CIESC Journal ›› 2025, Vol. 76 ›› Issue (6): 2451-2468.DOI: 10.11949/0438-1157.20241283
• Reviews and monographs • Previous Articles Next Articles
Jian PENG1,2( ), Lukai SHEN2, Likun WANG1,2(
), Lukai SHEN2, Likun WANG1,2( ), Lihong XIN3, Yong LIU2, Gaoling ZHAO2, Sainan MA1,2, Gaorong HAN2
), Lihong XIN3, Yong LIU2, Gaoling ZHAO2, Sainan MA1,2, Gaorong HAN2
Received:2024-11-12
Revised:2024-12-16
Online:2025-07-09
Published:2025-06-25
Contact:
Likun WANG
彭健1,2( ), 沈鲁恺2, 王立坤1,2(
), 沈鲁恺2, 王立坤1,2( ), 忻利宏3, 刘涌2, 赵高凌2, 马赛男1,2, 韩高荣2
), 忻利宏3, 刘涌2, 赵高凌2, 马赛男1,2, 韩高荣2
通讯作者:
王立坤
作者简介:彭健(2000—),男,硕士研究生,22360439@zju.edu.cn
基金资助:CLC Number:
Jian PENG, Lukai SHEN, Likun WANG, Lihong XIN, Yong LIU, Gaoling ZHAO, Sainan MA, Gaorong HAN. Preparation of tungstate nanomaterials and research progress in electrochromic field[J]. CIESC Journal, 2025, 76(6): 2451-2468.
彭健, 沈鲁恺, 王立坤, 忻利宏, 刘涌, 赵高凌, 马赛男, 韩高荣. 钨酸盐纳米材料的制备及其在电致变色领域的研究进展[J]. 化工学报, 2025, 76(6): 2451-2468.
Add to citation manager EndNote|Ris|BibTeX
| 纳米结构 | 制备方法 | 光调制幅度(波长/nm) | 响应时间 Tc、Tb/s | 着色效率/ (cm2·C-1) | 循环次数/次 | 电解液及浓度/ (mol·L-1) | 对电极 | 文献 |
|---|---|---|---|---|---|---|---|---|
| 纳米片 | 液相剥离 | 62.57%(700) | 10.74/6.97 @700 nm | — | 1000(6%调制衰减) | LiClO4-PC(1) | Pt | [ |
| 纳米棒 | 气相沉积 | 61%(680) | 2.05/0.74 @680 nm | 174 @680 nm | 1000(5%调制衰减) | LiClO4-PC(1) | NiO | [ |
| 89%(1000) | 0.85/1.0 @1000 nm | 386 @1000 nm | ||||||
| 纳米线 | 水热法 | 66%(650) | 1.2/2 @650 nm | 115.2 @650 nm | 10000(1.6%调制衰减) | KOH水溶液(3) | Pt | [ |
| 纳米花 | 溶剂热法 | 41.43%(700) | 6.67/1.54 @700 nm | — | 4000(2.25%调制衰减) | LiClO4-PC(1) | 碳棒 | [ |
纳米孔 网络 | 阳极氧化 | 75%(750) | 2.5/16.6 @750 nm | 141.5 @750 nm | 2000 | H2SO4水溶液(0.1) | Pt | [ |
| 纳米颗粒 | 电沉积法 | 88.51%(555) | 5.1/3.7 @632.8 nm | 137 @555 nm | — | LiClO4-PC(0.5) | ITO | [ |
Table 1 Electrochromic properties of WO3 thin films with different nanostructures
| 纳米结构 | 制备方法 | 光调制幅度(波长/nm) | 响应时间 Tc、Tb/s | 着色效率/ (cm2·C-1) | 循环次数/次 | 电解液及浓度/ (mol·L-1) | 对电极 | 文献 |
|---|---|---|---|---|---|---|---|---|
| 纳米片 | 液相剥离 | 62.57%(700) | 10.74/6.97 @700 nm | — | 1000(6%调制衰减) | LiClO4-PC(1) | Pt | [ |
| 纳米棒 | 气相沉积 | 61%(680) | 2.05/0.74 @680 nm | 174 @680 nm | 1000(5%调制衰减) | LiClO4-PC(1) | NiO | [ |
| 89%(1000) | 0.85/1.0 @1000 nm | 386 @1000 nm | ||||||
| 纳米线 | 水热法 | 66%(650) | 1.2/2 @650 nm | 115.2 @650 nm | 10000(1.6%调制衰减) | KOH水溶液(3) | Pt | [ |
| 纳米花 | 溶剂热法 | 41.43%(700) | 6.67/1.54 @700 nm | — | 4000(2.25%调制衰减) | LiClO4-PC(1) | 碳棒 | [ |
纳米孔 网络 | 阳极氧化 | 75%(750) | 2.5/16.6 @750 nm | 141.5 @750 nm | 2000 | H2SO4水溶液(0.1) | Pt | [ |
| 纳米颗粒 | 电沉积法 | 88.51%(555) | 5.1/3.7 @632.8 nm | 137 @555 nm | — | LiClO4-PC(0.5) | ITO | [ |
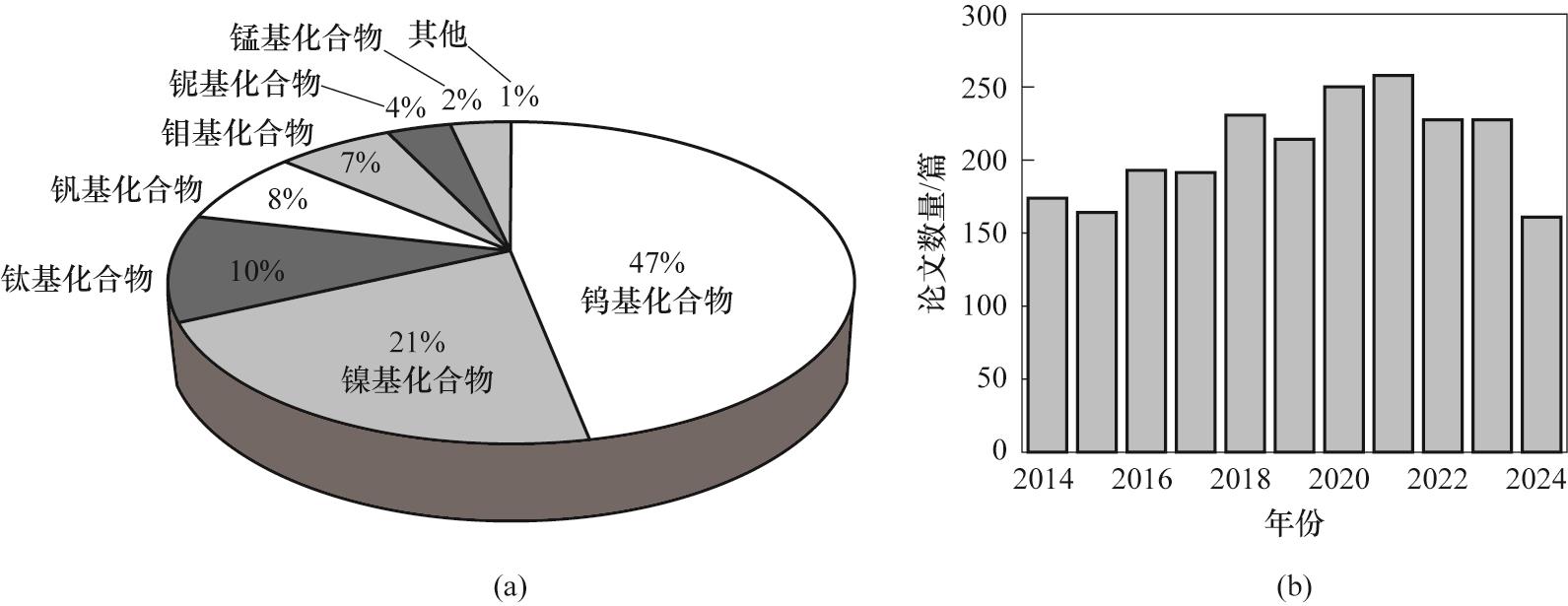
Fig.4 (a) The proportion of various inorganic material papers in the field of electrochromism; (b) The trend of the number of published papers on tungsten based compounds in the field of electrochromism from 2014 to 2024
| 制备方法 | 制备难度 | 优点 | 缺点 |
|---|---|---|---|
| 高温固相法[ | 较低 | 适用于大规模生产,操作简单; 产物结晶度高,稳定性好 | 需要高温条件,能耗大; 颗粒易团聚,形貌可控性差 |
| 沉淀法[ | 较低 | 工艺简单,设备要求低; 适合大规模制备,成本低 | 颗粒尺寸不均匀,形貌难以控制; 处理工艺复杂,产品纯度低 |
| 水热法[ | 中等 | 简单易行,成本较低; 反应条件温和(低温高压) | 颗粒尺寸较大,难以精确控制; 产率受限,安全性要求高 |
| 溶剂热法[ | 中等 | 颗粒尺寸较小,形貌可控; 溶剂种类多样,反应环境易调控 | 反应条件复杂(高温高压); 有机溶剂昂贵且对环境可能有污染 |
| 溶胶-凝胶法[ | 较低 | 可在低温进行,适合大规模生产; 多组分掺杂,均匀性较好 | 工艺较复杂,难以控制凝胶的均匀性; 产物颗粒易团聚 |
| 电化学沉积法[ | 较高 | 颗粒形貌可控; 可实现薄膜制备 | 设备复杂,对电极材料要求高; 需严格控制电化学参数,技术难度高 |
| 喷雾热解法[ | 较高 | 参数可控,生产效率高; 产物纯度高,粒径分布均匀 | 前体溶液分散性、稳定性要求高; 设备成本高,能耗较高 |
Table 2 Comparison of advantages and disadvantages of common preparation methods for tungstate nanomaterials
| 制备方法 | 制备难度 | 优点 | 缺点 |
|---|---|---|---|
| 高温固相法[ | 较低 | 适用于大规模生产,操作简单; 产物结晶度高,稳定性好 | 需要高温条件,能耗大; 颗粒易团聚,形貌可控性差 |
| 沉淀法[ | 较低 | 工艺简单,设备要求低; 适合大规模制备,成本低 | 颗粒尺寸不均匀,形貌难以控制; 处理工艺复杂,产品纯度低 |
| 水热法[ | 中等 | 简单易行,成本较低; 反应条件温和(低温高压) | 颗粒尺寸较大,难以精确控制; 产率受限,安全性要求高 |
| 溶剂热法[ | 中等 | 颗粒尺寸较小,形貌可控; 溶剂种类多样,反应环境易调控 | 反应条件复杂(高温高压); 有机溶剂昂贵且对环境可能有污染 |
| 溶胶-凝胶法[ | 较低 | 可在低温进行,适合大规模生产; 多组分掺杂,均匀性较好 | 工艺较复杂,难以控制凝胶的均匀性; 产物颗粒易团聚 |
| 电化学沉积法[ | 较高 | 颗粒形貌可控; 可实现薄膜制备 | 设备复杂,对电极材料要求高; 需严格控制电化学参数,技术难度高 |
| 喷雾热解法[ | 较高 | 参数可控,生产效率高; 产物纯度高,粒径分布均匀 | 前体溶液分散性、稳定性要求高; 设备成本高,能耗较高 |
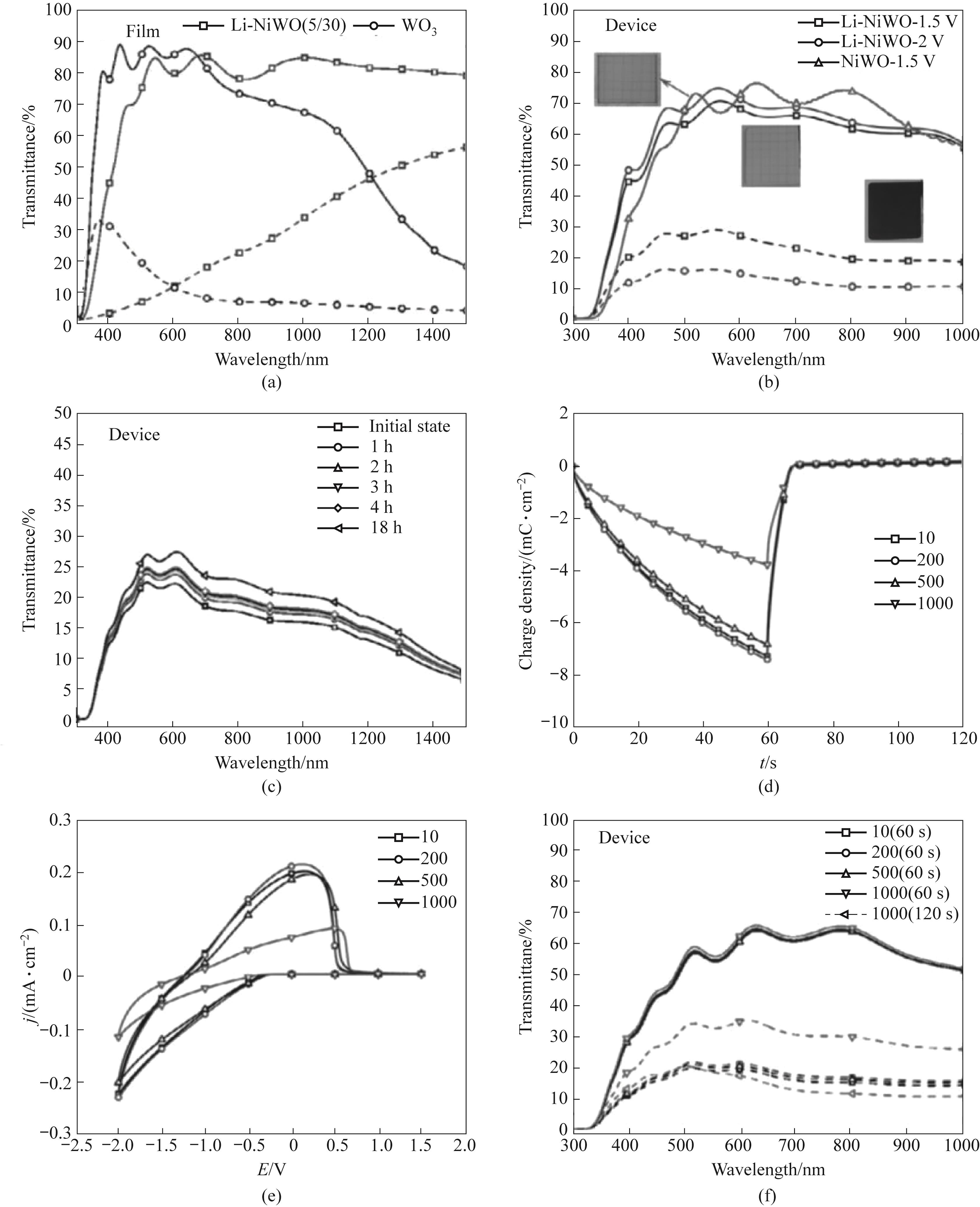
Fig.6 (a) Transmittance curves of Li doped NWO and WO3 films at 300—1500 nm; (b) Transmittance curves of the device at 300—1000 nm under different biases; (c) Transmittance curves of the device at 300—1500 nm after being left to stand for different periods of time; (d) Time variation curve of device charge density with the number of cycles; (e) The variation curve of the device CV curve with the number of cycles; (f) The variation curve of device transmittance with the number of cycles at 300—1000 nm[81]
