CIESC Journal ›› 2025, Vol. 76 ›› Issue (1): 120-130.DOI: 10.11949/0438-1157.20240597
• Thermodynamics • Previous Articles Next Articles
Lingyu LI1( ), Xin HU1, Huaigang CHENG1,2, Yun ZHAO1(
), Xin HU1, Huaigang CHENG1,2, Yun ZHAO1( ), Dong AN1, Yujun MA1, Jiahao JIN1, Xudong YU3, Weidong ZHANG1
), Dong AN1, Yujun MA1, Jiahao JIN1, Xudong YU3, Weidong ZHANG1
Received:2024-05-31
Revised:2024-09-27
Online:2025-02-08
Published:2025-01-25
Contact:
Yun ZHAO
李玲玉1( ), 胡鑫1, 成怀刚1,2, 赵云1(
), 胡鑫1, 成怀刚1,2, 赵云1( ), 安东1, 马玉军1, 金家豪1, 于旭东3, 张卫东1
), 安东1, 马玉军1, 金家豪1, 于旭东3, 张卫东1
通讯作者:
赵云
作者简介:李玲玉(2000—),女,硕士研究生,1844877730 @qq.com
基金资助:CLC Number:
Lingyu LI, Xin HU, Huaigang CHENG, Yun ZHAO, Dong AN, Yujun MA, Jiahao JIN, Xudong YU, Weidong ZHANG. Isothermal evaporation salt-forming regions of the ternary water-salt systems K+(Mg2+), Ca2+//Cl--H2O[J]. CIESC Journal, 2025, 76(1): 120-130.
李玲玉, 胡鑫, 成怀刚, 赵云, 安东, 马玉军, 金家豪, 于旭东, 张卫东. K+(Mg2+), Ca2+//Cl--H2O三元水盐体系的等温蒸发成盐相区[J]. 化工学报, 2025, 76(1): 120-130.
Add to citation manager EndNote|Ris|BibTeX
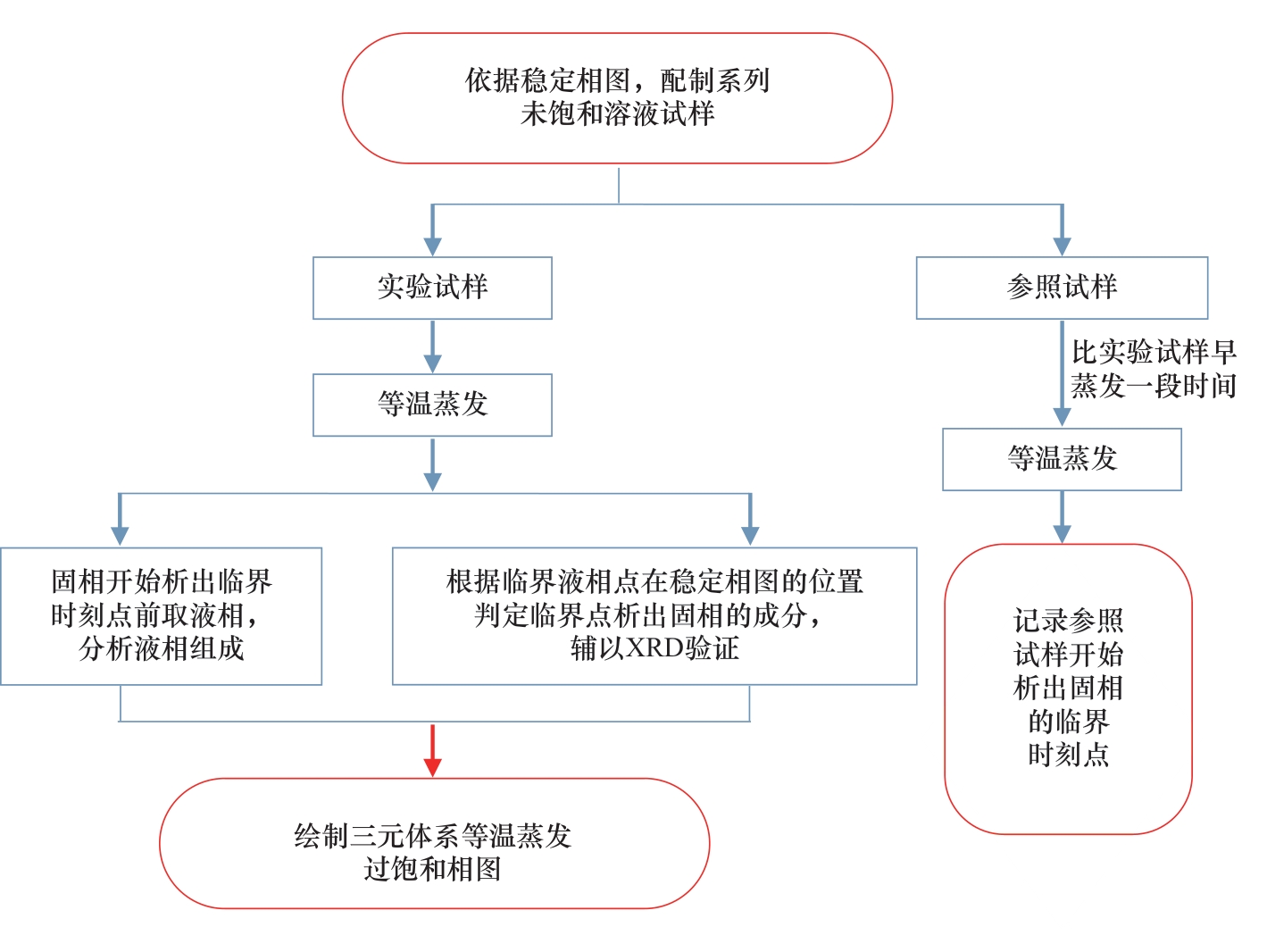
Fig.1 An improved experimental method for obtaining the isothermal evaporation supersaturation phase diagram of ternary water-salt system based on the traditional experimental method of metastable phase diagram
| 序号 | 液相点组成/%(质量分数) | 对应固相 | ||
|---|---|---|---|---|
| CaCl2 | KCl | H2O | ||
| 1,B | 0 | 23.48 | 76.52 | KCl |
| 2 | 2.50 | 21.20 | 76.30 | KCl |
| 3 | 4.62 | 19.40 | 75.97 | KCl |
| 4 | 7.00 | 17.30 | 75.70 | KCl |
| 5 | 8.10 | 16.87 | 75.03 | KCl |
| 6 | 13.79 | 12.94 | 73.28 | KCl |
| 7 | 17.73 | 9.92 | 72.35 | KCl |
| 8 | 19.62 | 9.18 | 71.19 | KCl |
| 9 | 22.23 | 7.66 | 70.11 | KCl |
| 10 | 23.43 | 6.87 | 69.71 | KCl |
| 11 | 28.41 | 5.32 | 66.27 | KCl |
| 12 | 31.66 | 4.52 | 63.82 | KCl |
| 13 | 33.62 | 3.71 | 62.67 | KCl |
| 14 | 37.28 | 3.19 | 59.53 | KCl+CaCl2·6H2O |
| 15 | 37.79 | 2.87 | 59.33 | KCl+CaCl2·6H2O |
| 16 | 38.16 | 2.55 | 59.29 | KCl+CaCl2·6H2O |
| 17 | 38.69 | 2.08 | 59.23 | KCl+CaCl2·6H2O |
| 18 | 38.87 | 2.11 | 59.02 | KCl+CaCl2·6H2O |
| 19 | 38.73 | 2.13 | 59.14 | KCl+CaCl2·6H2O |
| 20 | 38.92 | 2.07 | 59.02 | KCl+CaCl2·6H2O |
| 21 | 38.48 | 0.94 | 60.58 | CaCl2·6H2O |
| 22,A | 39.48 | 0 | 60.52 | CaCl2·6H2O |
Table 1 Supersaturation solubility measurement data and corresponding solid phase of K+, Ca2+//Cl--H2O ternary system at 273.2 K based on the new experimental method
| 序号 | 液相点组成/%(质量分数) | 对应固相 | ||
|---|---|---|---|---|
| CaCl2 | KCl | H2O | ||
| 1,B | 0 | 23.48 | 76.52 | KCl |
| 2 | 2.50 | 21.20 | 76.30 | KCl |
| 3 | 4.62 | 19.40 | 75.97 | KCl |
| 4 | 7.00 | 17.30 | 75.70 | KCl |
| 5 | 8.10 | 16.87 | 75.03 | KCl |
| 6 | 13.79 | 12.94 | 73.28 | KCl |
| 7 | 17.73 | 9.92 | 72.35 | KCl |
| 8 | 19.62 | 9.18 | 71.19 | KCl |
| 9 | 22.23 | 7.66 | 70.11 | KCl |
| 10 | 23.43 | 6.87 | 69.71 | KCl |
| 11 | 28.41 | 5.32 | 66.27 | KCl |
