CIESC Journal ›› 2025, Vol. 76 ›› Issue (2): 519-531.DOI: 10.11949/0438-1157.20240806
• Reviews and monographs • Previous Articles Next Articles
Xiaohang ZHONG1( ), Wei XU2, Wen ZHANG1, Li XU1, Yuxin WANG1(
), Wei XU2, Wen ZHANG1, Li XU1, Yuxin WANG1( )
)
Received:2024-07-17
Revised:2024-09-09
Online:2025-03-10
Published:2025-02-25
Contact:
Yuxin WANG
通讯作者:
王宇新
作者简介:钟晓航(1999—),女,硕士研究生,zhongxiaohang@tju.edu.cn
CLC Number:
Xiaohang ZHONG, Wei XU, Wen ZHANG, Li XU, Yuxin WANG. A critical review on the effects of Fe impurity on H2 production via alkaline water electrolysis[J]. CIESC Journal, 2025, 76(2): 519-531.
钟晓航, 许卫, 张文, 许莉, 王宇新. 碱性水电解制氢中铁杂质的影响研究进展[J]. 化工学报, 2025, 76(2): 519-531.
Add to citation manager EndNote|Ris|BibTeX
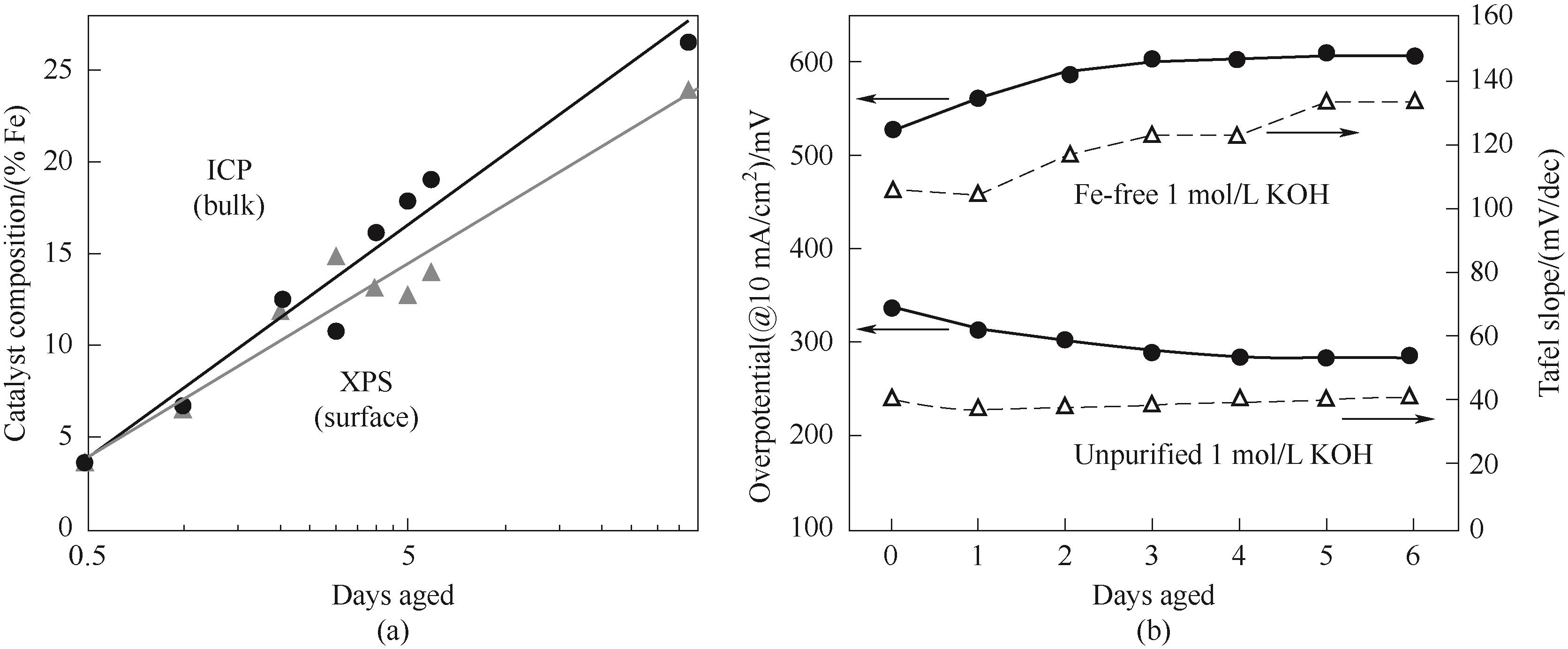
Fig.2 (a) Fe content of Ni(OH)2 films versus days of aging in unpurified 1 mol/L KOH;(b) Overpotentials and Tafel slopes of Ni(OH)2 films in Fe-free and unpurified 1 mol/L KOH at 10 mA/cm2[46]
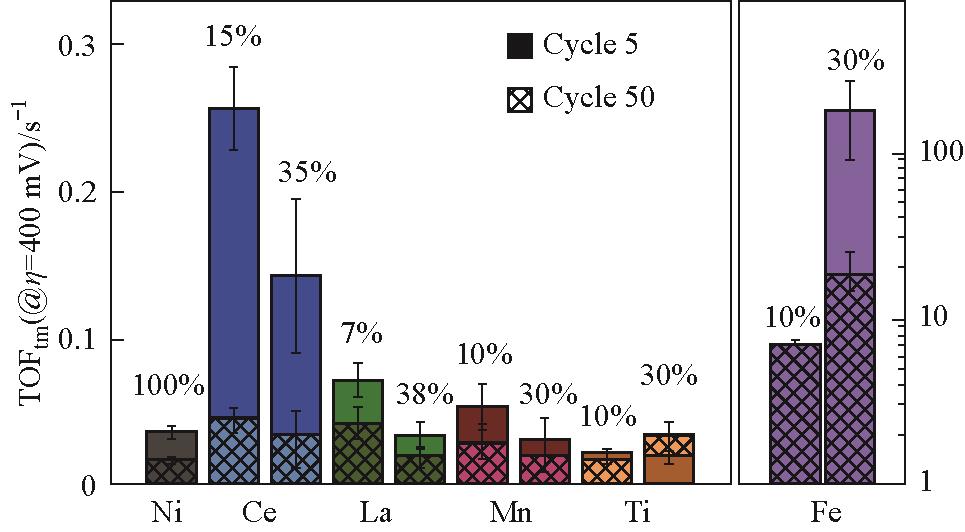