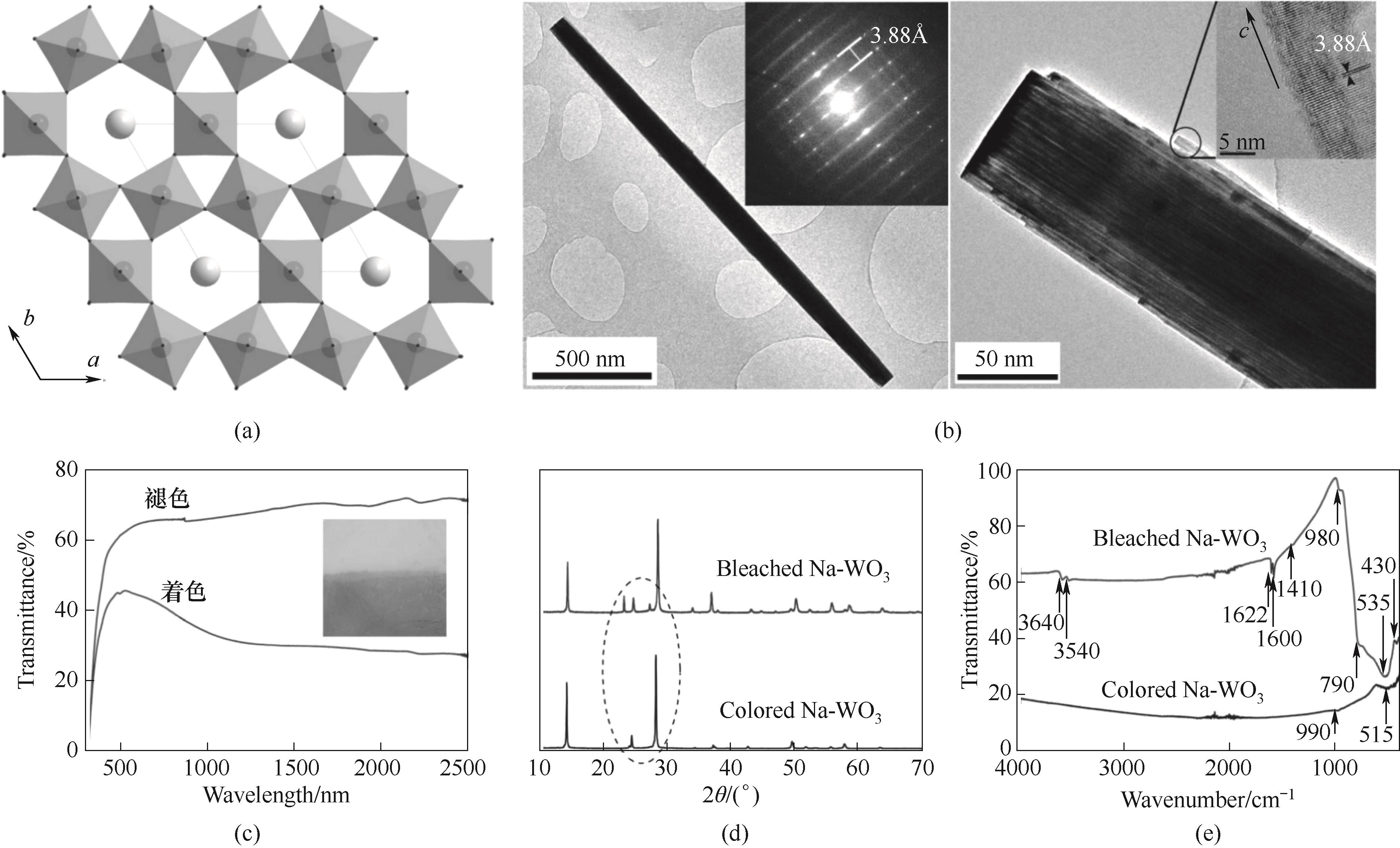
Fig.7 (a) Crystal structure of hexagonal sodium tungsten bronze; (b) Transmission electron microscopy image of sodium tungsten bronze; (c) Transmission spectra of sodium tungsten bronze in bleached and colored states (digital photo attached); (d) XRD patterns of sodium tungsten bronze in bleached and colored states; (e) Fourier transform infrared spectra of sodium tungsten bronze in bleached and colored states[19]

Fig.8 (a) Schematic diagram of H2W2O7, WO3·H2O, and WO3 structures; (b) CV curves at scanning speeds of 50, 100, 200, 500, and 1000 mV·s-1; (c) Scanning electron microscopy image of H2W2O7; (d) In situ Raman spectroscopy curve of H2W2O7; (e) Digital photos collected during in situ Raman spectroscopy of H2W2O7[89]
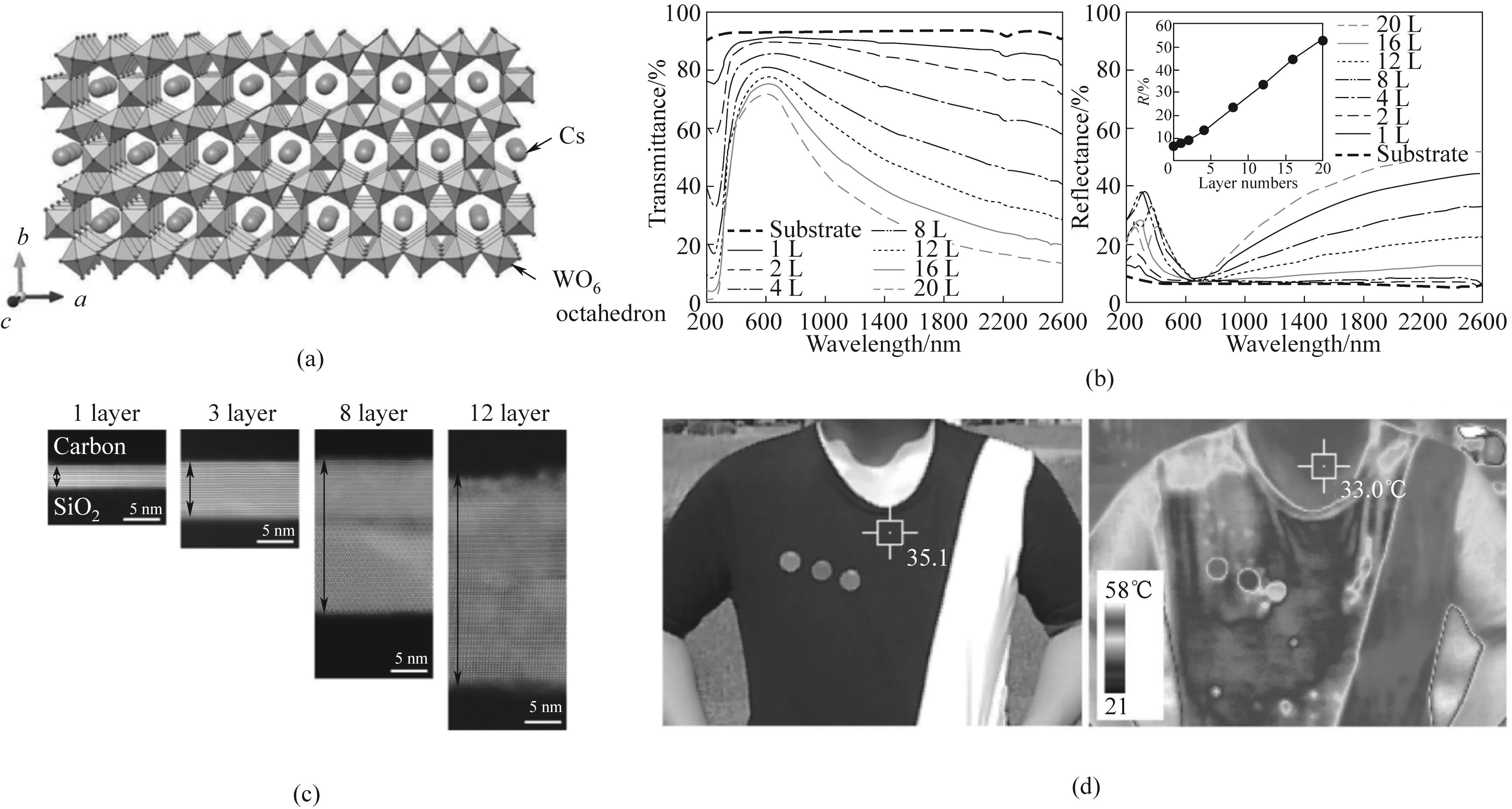
Fig.9 (a) Crystal structure of Cs3W11O35; (b) Transmittance and reflectance curves of Cs3W11O35 with different layers; (c) Transmission electron microscopy image of Cs3W11O35; (d) Visible and infrared images for thermal shielding test[94]
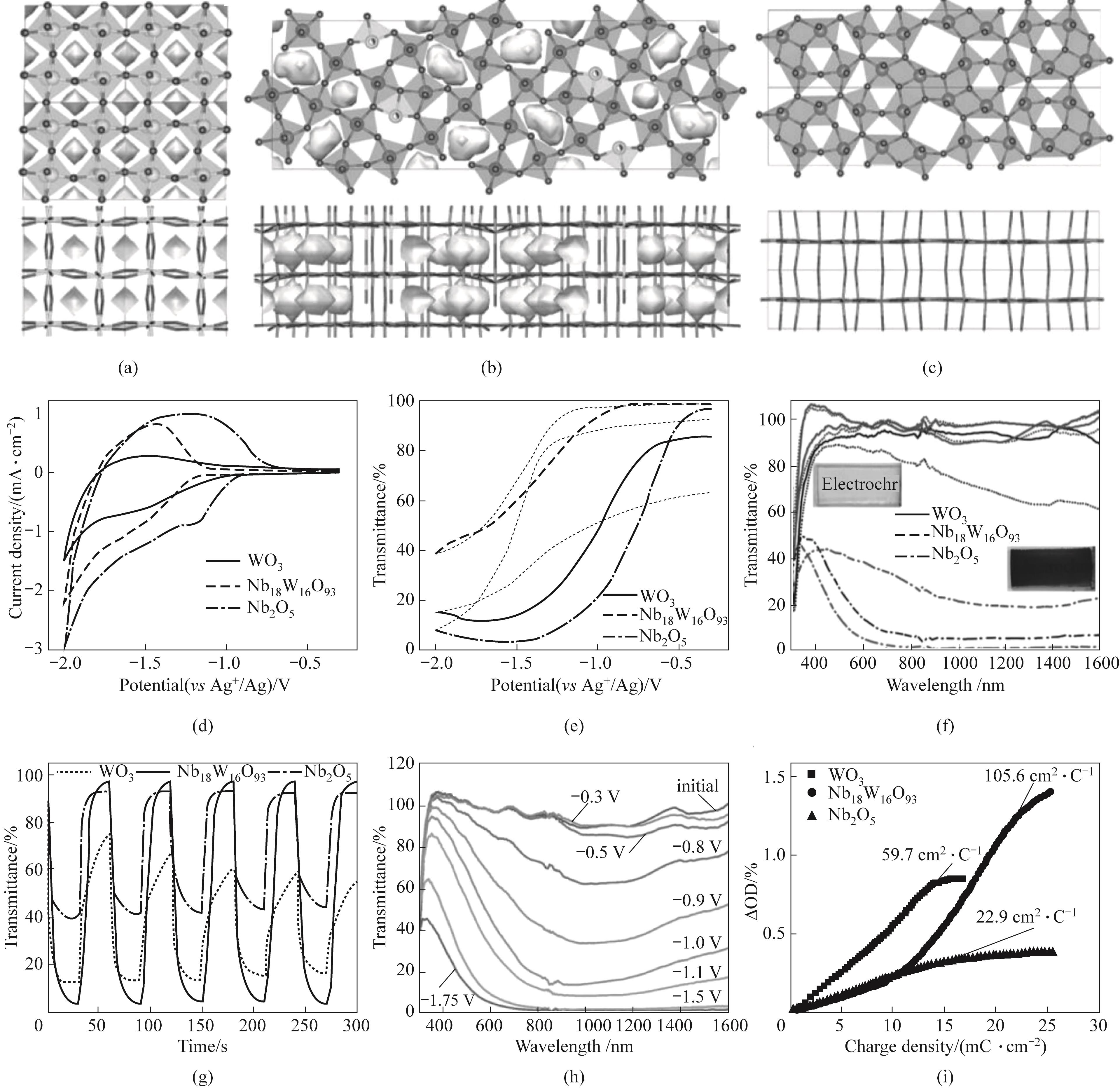
Fig.10 (a) Schematic diagram of WO3 structure; (b) Schematic diagram of Nb18W16O93 structure; (c) Schematic diagram of Nb2O5 structure; (d) CV curve at a scanning speed of 20 mV·s-1; (e) In situ transmittance voltage curve at 633 nm; (f) Transmittance curve of 300—1600 nm (corresponding photo in the illustration); (g) In situ transmittance curve with a square wave potential applied at 633 nm; (h) Transmittance curves at different voltages; (i) Coloring efficiency at 633 nm[39]

Fig.11 (a) Transmittance curves of Ce4W9O33 electrode under different applied potentials; (b) Electrochromic photo of Ce4W9O33 electrode; (c) Solar irradiation spectra of Ce4W9O33 electrode under different applied potentials; (d) In situ transmittance curve with a square wave potential applied at 633 nm; (e) In situ transmittance curve with a square wave potential applied at 1200 nm; (f) The cycling performance of Ce4W9O33 electrode[40]
| 纳米材料 | 制备方法 | 光调制幅度(波长/nm) | 响应时间 Tc、Tb/s | 着色效率/(cm2·C-1) | 循环次数/次 | 电解液及浓度/ (mol·L-1) | 对电极 | 文献 |
|---|---|---|---|---|---|---|---|---|
| WO3·0.1H2O | 光沉积法 | 55.1%(633) | 11.4/23.9 @633 nm | 47.7 @633 nm | — | ZnSO4 水溶液(1) | Zn | [ |
| WO3·0.5H2O | 69.0%(633) | 7.0/3.4 @633 nm | 61.9 @633 nm | 13000(3%调制衰减) | ||||
| WO3·H2O | 溶胶-凝胶法 | 82%(900) | 12.0/6.0 @900 nm | 97.8 @650 nm | 1000(13%调制衰减) | LiClO4/SPE | FTO | [ |
| WO3·H2O | 电沉积法 | 59%(1200) | <15 | 96.2 @1200 nm | — | LiClO4-PC(1) | Li | [ |
| TBAClO4-PC(0.1) | Pt | |||||||
| Ni-W@Li | 磁控溅射法 | 75%(550) | — | 115.2 @650 nm | 1000 | LiClO4-PC(0.5) | WO3 | [ |
| Bi2Na0.5La0.5TiWO9 | 高温固相法 | 30.3%(555) | — | 37.1 @555 nm | — | H2SO4水溶液(0.2) | Pt | [ |
| Nb18W16O93 | 溶胶-凝胶法 | 53.1%(633) | 4.7/4.0 @633 nm | 46.57 @633 nm | 8000(21.9%调制衰减) | LiClO4-PC(0.5) | FTO | [ |
| Nb18W16O93 | 水热法 | 49.4%(633) | 12.0/2.6 @633 nm | 33.7 @633 nm | 600(35%调制衰减) | LiClO4-PC(0.5) | Pt | [ |
| 90.5%(1600) | 6.5/6.1 @1600 nm | 66.1 @1600 nm | ||||||
| Nb18W16O93 | 溶胶-水热法 | 93%(633) | 10.1/12.7 @633 nm | 105.6 @633 nm | 1000(16.5%调制衰减) | LiClO4/PMMA(0.3) | NiO | [ |
| 89%(1200) | 7.4/5.4 @1200 nm | 6000(33.6%调制衰减) | ||||||
| Ce4W9O33 | 水热法 | 74.7%(633) | 10.5/4.5 @633 nm | 98.3 @1200nm | 500(11.1%调制衰减) | ZnSO4 水溶液(0.5) | NiO | [ |
| 85.8%(1200) | 6.5/4.1 @1200 nm |
Table 3 The electrochromic properties of common tungstate nanofilms
| 纳米材料 | 制备方法 | 光调制幅度(波长/nm) | 响应时间 Tc、Tb/s | 着色效率/(cm2·C-1) | 循环次数/次 | 电解液及浓度/ (mol·L-1) | 对电极 | 文献 |
|---|---|---|---|---|---|---|---|---|
| WO3·0.1H2O | 光沉积法 | 55.1%(633) | 11.4/23.9 @633 nm | 47.7 @633 nm | — | ZnSO4 水溶液(1) | Zn | [ |
| WO3·0.5H2O | 69.0%(633) | 7.0/3.4 @633 nm | 61.9 @633 nm | 13000(3%调制衰减) | ||||
| WO3·H2O | 溶胶-凝胶法 | 82%(900) | 12.0/6.0 @900 nm | 97.8 @650 nm | 1000(13%调制衰减) | LiClO4/SPE | FTO | [ |
| WO3·H2O | 电沉积法 | 59%(1200) | <15 | 96.2 @1200 nm | — | LiClO4-PC(1) | Li | [ |
| TBAClO4-PC(0.1) | Pt | |||||||
| Ni-W@Li | 磁控溅射法 | 75%(550) | — | 115.2 @650 nm | 1000 | LiClO4-PC(0.5) | WO3 | [ |
| Bi2Na0.5La0.5TiWO9 | 高温固相法 | 30.3%(555) | — | 37.1 @555 nm | — | H2SO4水溶液(0.2) | Pt | [ |