| 12 | 31.66 | 4.52 | 63.82 | KCl |
| 13 | 33.62 | 3.71 | 62.67 | KCl |
| 14 | 37.28 | 3.19 | 59.53 | KCl+CaCl2·6H2O |
| 15 | 37.79 | 2.87 | 59.33 | KCl+CaCl2·6H2O |
| 16 | 38.16 | 2.55 | 59.29 | KCl+CaCl2·6H2O |
| 17 | 38.69 | 2.08 | 59.23 | KCl+CaCl2·6H2O |
| 18 | 38.87 | 2.11 | 59.02 | KCl+CaCl2·6H2O |
| 19 | 38.73 | 2.13 | 59.14 | KCl+CaCl2·6H2O |
| 20 | 38.92 | 2.07 | 59.02 | KCl+CaCl2·6H2O |
| 21 | 38.48 | 0.94 | 60.58 | CaCl2·6H2O |
| 22,A | 39.48 | 0 | 60.52 | CaCl2·6H2O |
| 序号 | 液相点组成w/% | 对应固相 | ||
|---|---|---|---|---|
| CaCl2 | MgCl2 | H2O | ||
| 1,B | 39.52 | 0 | 60.48 | CaCl2·6H2O |
| 2 | 35.46 | 5.39 | 59.15 | CaCl2·6H2O |
| 3 | 33.68 | 7.56 | 58.76 | CaCl2·6H2O |
| 4 | 29.69 | 11.32 | 59.00 | CaCl2·6H2O |
| 5 | 25.91 | 15.37 | 58.72 | CaCl2·6H2O |
| 6 | 23.29 | 18.07 | 58.64 | CaCl2·6H2O+MgCl2·6H2O |
| 7 | 21.59 | 20.17 | 58.25 | CaCl2·6H2O+MgCl2·6H2O |
| 8 | 18.40 | 22.29 | 59.31 | CaCl2·6H2O+MgCl2·6H2O |
| 9 | 17.06 | 24.08 | 58.86 | CaCl2·6H2O+MgCl2·6H2O |
| 10 | 15.99 | 24.84 | 59.17 | CaCl2·6H2O+MgCl2·6H2O |
| 11 | 13.93 | 27.33 | 58.74 | CaCl2·6H2O+MgCl2·6H2O |
| 12 | 11.64 | 28.65 | 59.71 | CaCl2·6H2O+MgCl2·6H2O |
| 13 | 10.22 | 29.46 | 60.32 | MgCl2·6H2O |
| 14 | 7.89 | 31.54 | 60.57 | MgCl2·6H2O |
| 15 | 4.30 | 33.59 | 62.11 | MgCl2·6H2O |
| 16 | 2.14 | 34.77 | 63.09 | MgCl2·6H2O |
| 17 | 1.11 | 35.62 | 63.27 | MgCl2·6H2O |
| 18,A | 0 | 36.86 | 63.14 | MgCl2·6H2O |
Table 2 Supersaturation solubility measurement data and corresponding solid phase of Mg2+, Ca2+//Cl--H2O ternary system at 273.2 K based on the new experimental method
| 序号 | 液相点组成w/% | 对应固相 | ||
|---|---|---|---|---|
| CaCl2 | MgCl2 | H2O | ||
| 1,B | 39.52 | 0 | 60.48 | CaCl2·6H2O |
| 2 | 35.46 | 5.39 | 59.15 | CaCl2·6H2O |
| 3 | 33.68 | 7.56 | 58.76 | CaCl2·6H2O |
| 4 | 29.69 | 11.32 | 59.00 | CaCl2·6H2O |
| 5 | 25.91 | 15.37 | 58.72 | CaCl2·6H2O |
| 6 | 23.29 | 18.07 | 58.64 | CaCl2·6H2O+MgCl2·6H2O |
| 7 | 21.59 | 20.17 | 58.25 | CaCl2·6H2O+MgCl2·6H2O |
| 8 | 18.40 | 22.29 | 59.31 | CaCl2·6H2O+MgCl2·6H2O |
| 9 | 17.06 | 24.08 | 58.86 | CaCl2·6H2O+MgCl2·6H2O |
| 10 | 15.99 | 24.84 | 59.17 | CaCl2·6H2O+MgCl2·6H2O |
| 11 | 13.93 | 27.33 | 58.74 | CaCl2·6H2O+MgCl2·6H2O |
| 12 | 11.64 | 28.65 | 59.71 | CaCl2·6H2O+MgCl2·6H2O |
| 13 | 10.22 | 29.46 | 60.32 | MgCl2·6H2O |
| 14 | 7.89 | 31.54 | 60.57 | MgCl2·6H2O |
| 15 | 4.30 | 33.59 | 62.11 | MgCl2·6H2O |
| 16 | 2.14 | 34.77 | 63.09 | MgCl2·6H2O |
| 17 | 1.11 | 35.62 | 63.27 | MgCl2·6H2O |
| 18,A | 0 | 36.86 | 63.14 | MgCl2·6H2O |
| 序号 | 液相组成w/% | 湿固相组成w/% | 固相 | ||
|---|---|---|---|---|---|
| CaCl2 | KCl | CaCl2 | KCl | ||
| 1,B | 0 | 22.32 | 0 | 57.94 | KCl |
| 2 | 2.81 | 19.66 | 2.01 | 51.69 | KCl |
| 3 | 5.15 | 17.89 | 3.52 | 41.65 | KCl |
| 4 | 8.72 | 14.95 | 5.55 | 39.19 | KCl |
| 5 | 10.47 | 13.62 | 8.18 | 39.02 | KCl |
| 6 | 13.75 | 11.98 | 9.77 | 38.07 | KCl |