Fig.3 OER turnover frequency of Ni1-z M z O x H y films at 400 mV overpotential at cycle 5 and cycle 50 in Fe-free 1 mol/L KOH; Values reported are the average, and error bars are the standard deviation of three samples; The error bars of Ni0.9Fe0.1O x H y are small and not visible on this scale; TOFtm are calculated assuming all metal cations are active (and thus are lower limits)[53]
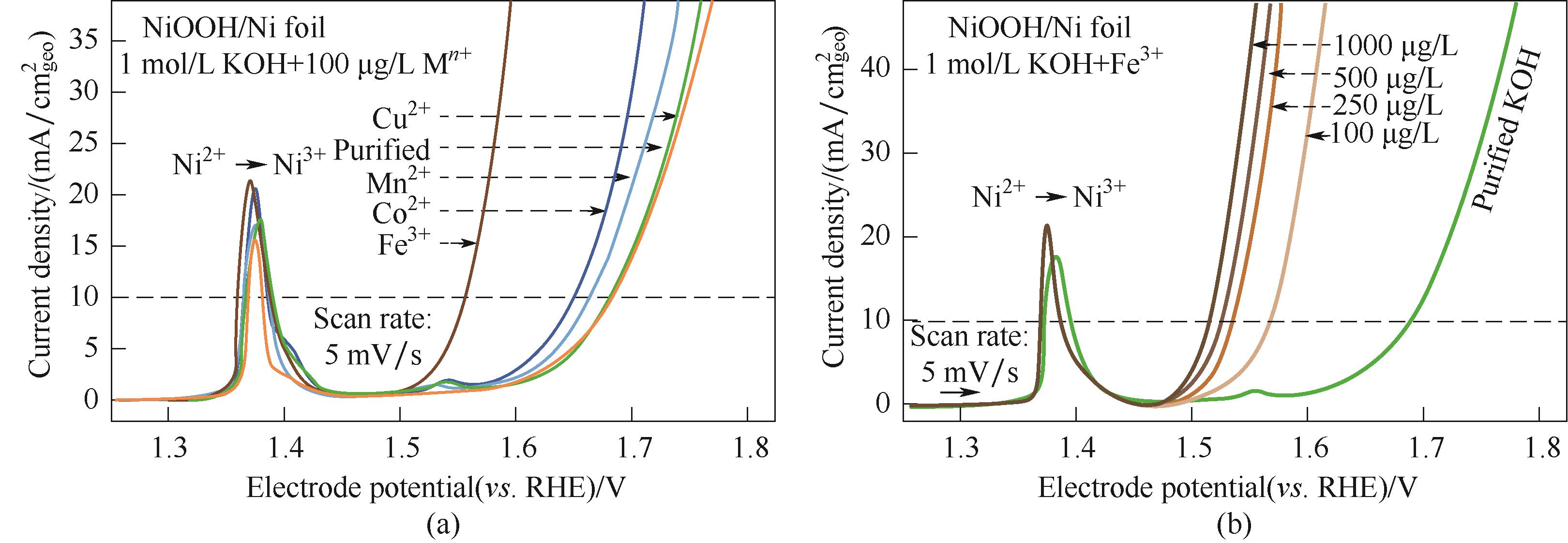
Fig.4 Linear sweep voltammograms: (a) variations of OER performance of NF-NiOOH electrodes after CP conditioning in purified 1 mol/L KOH electrolyte spiked with transition metal