| Nb18W16O93 | 溶胶-凝胶法 | 53.1%(633) | 4.7/4.0 @633 nm | 46.57 @633 nm | 8000(21.9%调制衰减) | LiClO4-PC(0.5) | FTO | [ |
| Nb18W16O93 | 水热法 | 49.4%(633) | 12.0/2.6 @633 nm | 33.7 @633 nm | 600(35%调制衰减) | LiClO4-PC(0.5) | Pt | [ |
| 90.5%(1600) | 6.5/6.1 @1600 nm | 66.1 @1600 nm | ||||||
| Nb18W16O93 | 溶胶-水热法 | 93%(633) | 10.1/12.7 @633 nm | 105.6 @633 nm | 1000(16.5%调制衰减) | LiClO4/PMMA(0.3) | NiO | [ |
| 89%(1200) | 7.4/5.4 @1200 nm | 6000(33.6%调制衰减) | ||||||
| Ce4W9O33 | 水热法 | 74.7%(633) | 10.5/4.5 @633 nm | 98.3 @1200nm | 500(11.1%调制衰减) | ZnSO4 水溶液(0.5) | NiO | [ |
| 85.8%(1200) | 6.5/4.1 @1200 nm |
| [1] | International Energy Agency. World Energy Outlook 2023[R]. Paris: OECD Publishing, 2023. |
| [2] | 中国建筑节能协会, 重庆大学城乡建设与发展研究院. 中国建筑能耗与碳排放研究报告(2023年)[J]. 建筑, 2024(2): 46-59. |
| China Association of Building Energy Efficiency, Institute of Urban-rural Construction and Development, Chongqing University. Research report on building energy consumption and carbon emissions in China (2023)[J]. Construction and Architecture, 2024(2): 46-59. | |
| [3] | Deb S K. A novel electrophotographic system[J]. Applied Optics, 1969, 8(S1): 192-195. |
| [4] | Svensson J S E M, Granqvist C G. Electrochromic tungsten oxide films for energy efficient windows[J]. Solar Energy Materials, 1984, 11(1/2): 29-34. |
| [5] | Granqvist C G. Oxide electrochromics: an introduction to devices and materials[J]. Solar Energy Materials and Solar Cells, 2012, 99: 1-13. |
| [6] | Llordés A, Garcia G, Gazquez J, et al. Tunable near-infrared and visible-light transmittance in nanocrystal-in-glass composites[J]. Nature, 2013, 500(7462): 323-326. |
| [7] | Mandal J, Du S C, Dontigny M, et al. Li4Ti5O12: a visible-to-infrared broadband electrochromic material for optical and thermal management[J]. Advanced Functional Materials, 2018, 28(36): 1802180. |
| [8] | Zhang S L, Cao S, Zhang T R, et al. Monoclinic oxygen-deficient tungsten oxide nanowires for dynamic and independent control of near-infrared and visible light transmittance[J]. Materials Horizons, 2018, 5(2): 291-297. |
| [9] | Manjakkal L, Pereira L, Kumi Barimah E, et al. Multifunctional flexible and stretchable electrochromic energy storage devices[J]. Progress in Materials Science, 2024, 142: 101244. |
| [10] | Gong H, Li A, Fu G X, et al. Ultrathin flexible electrochromic devices enabled by highly transparent ion-conducting films[J]. Journal of Materials Chemistry A, 2023, 11(16): 8939-8949. |
| [11] | Lee S J, Lee S H, Kang H W, et al. Flexible electrochromic and thermochromic hybrid smart window based on a highly durable ITO/graphene transparent electrode[J]. Chemical Engineering Journal, 2021, 416: 129028. |
| [12] | Ganesha M K, Hakkeem H, Mondal I, et al. An ITO free all tungsten-based electrochromic energy storage device as smart window[J]. Small, 2024, 20(48): e2405467. |
| [13] | Liu L, Zhen M S, Wang L Y, et al. Full-temperature all-solid-state dendrite-free Zn-ion electrochromic energy storage devices for intelligent applications[J]. Chemical Engineering Journal, 2023, 468: 143837. |
| [14] | Buch V R, Chawla A K, Rawal S K. Review on electrochromic property for WO3 thin films using different deposition techniques[J]. Materials Today: Proceedings, 2016, 3(6): 1429-1437. |
| [15] | Zhang X, Tian Y L, Li W J, et al. Preparation and performances of all-solid-state variable infrared emittance devices based on amorphous and crystalline WO3 electrochromic thin films[J]. Solar Energy Materials and Solar Cells, 2019, 200: 109916. |
| [16] | Zheng H D, Ou J Z, Strano M S, et al. Nanostructured tungsten oxide—properties, synthesis, and applications[J]. Advanced Functional Materials, 2011, 21(12): 2175-2196. |
| [17] | Gayathri P T G, Shaiju S S, Remya R, et al. Hydrated tungsten oxide nanosheet electrodes for broadband electrochromism and energy storage[J]. Materials Today Energy, 2018, 10: 380-387. |
| [18] | Azam A, Kim J, Park J, et al. Two-dimensional WO3 nanosheets chemically converted from layered WS2 for high-performance electrochromic devices[J]. Nano Letters, 2018, 18(9): 5646-5651. |
| [19] | Gao T, Jelle B P. Electrochromism of hexagonal sodium tungsten bronze nanorods[J]. Solar Energy Materials and Solar Cells, 2018, 177: 3-8. |
| [20] | Phan G T, Van Pham D, Patil R A, et al. Fast-switching electrochromic smart windows based on NiO-nanorods counter electrode[J]. Solar Energy Materials and Solar Cells, 2021, 231: 111306. |