| 7 | 16.74 | 10.12 | 11.82 | 38.28 | KCl |
| 8 | 20.23 | 7.58 | 12.85 | 41.54 | KCl |
| 9 | 23.42 | 6.53 | 15.80 | 36.07 | KCl |
| 10 | 25.14 | 5.45 | 17.02 | 32.37 | KCl |
| 11 | 26.90 | 4.49 | 17.91 | 35.41 | KCl |
| 12 | 32.40 | 3.89 | 27.59 | 19.38 | KCl |
| 13 | 34.69 | 3.45 | 18.38 | 47.32 | KCl |
| 14 | 35.48 | 2.83 | 29.58 | 20.22 | KCl |
| 15,E | 36.59 | 2.59 | 35.10 | 10.22 | KCl+CaCl2·6H2O |
| 16 | 37.46 | 1.75 | 42.17 | 1.30 | CaCl2·6H2O |
| 17,A | 38.73 | 0 | 45.31 | 0 | CaCl2·6H2O |
Table 3 Metastable experimental data of the ternary system K+, Ca2+//Cl--H2O at 273.2 K (traditional experimental method)
| 序号 | 液相组成w/% | 湿固相组成w/% | 固相 | ||
|---|---|---|---|---|---|
| CaCl2 | KCl | CaCl2 | KCl | ||
| 1,B | 0 | 22.32 | 0 | 57.94 | KCl |
| 2 | 2.81 | 19.66 | 2.01 | 51.69 | KCl |
| 3 | 5.15 | 17.89 | 3.52 | 41.65 | KCl |
| 4 | 8.72 | 14.95 | 5.55 | 39.19 | KCl |
| 5 | 10.47 | 13.62 | 8.18 | 39.02 | KCl |
| 6 | 13.75 | 11.98 | 9.77 | 38.07 | KCl |
| 7 | 16.74 | 10.12 | 11.82 | 38.28 | KCl |
| 8 | 20.23 | 7.58 | 12.85 | 41.54 | KCl |
| 9 | 23.42 | 6.53 | 15.80 | 36.07 | KCl |
| 10 | 25.14 | 5.45 | 17.02 | 32.37 | KCl |
| 11 | 26.90 | 4.49 | 17.91 | 35.41 | KCl |
| 12 | 32.40 | 3.89 | 27.59 | 19.38 | KCl |
| 13 | 34.69 | 3.45 | 18.38 | 47.32 | KCl |
| 14 | 35.48 | 2.83 | 29.58 | 20.22 | KCl |
| 15,E | 36.59 | 2.59 | 35.10 | 10.22 | KCl+CaCl2·6H2O |
| 16 | 37.46 | 1.75 | 42.17 | 1.30 | CaCl2·6H2O |
| 17,A | 38.73 | 0 | 45.31 | 0 | CaCl2·6H2O |
| 序号 | 液相组成w/% | 湿固相组成w/% | 固相 | ||
|---|---|---|---|---|---|
| CaCl2 | MgCl2 | CaCl2 | MgCl2 | ||
| 1,B | 38.72 | 0 | — | — | CaCl2·6H2O |
| 2 | 34.13 | 4.35 | 44.38 | 1.51 | CaCl2·6H2O |
| 3 | 28.26 | 10.20 | 37.15 | 5.69 | CaCl2·6H2O |
| 4 | 25.23 | 13.00 | 38.49 | 6.17 | CaCl2·6H2O |
| 5 | 20.43 | 17.66 | 27.51 | 13.43 | CaCl2·6H2O |
| 6 | 17.03 | 21.85 | 32.38 | 11.82 | CaCl2·6H2O |
| 7 | 14.79 | 23.63 | 26.18 | 16.29 | CaCl2·6H2O |
| 8,E | 13.75 | 25.22 | 18.71 | 24.91 | CaCl2·6H2O+MgCl2·6H2O |
| 9 | 10.64 | 27.59 | 5.68 | 36.09 | MgCl2·6H2O |
| 10 | 7.57 | 29.51 | 4.91 | 34.80 | MgCl2·6H2O |
| 11 | 3.57 | 33.25 | 3.05 | 36.79 | MgCl2·6H2O |
| 12 | 2.08 | 33.86 | 1.25 | 39.38 | MgCl2·6H2O |
| 13,A | 0 | 35.69 | — | — | MgCl2·6H2O |
Table 4 Metastable experimental data of the ternary system Mg2+, Ca2+//Cl--H2O at 273.2 K (traditional experimental method)
| 序号 | 液相组成w/% | 湿固相组成w/% | 固相 | ||
|---|---|---|---|---|---|
| CaCl2 | MgCl2 | CaCl2 | MgCl2 | ||
| 1,B | 38.72 | 0 | — | — | CaCl2·6H2O |
| 2 | 34.13 | 4.35 | 44.38 | 1.51 | CaCl2·6H2O |
| 3 | 28.26 | 10.20 | 37.15 | 5.69 | CaCl2·6H2O |
| 4 | 25.23 | 13.00 | 38.49 | 6.17 | CaCl2·6H2O |
| 5 | 20.43 | 17.66 | 27.51 | 13.43 | CaCl2·6H2O |
| 6 | 17.03 | 21.85 | 32.38 | 11.82 | CaCl2·6H2O |
| 7 | 14.79 | 23.63 | 26.18 | 16.29 | CaCl2·6H2O |
| 8,E | 13.75 | 25.22 | 18.71 | 24.91 | CaCl2·6H2O+MgCl2·6H2O |
| 9 | 10.64 | 27.59 | 5.68 | 36.09 | MgCl2·6H2O |