cations (100 μg/L M n+); (b) Influence of the Fe3+ concentration on the electrochemical performance of NF-NiOOH electrodes[55]
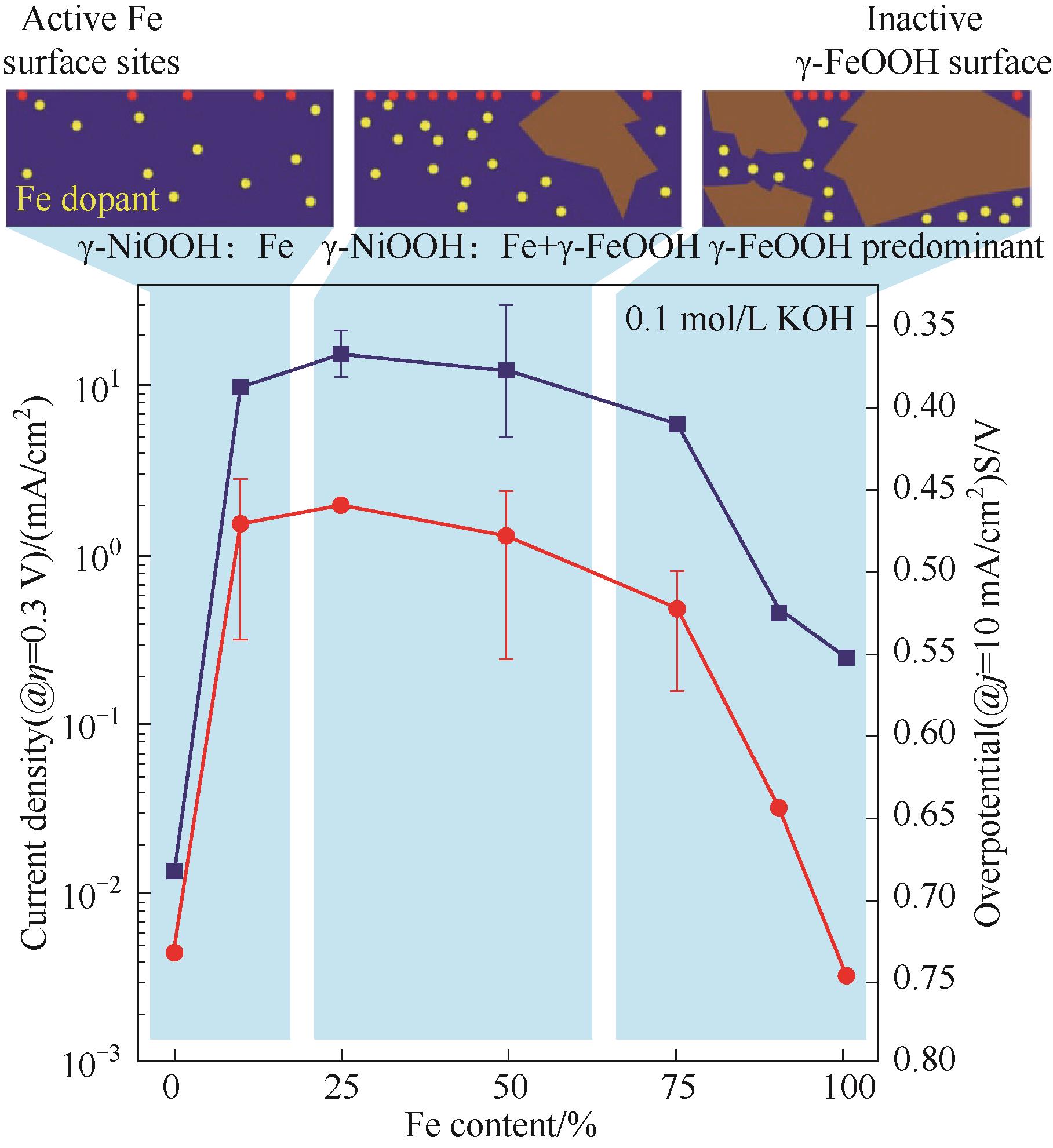
Fig.5 Measured OER activity of mixed Ni-Fe catalysts as a function of Fe content in 0.1 mol/L KOH; For 0% Fe, measurements were performed in electrolyte which was carefully purified to remove any Fe contamination; Top: a schematic illustrating the influence of Fe content on the competing formation of highly active Fe sites in γ-NiOOH and of phase-separated low-activity γ-FeOOH[56]