| [21] | Zhou D, Shi F, Xie D, et al. Bi-functional Mo-doped WO3 nanowire array electrochromism-plus electrochemical energy storage[J]. Journal of Colloid and Interface Science, 2016, 465: 112-120. |
| [22] | Liu X S, Wang G, Wang J, et al. Electrochromic and capacitive properties of WO3 nanowires prepared by one-step water bath method[J]. Coatings, 2022, 12(5): 595. |
| [23] | Liu P C, Wang B, Wang C C, et al. Amorphous tungsten oxide nanodots for chromatic applications[J]. Advanced Functional Materials, 2024, 34(34): 2400760. |
| [24] | Yao Y J, Zhao Q, Wei W, et al. WO3 quantum-dots electrochromism[J]. Nano Energy, 2020, 68: 104350. |
| [25] | Wang S, Xu H B, Zhao J P, et al. Two-dimensional WO3 nanosheets for high-performance electrochromic supercapacitors[J]. Inorganic Chemistry Frontiers, 2022, 9(3): 514-523. |
| [26] | Huang Y, Wang B S, Lyu P, et al. Oxygen-deficient tungsten oxide nanoflowers for dynamically tunable near-infrared light transmittance of smart windows[J]. Nano Research, 2023, 16(10): 12165-12172. |
| [27] | Ou J Z, Balendhran S, Field M R, et al. The anodized crystalline WO3 nanoporous network with enhanced electrochromic properties[J]. Nanoscale, 2012, 4(19): 5980-5988. |
| [28] | Dalavi D S, Devan R S, Patil R A, et al. Efficient electrochromic performance of nanoparticulate WO3 thin films[J]. Journal of Materials Chemistry C, 2013, 1(23): 3722-3728. |
| [29] | 陈莉蓉, 葛锐, 王杏如, 等. 无机电致变色材料多波段调控的研究进展[J]. 材料研究与应用, 2023, 17(5): 835-863. |
| Chen L R, Ge R, Wang X R, et al. Recent progress on inorganic electrochromic material with multi-band modulation[J]. Materials Research and Application, 2023, 17(5): 835-863. | |
| [30] | Wang L K, Liu Y, Han G R, et al. Dual-band electrochromic film based on mesoporous h-WO3/o-WO3·H2O/r-TiO2 for high performance smart windows[J]. Solar Energy Materials and Solar Cells, 2023, 250: 112053. |
| [31] | Wang L K, Liu Y, Han G R, et al. Controllable synthesis of hexagonal WO3 nanorod-cluster films with high electrochromic performance in NIR range[J]. Journal of Alloys and Compounds, 2022, 890: 161833. |
| [32] | Zhao F Y, Cheng Z Q, Xu G, et al. A facile electrochemical lithiation method to prepare porous nickel oxide electrodes with high electrochromic performance[J]. Electrochimica Acta, 2023, 441: 141863. |
| [33] | Zhao F Y, He H Y, Cheng Z Q, et al. Improving electrochromic performance of porous nickel oxide electrode via Cu doping[J]. Electrochimica Acta, 2022, 417: 140332. |
| [34] | Zhang S L, Cao S, Zhang T R, et al. Plasmonic oxygen-deficient TiO2- x nanocrystals for dual-band electrochromic smart windows with efficient energy recycling[J]. Advanced Materials, 2020, 32(43): e2004686. |
| [35] | Gao Y, Lei P Y, Zhang S Y, et al. A layer-stacked NiO nanowire/nanosheet homostructure for electrochromic smart windows with ultra-large optical modulation[J]. Nanoscale, 2023, 15(19): 8685-8692. |
| [36] | Huang J J, Zhang S Y, Qin Q, et al. Designing V2O5/MXene van der Waals heterostructure for complementary electrochromic dual-ion capacitor[J]. Chemical Engineering Journal, 2023, 476: 146626. |
| [37] | Ling Y, Fan H W, Wang K, et al. Electrochemical actuators with multicolor changes and multidirectional actuation[J]. Small, 2022, 18(15): e2107778. |
| [38] | Sun M T, Wang L K, Shi G H, et al. Niobium-tungsten bimetallic oxide electrodes with high dual-band electrochromic performance prepared by hydrothermal method[J]. Journal of the Electrochemical Society, 2023, 170(12): 126503. |
| [39] | Cai G F, Zhu R, Liu S Y, et al. Tunable intracrystal cavity in tungsten bronze-like bimetallic oxides for electrochromic energy storage[J]. Advanced Energy Materials, 2022, 12(5): 2103106. |
| [40] | Ma D Y, Yang T, Feng X Z, et al. Quadruple control electrochromic devices utilizing Ce4W9O33 electrodes for visible and near-infrared transmission intelligent modulation[J]. Advanced Science, 2024, 11(14): e2307223. |
| [41] | Sorouri A M, Sobhani-Nasab A, Ganjali M R, et al. Metal tungstates nanostructures for supercapacitors: a review[J]. Applied Materials Today, 2023, 32: 101819. |
| [42] | 武亚奇, 何恩节. 双钙钛矿钨酸盐荧光粉的递进式荧光增强及植物LED应用[J]. 宁夏师范学院学报, 2023, 44(10): 52-59. |
| Wu Y Q, He E J. Application in plant LED and progressive luminescence enhancement of double perovskite tungstate phosphor[J]. Journal of Ningxia Normal University, 2023, 44(10): 52-59. | |