| 10 | 7.57 | 29.51 | 4.91 | 34.80 | MgCl2·6H2O |
| 11 | 3.57 | 33.25 | 3.05 | 36.79 | MgCl2·6H2O |
| 12 | 2.08 | 33.86 | 1.25 | 39.38 | MgCl2·6H2O |
| 13,A | 0 | 35.69 | — | — | MgCl2·6H2O |
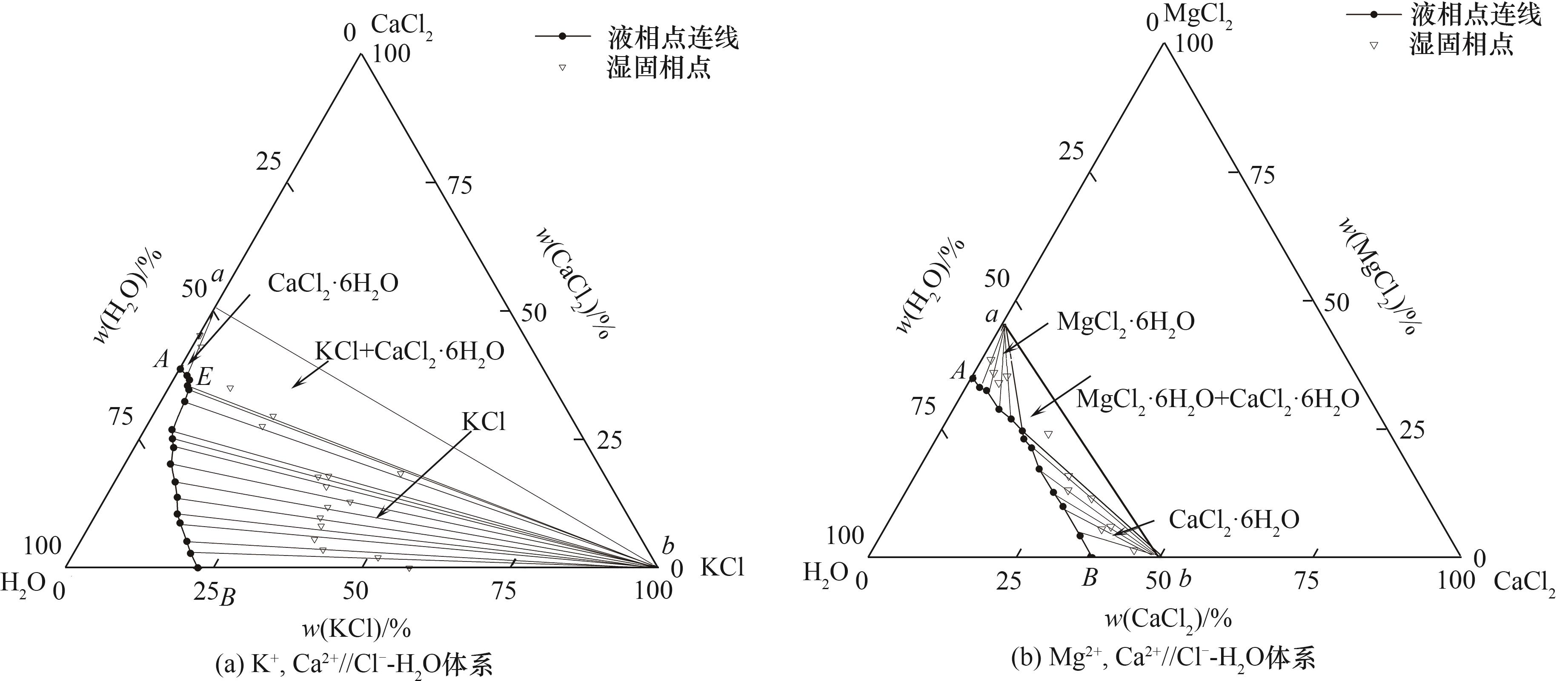
Fig.8 Determination of the solid phase corresponding to the metastable phase diagram of the ternary systems at 273.2 K by wet-residue method (traditional experimental method)
| 1 | 于旭东, 李琪, 陈念粗, 等. 三元体系KCl+CaCl2+H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| Yu X D, Li Q, Chen N C, et al. Phase equilibria and calculation of aqueous ternary system KCl+CaCl2+H2O at 298.2, 323.2, and 348.2 K[J]. CIESC Journal, 2023, 74(8): 3256-3265. | |
| 2 | 冯霞, 于雪峰, 姚智豪, 等. 三元体系Na+(Mg2+), Ca2+//Cl--H2O 278.2 K稳定相平衡研究[J]. 无机盐工业, 2024, 56(1): 47-52. |
| Feng X, Yu X F, Yao Z H, et al. Study on phase equilibria of aqueous ternary system of Na+(Mg2+), Ca2+//Cl--H2O at 278.2 K[J]. Inorganic Chemicals Industry, 2024, 56(1): 47-52. | |
| 3 | 赵志星, 姚智豪, 于雪峰, 等. 锂钠镁共存硫酸盐体系多温相图及其应用[J]. 化工学报, 2024, 75(6): 2123-2133. |
| Zhao Z X, Yao Z H, Yu X F, et al. Multi-temperature phase diagram of lithium-sodium-magnesium coexistence sulfate system and its application[J]. CIESC Journal, 2024, 75(6): 2123-2133. | |
| 4 | 杨游胜, 姚智豪, 赵志星, 等. 富锂硫酸盐型盐湖卤水蒸发实验研究进展[J]. 无机盐工业, 2024, 56(4): 1-7. |
| Yang Y S, Yao Z H, Zhao Z X, et al. Research progress of lithium-rich sulfate type salt lake brine evaporation experiment[J]. Inorganic Chemicals Industry, 2024, 56(4): 1-7. | |
| 5 | 陈科, 杜理, 曾英, 等. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| Chen K, Du L, Zeng Y, et al. Phase equilibria and calculation of quaternary system LiCl+MgCl2+CaCl2+H2O at 323.2 K[J]. CIESC Journal, 2023, 74(5): 1896-1903. | |
| 6 | 赵志星, 姚智豪, 黄琴, 等. 四元体系Li2SO4+Na2SO4+K2SO4+H2O 298.2 K相平衡研究[J]. 盐湖研究, 2022, 30(4): 41-49. |
| Zhao Z X, Yao Z H, Huang Q, et al. Phase equilibria of aqueous quaternary system Li2SO4+Na2SO4+K2SO4+H2O at 298.2 K[J]. Journal of Salt Lake Research, 2022, 30(4): 41-49. | |
| 7 | 任思颖, 于旭东, 罗军, 等. 298.2 K四元体系Li+, K+, N H 4 + //Cl--H2O相平衡研究[J]. 化工学报, 2022, 73(10): 4335-4344. |
| Ren S Y, Yu X D, Luo J, et al. Phase equilibria of aqueous quaternary system Li+, K+, N H 4 + //Cl--H2O at 298.2 K[J]. CIESC Journal, 2022, 73(10): 4335-4344. | |
| 8 | Li J, Zhou H, Yang J, et al. Solid-liquid phase equilibria of Li+, Na+, Ca2+//Cl--H2O quaternary system at 298.15 and 323.15 K[J]. Journal of Chemical & Engineering Data, 2024, 69(3): 1380-1386. |
| 9 | Steiger M, Voigt W. Solid-liquid metastable equilibria for solar evaporation of brines and solubility determination: a critical discussion[J]. Journal of Solution Chemistry, 2019, 48(7): 1009-1024. |
| 10 | 李栋婵. Na+, K+, Ca2+//Cl--H2O四元体系15℃、35℃介稳相平衡的研究[D]. 成都: 成都理工大学, 2007. |
| Li D C. Studies on the meta-stable equilibrium of the quaternary system Na+, K+, Ca2+//Cl--H2O at 15℃ and 35℃[D]. Chengdu: Chengdu University of Technology, 2007. | |