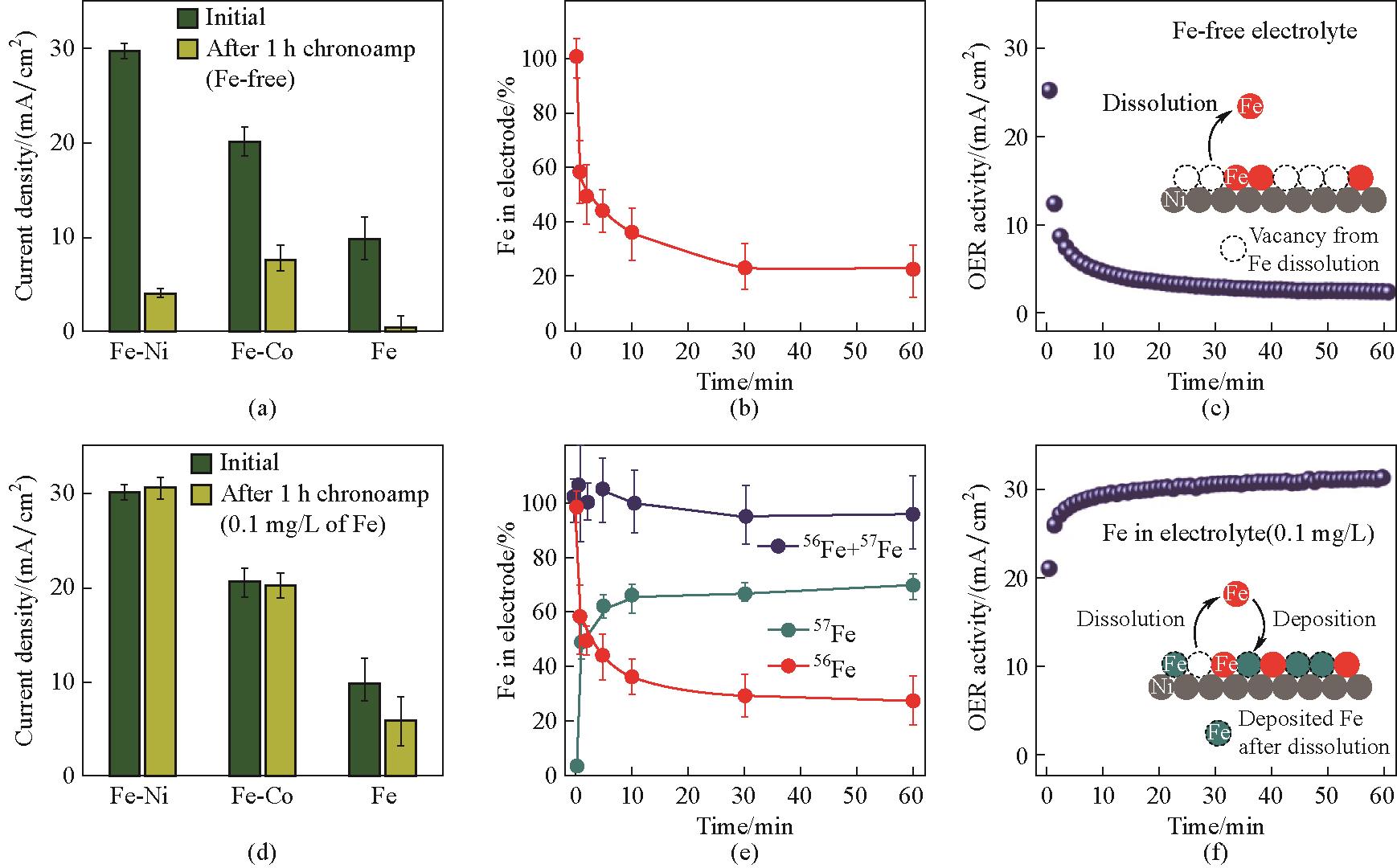
Fig.6 Activity-stability trend of Fe-M hydr(oxy)oxides and observation of dynamic Fe exchange by isotopic labelling experiments: summary of the results of the activity-stability study of Fe-M hydr(oxy)oxides during chronoamperometry experiments at 1.7 V for 1 h in ‘Fe-free’ purified KOH (a) and in a KOH solution containing 0.1 mg/L Fe (d); the total amount of Fe in the Fe-NiO x H y electrode [(b),(e)] and OER activity [(c),(f)] during chronoamperometry measurements at 1.7 V; the