|---|---|---|
 | -0.2 | 95 | 89 | 3 |
| -0.3 | 100 | 90 | 3 | |
| -0.4 | 94 | 89 | 4 | |
 | -0.3 | 91 | 80 | 3 |
| -0.4 | 100 | 93 | 3 | |
| -0.5 | 95 | 89 | 4 | |
 | -0.4 | 90 | 82 | 5 |
| -0.5 | 100 | 91 | 5 | |
| -0.6 | 94 | 85 | 6 |
| [1] | 穆振义. 乌尔曼反应在染料合成中的应用[J]. 染料工业, 1984, 21(3): 1-7. |
| Mu Z Y. Application of Ullman reaction in dye synthesis[J]. Dyestuffs and Coloration, 1984, 21(3): 1-7. | |
| [2] | 李光辉, 孔祥文, 王玲艳. Ullmann反应的绿色化研究进展[J]. 染料与染色, 2021, 58(3): 25-35. |
| Li G H, Kong X W, Wang L Y. Development of green chemical research on Ullmann reaction[J]. Dyestuffs and Coloration, 2021, 58(3): 25-35. | |
| [3] | Simon M S. Spectral shifts in anthraquinone dyes caused by non-conjugated substituents[J]. Journal of the American Chemical Society, 1963, 85(13): 1974-1977. |
| [4] | Baqi Y. Anthraquinone dyes: a synthetic and chemical characterization protocol for an industrial chemistry laboratory course[J]. Journal of Chemical Education, 2022, 99(3): 1441-1447. |
| [5] | 顾高炜, 邬伟国, 李建昌, 等. C. I. 酸性蓝324的乌尔曼缩合反应研究[J]. 染料与染色, 2020, 57(3): 8-9. |
| Gu G W, Wu W G, Li J C, et al. Study on Ullmann condensation by synthesis of C. I. acid blue 324[J]. Dyestuffs and Coloration, 2020, 57(3): 8-9. | |
| [6] | 刘慧, 陈煌煌, 高爱芹, 等. 纳米双核催化剂在溴氨蓝合成反应中的催化性能研究[J]. 染料与染色, 2018, 55(6): 20-24, 49. |
| Liu H, Chen H H, Gao A Q, et al. Catalytic performance of nano-sized catalyst on synthesis of bromine ammonia blue[J]. Dyestuffs and Coloration, 2018, 55(6): 20-24, 49. | |
| [7] | 姚蒙正, 王军宽. 溴氨酸的芳胺化条件研究[J]. 染料工业, 1984, 21(3):15-19. |
| Yao M Z, Wang J K. Study on the arylamination conditions of bromamic acid[J]. Dyestuffs and Coloration, 1984, 21(3): 15-19. | |
| [8] | Chen X, Ding K, Jun L. Synthesis, identification and application of aldehyde reactive dyes[J]. Dyes and Pigments, 2015, 123: 404-412. |
| [9] | Sperotto E, van Klink G P M, van Koten G, et al. The mechanism of the modified Ullmann reaction[J]. Dalton Transactions, 2010, 39(43): 10338-10351. |
| [10] | Ma Z D, Li Y X, Jin M M, et al. Fabrication of adsorbents with enhanced CuI stability: creating a superhydrophobic microenvironment through grafting octadecylamine[J]. Chinese Journal of Chemical Engineering, 2023, 55: 41-48. |
| [11] | Xin Y Y, Zhou L, Ma K K, et al. Removal of bromoamine acid in dye wastewater by gas-liquid plasma: the role of ozone and hydroxyl radical[J]. Journal of Water Process Engineering, 2020, 37: 101457. |
| [12] | Jedinák L, Zátopková R, Zemánková H, et al. The Suzuki-Miyaura cross-coupling reaction of halogenated aminopyrazoles: method development, scope, and mechanism of dehalogenation side reaction[J]. The Journal of Organic Chemistry, 2017, 82(1): 157-169. |
| [13] | Yang Q, Zhao Y S, Ma D W. Cu-mediated Ullmann-type cross-coupling and industrial applications in route design, process development, and scale-up of pharmaceutical and agrochemical processes[J]. Organic Process Research & Development, 2022, 26(6): 1690-1750. |
| [14] | Chen W, Wu Y D, Jiang Y M, et al. Catalyst selection over an electrochemical reductive coupling reaction toward direct electrosynthesis of oxime from NO x and aldehyde[J]. Journal of the American Chemical Society, 2024, 146(9): 6294-6306. |
| [15] | Hu J Y, Ma R, Hu J C, et al. Electrochemical oxidative dehydrogenation aromatization of cyclohex-2-enone and amines to 1,4-phenylenediamine[J]. Green Chemistry, 2024, 26(8): 4684-4690. |
| [16] | 卢林德, 孟胜锋, 上官开泰, 等. 溴氨酸类染料合成的机理及生产实践[J]. 染料与染色, 2024, 61(1): 25-29, 39. |
| Lu L D, Meng S F, Shangguan K T, et al. Mechanism and manufacture of dyes synthesized from 1-amino-4-bromoanthraquinone-2-sulfonic acid[J]. Dyestuffs and Coloration, 2024, 61(1): 25-29, 39. | |