| 11 | Deng T L, Li D C, Wang S Q. Metastable phase equilibrium in the aqueous ternary system (KCl-CaCl2-H2O) at (288.15 and 308.15) K[J]. Journal of Chemical & Engineering Data, 2008, 53(4): 1007-1011. |
| 12 | Edanovsky A B, Lyakhovskaya E, Shleymovich R E. Handbook of Experimental Data on the Solubility of Multicomponent Water-Salt Systems (Volume One: Three-Component Systems)[M]. Leningrad: Goskhimizdat Publ., 1953. |
| 13 | Edanovsky A B, Lyakhovskaya E I, Shleymovich R E. Handbook of Experimental Data on the Solubility of Multicomponent Water-Salt Systems (Volume Two: Four-Component and More Complex Systems)[M]. Leningrad: Goskhimizdat Publ., 1954. |
| 14 | Silcock H L. Solubilities of Inorganic and Organic Compounds (Volume Three Part Two)[M]. Oxford: Pergamon Press, 1979. |
| 15 | Silcock H L. Solubilities of Inorganic and Organic Compounds (Volume Three Part Three)[M]. Oxford: Pergamon Press, 1979. |
| 16 | Pelsh A D. Handbook of Experimental Data on Solubility Multi-Component Salt-Water Systems[M]. Leningrad Department: Leningrad Publishing, 1973. |
| 17 | Igelsrud I, Thompson T G. Equilibria in the saturated solutions of salts occurring in sea water (Ⅰ): The ternary systems MgCl2-KCl-H2O, MgCl2-CaCl2-H2O, CaCl2-KCl-H2O and CaCl2-NaCl-H2O at 0°[J]. Journal of the American Chemical Society, 1936, 58(2): 318-322. |
| 18 | Lightfoot W J, Prutton C F. Equilibria in saturated solutions(1): The ternary systems CaCl2-MgCl2-H2O, CaCl2-KCl-H2O, and MgCl2-KCl-H2O at 35-degrees[J]. Journal of the American Chemical Society, 1946, 68(6): 1001-1002. |
| 19 | Lightfoot W J, Prutton C F. Equilibria in saturated salt solutions(2): The ternary systems CaCl2-MgCl2-H2O, CaCl2-KCl-H2O and MgCl2-KCl-H2O at 75-degrees[J]. Journal of the American Chemical Society, 1947, 69(9): 2098-2100. |
| 20 | Assarsson G O. Equilibria in aqueous systems containing K+, Na+, Ca2+, Mg2+ and Cl-(1): The ternary system CaCl2-KCl-H2O[J]. Journal of the American Chemical Society, 1950, 72(4): 1433-1436. |
| 21 | Assarsson G O. Equilibria in aqueous systems containing K+, Na+, Ca2+, Mg2+ and Cl-(3): The ternary system CaCl2-MgCl2-H2O[J]. Journal of the American Chemical Society, 1950, 72(4): 1442-1444. |
| 22 | Yang J M, Liu X L, Liang P P. Solubilities of salts in the ternary systems NaCl+CaCl2+H2O and KCl+CaCl2+H2O at 75℃[J]. Russian Journal of Physical Chemistry A, 2011, 85(7): 1149-1154. |
| 23 | 姚智豪, 孟浩, 于旭东, 等. 三元体系KCl+CaCl2+H2O在278.2 K及308.2 K下的稳定相平衡研究[J]. 矿产保护与利用, 2021, 41(6): 112-116. |
| Yao Z H, Meng H, Yu X D, et al. Stable phase equilibria of ternary system KCl+CaCl2+H2O at 278.2 K and 308.2 K[J]. Conservation and Utilization of Mineral Resources, 2021, 41(6): 112-116. | |
| 24 | Assarsson G O. Equilibria in aqueous systems containing K+, Na+, Ca2+, Mg2+ and Cl-(2): The quaternary system CaCl2-KCl-NaCl-H2O[J]. Journal of the American Chemical Society, 1950, 72(4): 1437-1441. |