| [43] | Fukuda K, Akatsuka K, Ebina Y, et al. Photochromogenic nanosheet crystallites of tungstate with a 2D bronze structure[J]. Inorganic Chemistry, 2012, 51(3): 1540-1543. |
| [44] | Schaak R E, Mallouk T E. Exfoliation of layered rutile and perovskite tungstates[J]. Chemical Communications, 2002(7): 706-707. |
| [45] | Huang L L, Liu M H, Lin H X, et al. Shape regulation of high-index facet nanoparticles by dealloying[J]. Science, 2019, 365(6458): 1159-1163. |
| [46] | Gadiyar C, Loiudice A, D'Ambra F, et al. Nanocrystals as precursors in solid-state reactions for size- and shape-controlled polyelemental nanomaterials[J]. Journal of the American Chemical Society, 2020, 142(37): 15931-15940. |
| [47] | Thongtem T, Kungwankunakorn S, Kuntalue B, et al. Luminescence and absorbance of highly crystalline CaMoO4, SrMoO4, CaWO4 and SrWO4 nanoparticles synthesized by co-precipitation method at room temperature[J]. Journal of Alloys and Compounds, 2010, 506(1): 475-481. |
| [48] | Chen J H, Feng S Y, Deng J H, et al. Application of precursor with ultra-small particle size and uniform particle distribution for ultra-high nickel single-crystal cathode materials by coprecipitation method[J]. Journal of Colloid and Interface Science, 2025, 679: 798-810. |
| [49] | Kanade V K, Kanade C K, Pujari R B, et al. Surface and diffusive capacity controlled electrochemistry in nickel boride/nickel borate[J]. Journal of Industrial and Engineering Chemistry, 2022, 116: 351-358. |
| [50] | Rahimi-Nasrabadi M, Pourmortazavi S M, Ganjali M R, et al. Optimizing the synthesis procedure and characterization of t e r b i u m ( Ⅲ ) tungstate nanoparticles as high performance photocatalysts[J]. Journal of Materials Science: Materials in Electronics, 2017, 28(13): 9724-9731. |
| [51] | Rahimi-Nasrabadi M, Pourmortazavi S M, Ganjali M R, et al. Synthesis procedure optimization and characterization of e u r o p i u m ( Ⅲ ) tungstate nanoparticles[J]. Journal of Molecular Structure, 2014, 1074: 85-91. |
| [52] | Pourmortazavi S M, Rahimi-Nasrabadi M, Khalilian-Shalamzari M, et al. Synthesis, structure characterization and catalytic activity of nickel tungstate nanoparticles[J]. Applied Surface Science, 2012, 263: 745-752. |
| [53] | Huang B, Wang H Y, Liang S F, et al. Two-dimensional porous cobalt-nickel tungstate thin sheets for high performance supercapattery[J]. Energy Storage Materials, 2020, 32: 105-114. |
| [54] | Rahimi-Nasrabadi M, Pourmohamadian V, Karimi M S, et al. Assessment of supercapacitive performance of europium tungstate nanoparticles prepared via hydrothermal method[J]. Journal of Materials Science: Materials in Electronics, 2017, 28(17): 12391-12398. |
| [55] | Zeng S Y, Tang R F, Duan S X, et al. Kinetically controlled synthesis of bismuth tungstate with different structures by a NH4F assisted hydrothermal method and surface-dependent photocatalytic properties[J]. Journal of Colloid and Interface Science, 2014, 432: 236-245. |
| [56] | Wu Z C, Wang X, Huang J S, et al. A Co-doped Ni-Fe mixed oxide mesoporous nanosheet array with low overpotential and high stability towards overall water splitting[J]. Journal of Materials Chemistry A, 2018, 6(1): 167-178. |
| [57] | Chen L P, Li C E, Zhao Y F, et al. Constructing 3D Bi/Bi4O5I2 microspheres with rich oxygen vacancies by one-pot solvothermal method for enhancing photocatalytic activity on mercury removal[J]. Chemical Engineering Journal, 2021, 425: 131599. |
| [58] | Chen T, Meng J, Lin Q Y, et al. One-step synthesis of hollow BaZrO3 nanocrystals with oxygen vacancies for photocatalytic hydrogen evolution from pure water[J]. Journal of Alloys and Compounds, 2019, 780: 498-503. |
| [59] | Jiang L, Ding H Z, Xu M S, et al. UV-vis-NIR full-range responsive carbon dots with large multiphoton absorption cross sections and deep-red fluorescence at nucleoli and in vivo [J]. Small, 2020, 16(19): e2000680. |
| [60] | Xie Y, Qian Y, Wang W, et al. A benzene-thermal synthetic route to nanocrystalline GaN[J]. Science, 1996, 272(5270): 1926-1927. |
| [61] | Li Q Y, Deng S S, Li D L, et al. Tungsten bronze Cs x WO3 nanopowders doped by Ti to enhance transparent thermal insulation ability for energy saving[J]. Journal of Alloys and Compounds, 2023, 944: 169164. |
| [62] | Ahmadi F, Rahimi-Nasrabadi M, Behpour M. Synthesis Nd2TiO5 nanoparticles with different morphologies by novel approach and its photocatalyst application[J]. Journal of Materials Science: Materials in Electronics, 2017, 28(2): 1531-1536. |