schematic diagram in (c) depicts the dissolution process; similar chronoamperometry experiments performed in electrolyte containing 0.1 mg/L 57Fe[(e),(f)] reveal Fe dynamic exchange (dissolution and redeposition) at the interface during OER catalysis; the schematic diagram in (f) depicts both the Fe dissolution and redeposition processes[26]
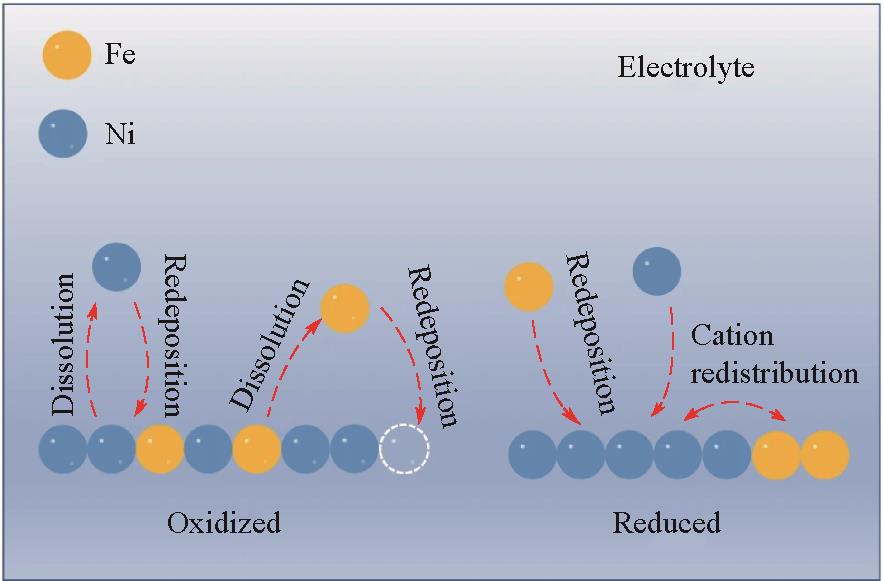
Fig.7 Mechanism for reversible phase segregation: at the OER potential, the dissolution of Fe and site-selective redeposition of Fe lead to phase segregation, whereas