| 25 | 李华山, 张游, 李飞, 等. 303.15 K三元体系 CaCl2-MgCl2-H2O相平衡实验及计算[J]. 化学工程, 2022, 50(8): 40-45. |
| Li H S, Zhang Y, Li F, et al. Phase equilibrium experiments and calculations in ternary system CaCl2-MgCl2-H2O at 303.15 K[J]. Chemical Engineering (China), 2022, 50(8): 40-45. | |
| 26 | 郑秋风. 五元体系 N H 4 + , Mg2+, Ca2+(Sr2+), Al3+//Cl--H2O及其相关子体系298.2 K相平衡研究[D]. 成都: 成都理工大学, 2021. |
| Zheng Q F. Stable phase equilibria of the quinary system N H 4 + , Mg2+, Ca2+(Sr2+), Al3+//Cl--H2O and its related subsystems at 298.2 K[D]. Chengdu: Chengdu University of Technology, 2021. | |
| 27 | 刘敏. 五元体系 N H 4 + , Mg2+, Ca2+, Sr2+//Cl--H2O及其部分四元子体系298.2 K稳定相平衡研究[D]. 成都: 成都理工大学, 2020. |
| Liu M. Stable phase equilibria of the quinary system N H 4 + , Mg2+, Ca2+, Sr2+//Cl--H2O and its partial quaternary subsystems at 298.2 K[D]. Chengdu: Chengdu University of Technology, 2020. | |
| 28 | Zhang F, Zeng Y, Yu X D, et al. Solid-liquid phase equilibria determination of the quaternary system Na+, K+, Ca2+//Cl--H2O at 348.2 and 363.2 K[J]. Journal of Chemical & Engineering Data, 2024, 69(4): 1740-1746. |
| 29 | Li D C, Shi T, Zhao, H X, et al. Phase equilibria in the quaternary systems (KCl+CaCl2+SrCl2+H2O) and (LiCl+NaCl+SrCl2+ H2O) at 288.15 K and 0.1 MPa[J]. Journal of Chemical & Engineering Data, 2020, 65(11): 5266-5274. |
| 30 | Sun L J, Zeng Y, Yu X D, et al. Solid-liquid equilibria of the quaternary water-salt system Na+, Mg2+, Ca2+//Cl--H2O at 348.2 and 363.2 K[J]. Journal of Chemical & Engineering Data, 2024, 69(3): 1263-1272. |
| 31 | 黄志强, 李健, 苏杭, 等. 癸二酸溶解度、介稳区及晶体生长速率的测定[J]. 中国油脂, 2024, 49(7): 96-100. |
| Huang Z Q, Li J, Su H, et al. Determination of solubility, metastable zone and crystal growth rate for sebacic acid[J]. China Oils and Fats, 2024, 49(7): 96-100. | |
| 32 | 邹洋, 陆志艳, 胡志林, 等. KNO3介稳区宽度的研究及初级成核动力学计算[J]. 无机盐工业, 2024, 56(9): 67-74. |
| Zou Y, Lu Z Y, Hu Z L, et al. Study on the metastable zone width and the primary nucleation kinetics for cooling crystallization of KNO3 [J]. Inorganic Chemicals Industry, 2024, 56(9): 67-74. | |
| 33 | 张梁, 马骥, 贺高红, 等. 膜调控的头孢呋辛钠溶析-冷却耦合结晶成核介稳区测定及分析[J]. 化工进展, 2024, 43(1): 260-268. |
| Zhang L, Ma J, He G H, et al. Determination and analysis of combined cooling and antisolvent crystallization metastable zone width of cefuroxime sodium with membrane regulation[J]. Chemical Industry and Engineering Progress, 2024, 43(1): 260-268. | |
| 34 | 孙雅, 周通, 陈广源, 等. 鸟粪石结晶过程溶解度、介稳区及诱导期的测定[J]. 南京工业大学学报(自然科学版), 2023, 45(1): 52-59. |
| Sun Y, Zhou T, Chen G Y, et al. Determination of solubility, metastability zone and induction period of struvite during crystallization[J]. Journal of Nanjing Tech University (Natural Science Edition), 2023, 45(1): 52-59. | |
| 35 | 周星屹, 闫樊钰慧, 曹金乐, 等. HNS溶解度与介稳区的光流控测定方法研究[J]. 含能材料, 2022, 30(5): 431-438. |