| [63] | 魏芳, 李玉宏, 张振乾. 溶胶凝胶法制备纳米二氧化硅及性能研究[J]. 山东化工, 2024, 53(17): 13-16. |
| Wei F, Li Y H, Zhang Z Q. Study on preparation and properties of nano silica by sol gel method[J]. Shandong Chemical Industry, 2024, 53(17): 13-16. | |
| [64] | 龚圣, 周新华, 尹国强, 等. 溶胶-凝胶法制备纳米锑掺杂氧化锡的团聚消除[J]. 化工学报, 2011, 62(5): 1460-1465. |
| Gong S, Zhou X H, Yin G Q, et al. Agglomeration eliminating nano-sized antimony doped tin oxide prepared by sol-gel method[J]. CIESC Journal, 2011, 62(5): 1460-1465. | |
| [65] | Zhang L H, Ma H R, Ying Z H, et al. Lowering charge transport barriers by eliminating the electric double layer residues to reconstruct adjacent SnO2 nanocrystals for high-efficiency flexible perovskite solar cells[J]. Advanced Functional Materials, 2024, 34(45): 2406946. |
| [66] | Li L, Xu H, Chen Y H, et al. Preparation and optical properties of hexa-tungsten bronze-type CsNbW2O9 semiconductor[J]. Optical Materials, 2017, 66: 361-366. |
| [67] | Rahmani M, Sedaghat T. A facile sol-gel process for synthesis of ZnWO4 nanopartices with enhanced band gap and study of its photocatalytic activity for degradation of methylene blue[J]. Journal of Inorganic and Organometallic Polymers and Materials, 2019, 29(1): 220-228. |
| [68] | Prasad A K, Park J Y, Jung H Y, et al. Electrochemical deposition of Ni-WO3 thin-film composites for electrochromic energy storage applications: novel approach toward quantum-dot-sensitized solar cell-assisted Ni-WO3 electrochromic device[J]. Journal of Industrial and Engineering Chemistry, 2023, 117: 500-509. |
| [69] | Yourey J E, Bartlett B M. Electrochemical deposition and photoelectrochemistry of CuWO4, a promising photoanode for wateroxidation[J]. Journal of Materials Chemistry, 2011, 21(21): 7651-7660. |
| [70] | Santiago A A G, Fernandes Y L R L, Tranquilin R L, et al. Influence of Zn1- x Ca x WO4 heterostructures synthesized by spray pyrolysis on photoluminescence property[J]. Ceramics International, 2019, 45(17): 23256-23264. |
| [71] | Patil A R, Dongale T D, Namade L D, et al. Sprayed FeWO4 thin film-based memristive device with negative differential resistance effect for non-volatile memory and synaptic learning applications[J]. Journal of Colloid and Interface Science, 2023, 642: 540-553. |
| [72] | Pourmortazavi S M, Rahimi-Nasrabadi M, Fazli Y, et al. Taguchi method assisted optimization of electrochemical synthesis and structural characterization of copper tungstate nanoparticles[J]. International Journal of Refractory Metals and Hard Materials, 2015, 51: 29-34. |
| [73] | Nakakura S, Ogi T. Hexagonal cesium tungsten bronze nanoparticles produced by solvent-free spray pyrolysis and their near infrared absorption properties[J]. Journal of Materials Chemistry C, 2021, 9(25): 8037-8042. |
| [74] | Jiang X N, Chen S, Zhang X R, et al. Carbon-doped flower-like Bi2WO6 decorated carbon nanosphere nanocomposites with enhanced visible light photocatalytic degradation of tetracycline[J]. Advanced Composites and Hybrid Materials, 2023, 6(1): 47. |
| [75] | Singh V P, Singh G, Patel R, et al. Highly sensitive detection of hazardous hydroquinone and chloramphenicol in the presence of paracetamol using cobalt tungstate (CoWO4) nanoplates modified electrode[J]. Journal of Environmental Chemical Engineering, 2023, 11(6): 111208. |
| [76] | Ravi G, Mamidi S, Sreenu K, et al. Layered Na2W4O13 and its cation/anion doped analogues for the treatment of polluted water[J]. FlatChem, 2019, 13: 1-7. |
| [77] | Ofori F A, Sheikh F A, Appiah-Ntiamoah R, et al. A simple method of electrospun tungsten trioxide nanofibers with enhanced visible-light photocatalytic activity[J]. Nano-Micro Letters, 2015, 7(3): 291-297. |
| [78] | Balaji S, Djaoued Y, Albert A S, et al. Construction and characterization of tunable meso-/macroporous tungsten oxide-based transmissive electrochromic devices[J]. Journal of Materials Science, 2009, 44(24): 6608-6616. |
| [79] | Pugolovkin L V, Cherstiouk O V, Plyasova L M, et al. Electrodeposited non-stoichiometric tungstic acid for electrochromic applications: film growth modes, crystal structure, redox behavior and stability[J]. Applied Surface Science, 2016, 388: 786-793. |
| [80] | Kuzmin A, Purans J, Kalendarev R, et al. XAS, XRD, AFM and Raman studies of nickel tungstate electrochromic thin films[J]. Electrochimica Acta, 2001, 46(13/14): 2233-2236. |