at the reduction state, the redistribution of metal cations and homogeneous Fe redeposition alleviate the phase segregation[59]
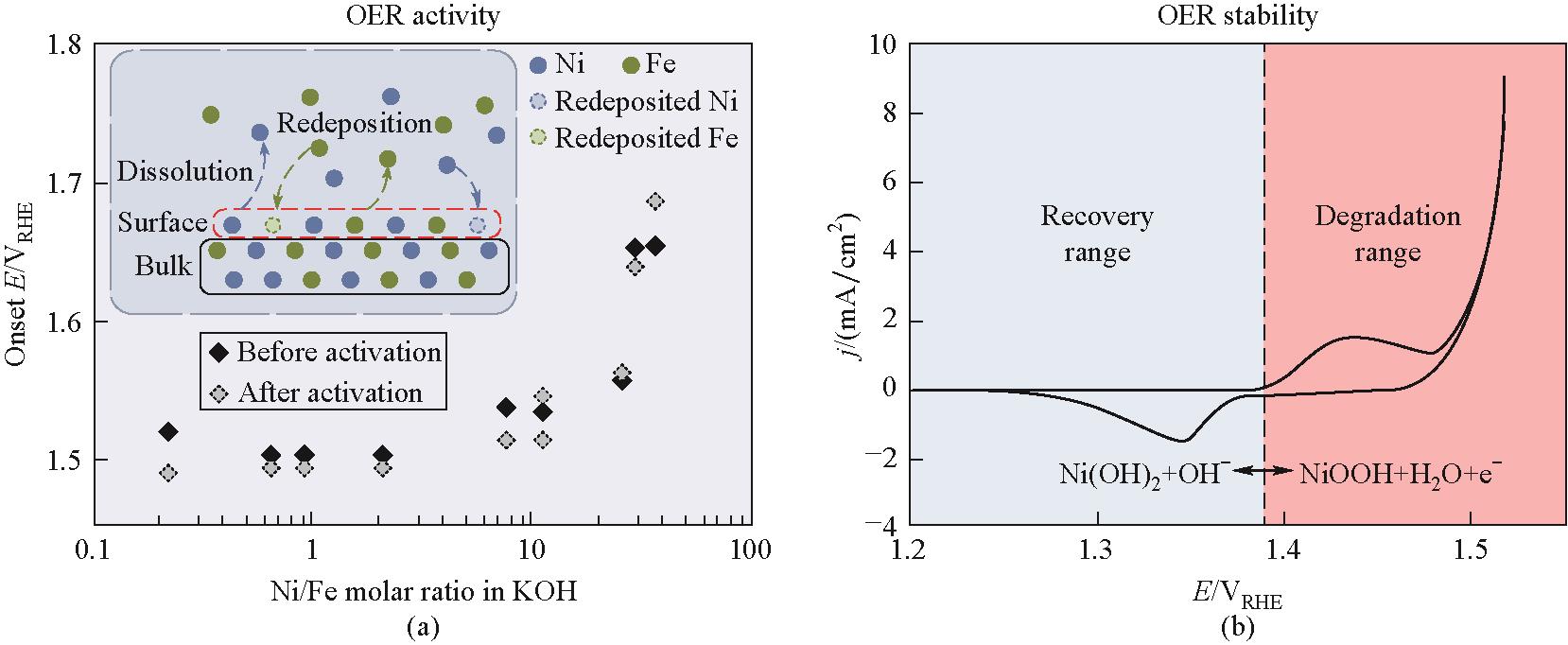
Fig.9 (a)Change of OER onset potential with Ni/Fe molar ratio in KOH solution; (b)Schematic of electrode degradation and recovery potential ranges[62]
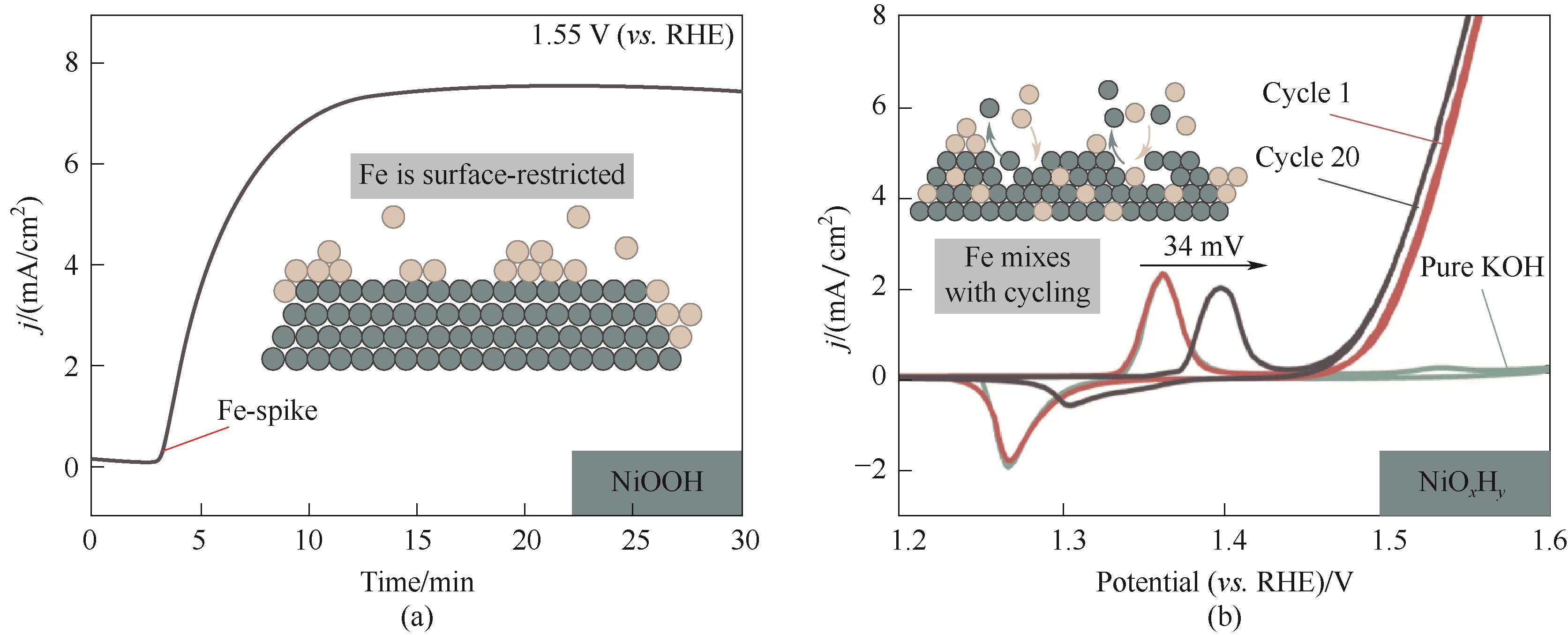
Fig.10 (a) CA measurements of NiOOH at 1.55 V (vs. RHE), after starting the measurement in purified Fe-free 1 mol/L KOH electrolyte, aqueous Fe(NO3)3 was added to a concentration of 0.1 mg/L; (b) CV test after peak Fe doping in (a)[58]