| [17] | He J B, Lu D Y, Jin G P. Potential dependence of cuprous/cupric duplex film growth on copper electrode in alkaline media[J]. Applied Surface Science, 2006, 253(2): 689-697. |
| [18] | Fan H H, Weng W L, Lee C Y, et al. Electrochemical cycling-induced spiky Cu x O/Cu nanowire array for glucose sensing[J]. ACS Omega, 2019, 4(7): 12222-12229. |
| [19] | Tromans D, Sun R H. Anodic behavior of copper in weakly alkaline solutions[J]. Journal of Electrochemical Society, 1992, 139(7): 1945-1951. |
| [20] | Liu F L, Gao X T, Guo Z X, et al. Sustainable adipic acid production via paired electrolysis of lignin-derived phenolic compounds with water as hydrogen and oxygen sources[J]. Journal of the American Chemical Society, 2024, 146(22): 15275-15285. |
| [21] | Dong L, Ge W X, Fan Y, et al. Surfactant‐modified electrode-electrolyte interface for steering CO2 electrolysis on Cu electrodes[J]. American Institute of Chemical Engineers Journal, 2024, 70(1): e18271. |
| [22] | Shao B B, Du H Y, Hao X Y, et al. Ligand assisted copper-catalyzed Ullmann cross coupling reaction of bromaminic acid with amines[J]. Chinese Journal of Chemical Engineering, 2016, 24(8): 1000-1006. |
| [23] | 舒余德, 孟爱东. 碱性NaCl溶液中铜阳极生成Cu2O的机理[J]. 有色金属, 1996, 48(4): 58-62. |
| Shu Y D, Meng A D. Mechanism of forming Cu2O on copper anode in alkaline[J]. Nonferrous Metals Engineering, 1996, 48(4): 58-62. | |
| [24] | 雷惊雷, 李凌杰, 张胜涛, 等. 铜电极在弱碱性介质中腐蚀行为的研究[J]. 化学学报, 2001, 59(8): 1216-1221. |
| Lei J L, Li L J, Zhang S T, et al. Studies on corrosion behavior of copper electrode in weak alkaline solution[J]. Acta Chimica Sinica, 2001, 59(8): 1216-1221. | |
| [25] | Chen F Y, Elgazzar A, Pecaut S, et al. Electrochemical nitrate reduction to ammonia with cation shuttling in a solid electrolyte reactor[J]. Nature Catalysis, 2024, 7(9): 1032-1043. |
| [26] | Thanh N T K, Maclean N, Mahiddine S. Mechanisms of nucleation and growth of nanoparticles in solution[J]. Chemical Reviews, 2014, 114(15): 7610-7630. |
| [27] | Candal R J, Regazzoni A E, Blesa M A. Precipitation of copper(Ⅱ) hydrous oxides and copper(Ⅱ) basic salts[J]. Journal of Materials Chemistry, 1992, 2(6): 657-661. |
| [28] | Grujicic D, Pesic B. Electrodeposition of copper: the nucleation mechanisms[J]. Electrochimica Acta, 2002, 47(18): 2901-2912. |
| [29] | Lazanas A C, Prodromidis M I. Electrochemical impedance spectroscopy—a tutorial[J]. ACS Measurement Science Au, 2023, 3(3): 162-193. |
| [30] | Sagar P, Arun Kumar N S, Shreenivasa L, et al. Citric acid assisted one-pot approach to synthesize CuO, CuO/Cu2O, Cu/Cu2O, and metallic Cu: potential electrocatalyst for enhanced OER[J]. Ionics, 2023, 29(2): 711-719. |
| [31] | Huang J. Diffusion impedance of electroactive materials, electrolytic solutions and porous electrodes: Warburg impedance and beyond[J]. Electrochimica Acta, 2018, 281: 170-188. |
| [32] | He H, Huang C, Luo C W, et al. Dynamic study of Li intercalation into graphite by in situ high energy synchrotron XRD[J]. Electrochimica Acta, 2013, 92: 148-152. |
| [33] | Poulston S, Parlett P M, Stone P, et al. Surface oxidation and reduction of CuO and Cu2O studied using XPS and XAES[J]. Surface and Interface Analysis, 1996, 24(12): 811-820. |
| [34] | Biesinger M C, Lau L W M, Gerson A R, et al. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn[J]. Applied Surface Science, 2010, 257(3): 887-898. |
| [35] | Malik E M, Rashed M, Wingen L, et al. Ullmann reactions of 1-amino-4-bromoanthraquinones bearing various 2-substituents furnishing novel dyes[J]. Dyes and Pigments, 2016, 131: 33-40. |