| [81] | Wei Y X, Liu W M, Li J Y, et al. Investigation on the properties of Li doped Ni-W oxide film and application for black electrochromic device[J]. Electrochimica Acta, 2022, 406: 139833. |
| [82] | Green S V, Granqvist C G, Niklasson G A. Structure and optical properties of electrochromic tungsten-containing nickel oxide films[J]. Solar Energy Materials and Solar Cells, 2014, 126: 248-259. |
| [83] | Liu G B, Liu S K, Li X L, et al. Optimized W-d band configuration in porous sodium tungsten bronze octahedron enabling Pt-like and wide-pH hydrogen evolution[J]. Nano Energy, 2024, 123: 109442. |
| [84] | Nicolosi V, Chhowalla M, Kanatzidis M G, et al. Liquid exfoliation of layered materials[J]. Science, 2013, 340(6139): 1226419. |
| [85] | Schaak R E, Mallouk T E. Perovskites by design: a toolbox of solid-state reactions[J]. Chemistry of Materials, 2002, 14(4): 1455-1471. |
| [86] | Kishimoto F, Takanabe K. Electron storage in monolayer tungstate nanosheets produced via a scalable exfoliation method[J]. The Journal of Physical Chemistry Letters, 2024, 15(13): 3509-3515. |
| [87] | Pope T R, Lassig M N, Neher G, et al. Chromism of Bi2WO6 in single crystal and nanosheet forms[J]. Journal of Materials Chemistry C, 2014, 2(17): 3223-3230. |
| [88] | Iimura R, Hasegawa T, Yin S. Electrochromic behavior originating from the W6+/W5+ redox in aurivillius-type tungsten-based layered perovskites[J]. Inorganic Chemistry, 2022, 61(5): 2509-2516. |
| [89] | Wang R C, Sun Y Y L, Brady A, et al. Fast proton insertion in layered H2W2O7 via selective etching of an aurivillius phase[J]. Advanced Energy Materials, 2021, 11(1): 2003335. |
| [90] | Niu J L, Wang Y, Zou X L, et al. Infrared electrochromic materials, devices and applications[J]. Applied Materials Today, 2021, 24: 101073. |
| [91] | Fukuda K, Akatsuka K, Ebina Y, et al. Exfoliated nanosheet crystallite of cesium tungstate with 2D pyrochlore structure: synthesis, characterization, and photochromic properties[J]. ACS Nano, 2008, 2(8): 1689-1695. |
| [92] | Shen B X, Ding S Y, Wang Y H, et al. Novel one-pot solvothermal synthesis and phase-transition mechanism of hexagonal Cs x WO3 nanocrystals with superior near-infrared shielding property for energy-efficient windows[J]. Solar Energy, 2021, 230: 401-408. |
| [93] | He Z M, Yu P, Gao J J, et al. An energy-efficient and low-driving-voltage flexible smart window enhanced by POSS and Cs x WO3 [J]. Solar Energy Materials and Solar Cells, 2023, 250: 112096. |
| [94] | Tsunematsu H, Shi Y, Yamamoto E, et al. Gigantic thermal shielding in 2D oxide nanosheets[J]. ACS Nano, 2023, 17(12): 11396-11405. |
| [95] | Griffith K J, Wiaderek K M, Cibin G, et al. Niobium tungsten oxides for high-rate lithium-ion energy storage[J]. Nature, 2018, 559(7715): 556-563. |
| [96] | Ren R R, Liu S Y, Gao Y, et al. Tunable interaction between Zn2+ and superstructured Nb18W16O93 bimetallic oxide for multistep tinted electrochromic device[J]. ACS Energy Letters, 2023, 8(5): 2300-2307. |
| [97] | Wu C, Shao Z W, Zhai W B, et al. Niobium tungsten oxides for electrochromic devices with long-term stability[J]. ACS Nano, 2022, 16(2): 2621-2628. |
| [98] | Diao Q, Yin Y N, Jia W S, et al. Highly sensitive ethanol sensor based on Ce-doped WO3 with raspberry-like architecture[J]. Materials Research Express, 2020, 7(11): 115012. |
| [99] | Zhuang D S, Zhang Z X, Weng J B, et al. Amorphous hydrated tungsten oxides with enhanced pseudocapacitive contribution for aqueous zinc-ion electrochromic energy storage[J]. Advanced Energy Materials, 2024, 14(40): 2402603. |
| [100] | Fortunato J, Zydlewski B Z, Lei M, et al. Dual-band electrochromism in hydrous tungsten oxide[J]. ACS Photonics, 2023, 10(9): 3409-3418. |
| [101] | Sun Y Q, Fu W, Hu Y X, et al. The role of tungsten-related elements for improving the electrochemical performances of cathode materials in lithium ion batteries[J]. Tungsten, 2021, 3(3): 245-259. |
| [102] | Xiao S X, Zhang Y J, Ma L, et al. Easy-to-make sulfonatoalkyl viologen/sodium carboxymethylcellulose hydrogel-based electrochromic devices with high coloration efficiency, fast response and excellent cycling stability[J]. Dyes and Pigments, 2020, 174: 108055. |
| [103] | Lu H C, Kao S Y, Yu H F, et al. Achieving low-energy driven viologens-based electrochromic devices utilizing polymeric ionic liquids[J]. ACS Applied Materials & Interfaces, 2016, 8(44): 30351-30361. |