| Zhou X Y, Yan F Y H, Cao J L, et al. Measuring method of solubility and metastable zone of HNS based on optofluidics[J]. Chinese Journal of Energetic Materials, 2022, 30(5): 431-438. | |
| 36 | 王逸夫, 金央, 李军. 微尺度流动体系的介稳区测定方法及影响[J]. 无机盐工业, 2022, 54(2): 45-49. |
| Wang Y F, Jin Y, Li J. Measurement method and effect of metastable zone of microscale flow system[J]. Inorganic Chemicals Industry, 2022, 54(2): 45-49. | |
| 37 | 丁瑞, 马钧亮, 鹿桂花, 等. 2-金刚烷酮在4种溶剂中的溶解度关联及介稳区测定[J]. 化学工程, 2021, 49(9): 34-39. |
| Ding R, Ma J L, Lu G H, et al. Correlation of solubility and determination of metastable zone for 2-adamantinonec in four kinds of solvents[J]. Chemical Engineering (China), 2021, 49(9): 34-39. | |
| 38 | 郭盛争, 吴送姑, 苏鑫, 等. 莱鲍迪苷A溶解度与介稳区宽度的测定及其结晶过程研究[J]. 化工学报, 2021, 72(8): 3997 -4008. |
| Guo S Z, Wu S G, Su X, et al. Determination of solubility and metastable zone width of rebaudioside A and study on its crystallization process[J]. CIESC Journal, 2021, 72(8): 3997-4008. | |
| 39 | 中国科学院青海盐湖研究所. 卤水和盐的分析方法[M]. 2版. 北京: 科学出版社, 1988. |
| Qinghai Institute of Salt Lakes of Chinese Academy of Sciences. Analysis Method of Brine and Salt[M]. 2nd ed. Beijing: Science Press, 1988. | |
| 40 | 成怀刚, 程芳琴. 水盐体系相分离[M]. 北京: 冶金工业出版社, 2022. |
| Cheng H G, Cheng F Q. Phase Separation of Salt-Water System[M]. Beijing: Metallurgical Industry Press, 2022. | |
| 41 | 冉广芬, 马海州, 孟瑞英, 等. 四苯硼钠-季铵盐容量法快速测钾[J]. 盐湖研究, 2009, 17(2): 39-42. |
| Ran G F, Ma H Z, Meng R Y, et al. Rapid determination of potassium content by sodium tetraphenylboron quaternary ammonium salt volumetric method[J]. Journal of Salt Lake Research, 2009, 17(2): 39-42. | |
| 42 | 苟国敬, 高世扬, 夏树屏, 等. MgO·nB2O3-18%MgSO4-H2O体系0°C时的热力学非平衡态液固相关系[J]. 化学学报, 2003, 61(9): 1434-1440. |
| Gou G J, Gao S Y, Xia S P, et al. Liquid-solid phase diagram of thermodynamic non-equilibrium state of MgO·nB2O3-18%MgSO4-H2O system at 0℃[J]. Acta Chimica Sinica, 2003, 61(9): 1434-1440. | |
| 43 | Zhou H, Zhang H L, Chen Y D, et al. Salt-forming regions of the Na+, Mg2+//Cl-, S O 4 2 - -H2O system at 348.15 K in the nonequilibrium state of isothermal boiling evaporation[J]. Journal of Chemical & Engineering Data, 2012, 57(3): 943-951. |
| 44 | Zhou H, Zhang J B, Zhang H L, et al. Salt-forming regions of Na+, Mg2+//Cl-, S O 4 2 - -H2O system at 373.15 K in the nonequilibrium state of isothermal boiling evaporation[J]. Journal of Chemical & Engineering Data, 2012, 57(4): 1192-1202. |
| 45 | Zhou H, Bao Y J, Bai X Q, et al. Salt-forming regions of seawater type solution in the evaporation and fractional crystallization process[J]. Fluid Phase Equilibria, 2014, 362: 281-287. |
| [1] | Chuangde ZHANG, Li CHEN. Pore-scale study of effects of preferential path on multiphase reactive transport process in porous media [J]. CIESC Journal, 2025, 76(1): 161-172. |