| [36] | Beletskaya I P, Cheprakov A V. The complementary competitors: palladium and copper in C—N cross-coupling reactions[J]. Organometallics, 2012, 31(22): 7753-7808. |
| [1] | Guoxiang HU, Yikui ZHU, Hua LONG, Xiaowen LIU, Qingang XIONG. Study on the underlying mechanism of choline chloride-lactic acid molar ratio influencing alkali lignin solubility in choline chloride-lactic acid deep eutectic solvents [J]. CIESC Journal, 2025, 76(9): 4449-4461. |
| [2] | Huiqin ZHANG, Hongjun ZHAO, Zhengjun FU, Li ZHUANG, Kai DONG, Tianzhi JIA, Xueli CAO, Shipeng SUN. Application of nanofiltration membrane in concentration of ionic rare earth leach solution [J]. CIESC Journal, 2025, 76(8): 4095-4107. |
| [3] | Chenrui MA, Xiang WANG, Minhang SONG, Jun JING, Qiong WU, Yun HUANG. Theoretical research on collision behavior and production evolution of titania particles in an industrial oxidation reactor of chlorination process [J]. CIESC Journal, 2025, 76(7): 3316-3324. |
| [4] | Wei LIN, Jian DU, Chen YAO, Jiahao ZHU, Wei WANG, Xiaotao ZHENG, Jianmin XU, Jiuyang YU. Study on ion transport and nucleation mechanism in electrochemical water softening process [J]. CIESC Journal, 2025, 76(4): 1788-1799. |
| [5] | Junying WANG, Hui JIN. Molecular dynamics investigation on the solubility parameters of supercritical CO2 and petroleum hydrocarbon [J]. CIESC Journal, 2025, 76(11): 5788-5798. |
| [6] | Yansong HU, Zhao YANG, Lei GAO, Bujian ZHANG. Experimental study of solubility and viscosity of R513A and PVE lubricants [J]. CIESC Journal, 2025, 76(10): 5015-5023. |
| [7] | Yupeng DU, Chunliang GE, Leilin DING, Li ZHANG, Jiajun HU, Fengping YU, Yi LIN, Feng WANG, Shi JIANG, Yu GUO. CO oxidation performance of Pt catalysts supported on CeO2-Al2O3 supports synthesized via urea homogeneous precipitation [J]. CIESC Journal, 2025, 76(10): 5114-5127. |
| [8] | Lingyu LI, Xin HU, Huaigang CHENG, Yun ZHAO, Dong AN, Yujun MA, Jiahao JIN, Xudong YU, Weidong ZHANG. Isothermal evaporation salt-forming regions of the ternary water-salt systems K+(Mg2+), Ca2+//Cl--H2O [J]. CIESC Journal, 2025, 76(1): 120-130. |
| [9] | Xinyue WANG, Xiaohu XU, Haiyang ZHANG, Chunhua YIN. Study on encapsulation and properties vitamin A acetate/cyclodextrin [J]. CIESC Journal, 2024, 75(S1): 321-328. |
| [10] | Siyu QIN, Yijia LIU, Jiacheng YANG, Wei TONG, Liwen JIN, Xiangzhao MENG. Characteristics of gas-liquid two-phase heat transfer in a confined vapor chamber [J]. CIESC Journal, 2024, 75(S1): 47-55. |
| [11] | Mingjun YANG, Wei SONG, Lei ZHANG, Zheng LING, Bingbing CHEN, Yongchen SONG. Research on the enhanced method of CO2-seawater hydrate generation [J]. CIESC Journal, 2024, 75(8): 2939-2948. |
| [12] | Zhixing ZHAO, Zhihao YAO, Xuefeng YU, Yousheng YANG, Ying ZENG, Xudong YU. Multi-temperature phase diagram of lithium-sodium-magnesium coexistence sulfate system and its application [J]. CIESC Journal, 2024, 75(6): 2123-2133. |
| [13] | Youming SI, Lingfeng ZHENG, Pengzhong CHEN, Jiangli FAN, Xiaojun PENG. Performance and mechanism of novel antimony oxo cluster photoresist [J]. CIESC Journal, 2024, 75(4): 1705-1717. |
| [14] | Yuxiang CHEN, Chuanlei LIU, Zijun GONG, Qiyue ZHAO, Guanchu GUO, Hao JIANG, Hui SUN, Benxian SHEN. Machine learning-assisted solvent molecule design for efficient absorption of ethanethiol [J]. CIESC Journal, 2024, 75(3): 914-923. |
| [15] | Han TANG, Jin CAI, Haihang QIN, Guangjin CHEN, Changyu SUN. Predictive model on gas solubility in water-rich phase coexisted with gas hydrates [J]. CIESC Journal, 2024, 75(11): 4348-4358. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||