| [104] | Zhou X, Zhou K J, Tang L, et al. A strong and highly transparent ionogel electrolyte enabled by in situ polymerization-induced microphase separation for high-performance electrochromic devices[J]. Macromolecular Rapid Communications, 2024, 45(13): 2300736. |
| [105] | Zhou Z, Tang Y K, Zhao F Y, et al. Transparent succinonitrile-modified polyacrylate gel polymer electrolyte for solid electrochromic devices[J]. Chemical Engineering Journal, 2024, 481: 148724. |
| [106] | Li H Z, Firby C J, Elezzabi A Y. Rechargeable aqueous hybrid Zn2+/Al3+ electrochromic batteries[J]. Joule, 2019, 3(9): 2268-2278. |
| [107] | Zhang S L, Cao S, Zhang T R, et al. Al3+ intercalation/de-intercalation-enabled dual-band electrochromic smart windows with a high optical modulation, quick response and long cycle life[J]. Energy & Environmental Science, 2018, 11(10): 2884-2892. |
| [108] | Li C A, Ko B, Park K H, et al. High-performance electrochromic devices based on size-controlled 2D WO3 nanosheets prepared using the intercalation method[J]. Materials, 2023, 17(1): 41. |
| [1] | Shenghua YANG, Yangjie SUN, Xiaojun XUE, Jie MI, Jiancheng WANG, Yu FENG. Research progress on gas pollutants removal by defective metal oxides [J]. CIESC Journal, 2025, 76(6): 2469-2482. |
| [2] | Haotian AN, Zhangye HAN, Muyao LU, Awu ZHOU, Jianrong LI. Promoting industrial application of MOF: scale-up preparation and shaping [J]. CIESC Journal, 2025, 76(5): 2011-2025. |
| [3] | Jinyue WANG, Enze XIE, Hanze MA, Sheng YUAN, Guangwei HE, Zhongyi JIANG. Monoatomic layer separation membrane: progress and prospect [J]. CIESC Journal, 2025, 76(5): 1943-1959. |
| [4] | Yingdong ZHAO, Peijun JI, Riyao CONG, Haichao FU, Jialong ZHANG, Pengzhong CHEN, Xiaojun PENG. Preparation and high-resolution lithography study of organic tin photoresists containing acrylates [J]. CIESC Journal, 2025, 76(4): 1820-1830. |
| [5] | Ben’an CAI, Jianxin ZHANG, Chengjun LONG, Qiaochen DU, Xunjian CHE, Yiying ZHANG, Weihua CAI. Spray flash evaporation preparation of micro/nanoparticles [J]. CIESC Journal, 2025, 76(3): 1334-1345. |
| [6] | Ziyi XU, Yang XI, Zewen SONG, Haijun ZHOU. Advances in the application of carbon nanomaterials for zinc ion batteries [J]. CIESC Journal, 2025, 76(1): 40-52. |
| [7] | Shugang HU, Guoqing TIAN, Wenjuan LIU, Guangfei XU, Huaqing LIU, Jian ZHANG, Yanlong WANG. Preparation of nanoscale zero-valent iron and its application of reduction and oxidation technology [J]. CIESC Journal, 2024, 75(9): 3041-3055. |
| [8] | Yanhui DAI, Qizhao XIONG, Qiang FANG, Dongxiao YANG, Yi WANG, Yang CHEN, Jinping LI, Libo LI. In situ steam-assisted method for one-step synthesis of hierarchically porous Cu-BTC [J]. CIESC Journal, 2024, 75(9): 3329-3337. |
| [9] | Binglin BAI, Shen DU, Mingjia LI, Chuanqi ZHANG. Optical transmittance and electrical conductivity characteristics of single-walled carbon nanotube films based on water-phase exfoliation method [J]. CIESC Journal, 2024, 75(7): 2680-2687. |
| [10] | Lulu ZHAO, Erjun TANG, Xuteng XING, Shaojie LIU, Xiaomeng CHU, Na HU, Ze ZHANG. Effects of POSS modified graphene oxide in anti-corrosion and hydrophobic properties of coatings [J]. CIESC Journal, 2024, 75(5): 1977-1986. |
| [11] | Youming SI, Lingfeng ZHENG, Pengzhong CHEN, Jiangli FAN, Xiaojun PENG. Performance and mechanism of novel antimony oxo cluster photoresist [J]. CIESC Journal, 2024, 75(4): 1705-1717. |
| [12] | Xiaoqing YAN, Ying ZHAO, Yuzhe ZHANG, Honghui OU, Qizhong HUANG, Huagui HU, Guidong YANG. Preparation of five-fold twinned copper nanowires@polypyrrole and their electrocatalytic conversion of nitrate to ammonia [J]. CIESC Journal, 2024, 75(4): 1519-1532. |
| [13] | Yuwei YANG, Min LI, Zhiying YAO, Qinlin SUN, Yang LIU, Dan GE, Bingbing SUN. Application and prospect of organoids-on-chip in the study of nano-drug delivery systems [J]. CIESC Journal, 2024, 75(4): 1209-1221. |
| [14] | Rui CHANG, Ruirui XING, Xuehai YAN. Green and biorecyclable materials based on peptide noncovalent chemistry [J]. CIESC Journal, 2024, 75(4): 1317-1332. |
| [15] | Xi WU, Bo SUN, Yindong LIU, Chuanlei QI, Kaiyi CHEN, Luhai WANG, Chong XU, Yongfeng LI. Research progress in preparation technology of pitch-based carbon anode materials for sodium-ion batteries [J]. CIESC Journal, 2024, 75(4): 1270-1283. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||