| [2] | Xinyue WANG, Xiaohu XU, Haiyang ZHANG, Chunhua YIN. Study on encapsulation and properties vitamin A acetate/cyclodextrin [J]. CIESC Journal, 2024, 75(S1): 321-328. |
| [3] | Junyong HU, Yali HU, Xueyi TAN, Jiaxin HUANG, Lewei ZHANG, Junli ZENG, Xiaoyi LIU, Yuan TAO. Experimental study on the performance of multi-stage reverse electrodialysis based on LiCl-NH4Cl aqueous solution [J]. CIESC Journal, 2024, 75(7): 2670-2679. |
| [4] | Zhixing ZHAO, Zhihao YAO, Xuefeng YU, Yousheng YANG, Ying ZENG, Xudong YU. Multi-temperature phase diagram of lithium-sodium-magnesium coexistence sulfate system and its application [J]. CIESC Journal, 2024, 75(6): 2123-2133. |
| [5] | Wenchao JIANG, Zhaochao XU. Fluorescent dyes for super-resolution imaging of organelles [J]. CIESC Journal, 2024, 75(4): 1333-1354. |
| [6] | Youming SI, Lingfeng ZHENG, Pengzhong CHEN, Jiangli FAN, Xiaojun PENG. Performance and mechanism of novel antimony oxo cluster photoresist [J]. CIESC Journal, 2024, 75(4): 1705-1717. |
| [7] | Yuxiang CHEN, Chuanlei LIU, Zijun GONG, Qiyue ZHAO, Guanchu GUO, Hao JIANG, Hui SUN, Benxian SHEN. Machine learning-assisted solvent molecule design for efficient absorption of ethanethiol [J]. CIESC Journal, 2024, 75(3): 914-923. |
| [8] | Yuxi WU, Yuanhui TANG, Qiang GUO, Yakai LIN, Lixin YU, Xiaolin WANG. Experimental study and simulation on nanofiltration separation of lithium and magnesium from sulfate desorption solution [J]. CIESC Journal, 2024, 75(12): 4563-4575. |
| [9] | Han TANG, Jin CAI, Haihang QIN, Guangjin CHEN, Changyu SUN. Predictive model on gas solubility in water-rich phase coexisted with gas hydrates [J]. CIESC Journal, 2024, 75(11): 4348-4358. |
| [10] | Mi FENG, Jie ZHANG, Xingmei LYU. One-step extraction and separation of high purity chitin based on choline ionic liquid [J]. CIESC Journal, 2024, 75(11): 4286-4297. |
| [11] | Ning XU, Qinglong QIAO, Zhaochao XU. Advancements of fluorescent dyes for advanced biological imaging applications [J]. CIESC Journal, 2024, 75(11): 4082-4094. |
| [12] | Jialin ZHANG, Dawei XU, Yue GAO, Xingang LI. Performance of soot combustion over CeO2 modified CuO catalysts supported on nickel foams [J]. CIESC Journal, 2024, 75(1): 312-321. |
| [13] | Minghui CHANG, Lin WANG, Jiajia YUAN, Yifei CAO. Study on the cycle performance of salt solution-storage-based heat pump [J]. CIESC Journal, 2023, 74(S1): 329-337. |
| [14] | Chao HU, Yuming DONG, Wei ZHANG, Hongling ZHANG, Peng ZHOU, Hongbin XU. Preparation of high-concentration positive electrolyte of vanadium redox flow battery by activating vanadium pentoxide with highly concentrated sulfuric acid [J]. CIESC Journal, 2023, 74(S1): 338-345. |
| [15] | Zhenghao JIN, Lijie FENG, Shuhong LI. Energy and exergy analysis of a solution cross-type absorption-resorption heat pump using NH3/H2O as working fluid [J]. CIESC Journal, 2023, 74(S1): 53-63. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||