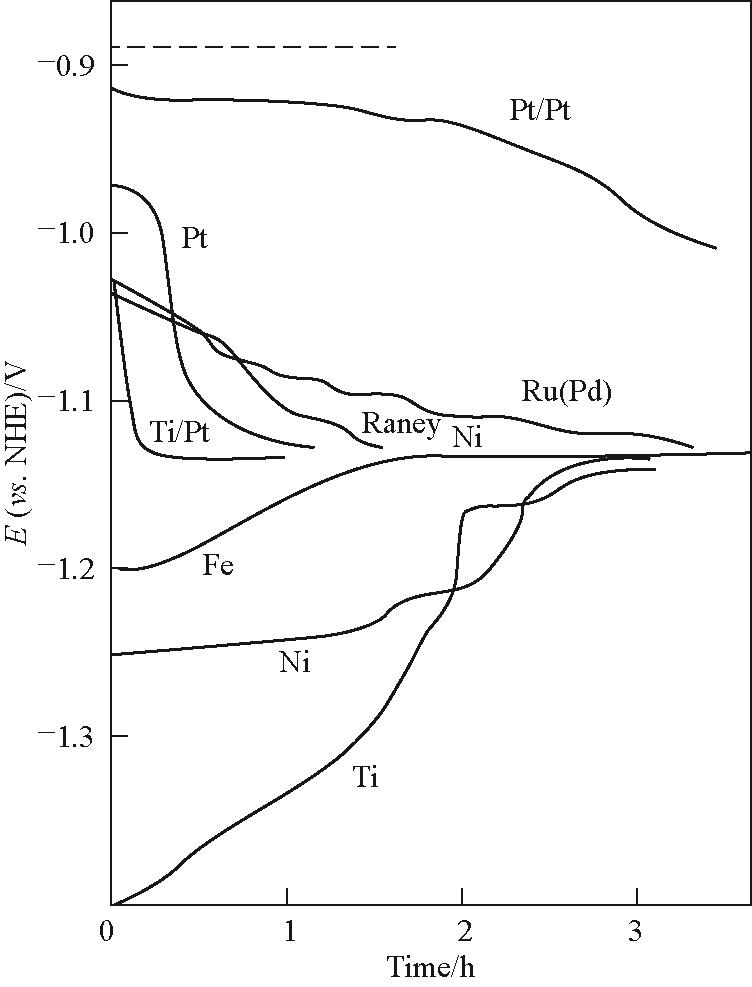
Fig.11 Variation of the cathode potential with time during the course of the HER from 33%(mass) NaOH at 90℃ and 3 kA/m2 in the presence of 10 mg/L Fe; The dashed line represents the reversible potential[63]
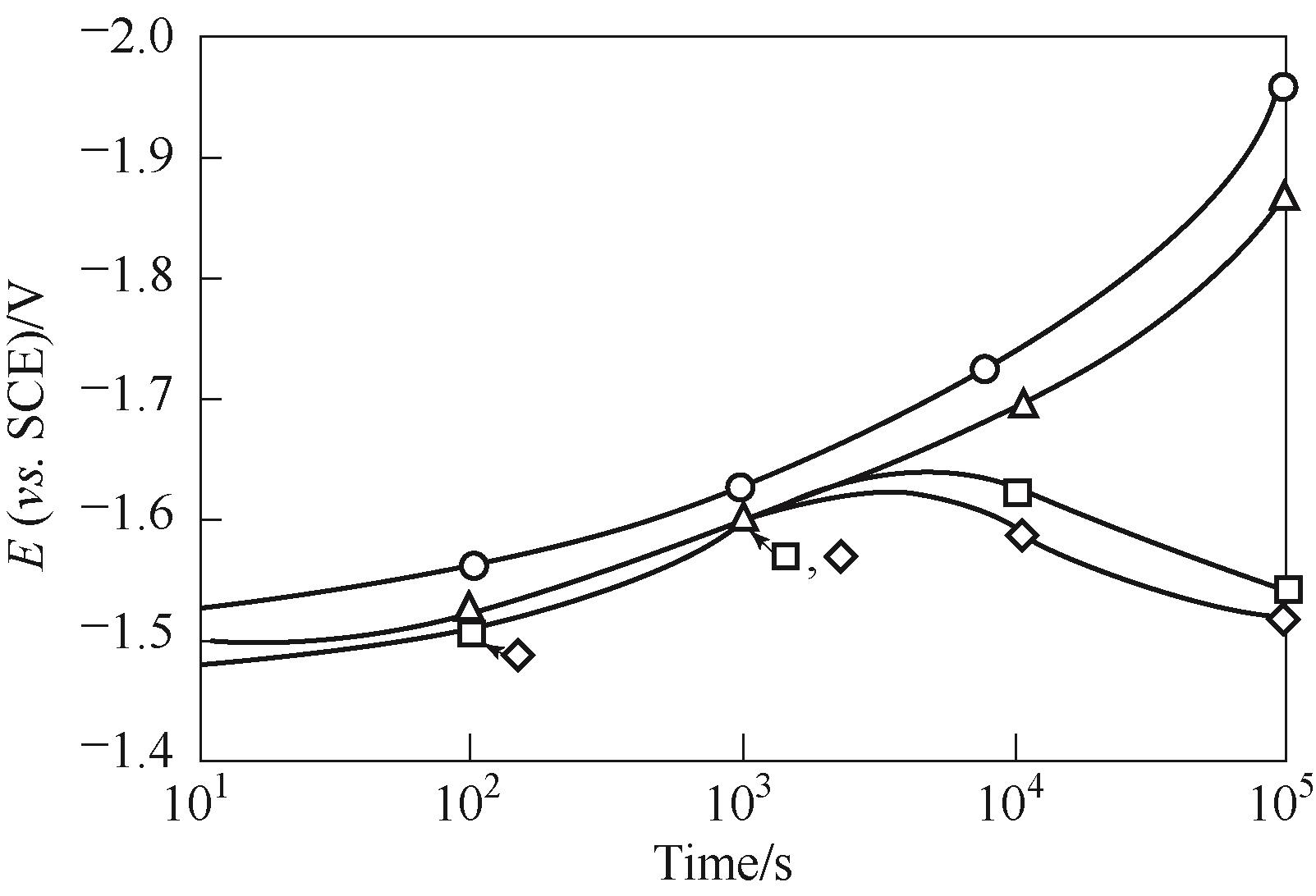
Fig.12 Nickel cathode potential behavior with time under galvanostatic control of 250 mA/cm2 in 30%(mass) KOH at 37℃ ( for 0.03 mg/L dissolved iron; for 3.0 mg/L dissolved iron)[64]
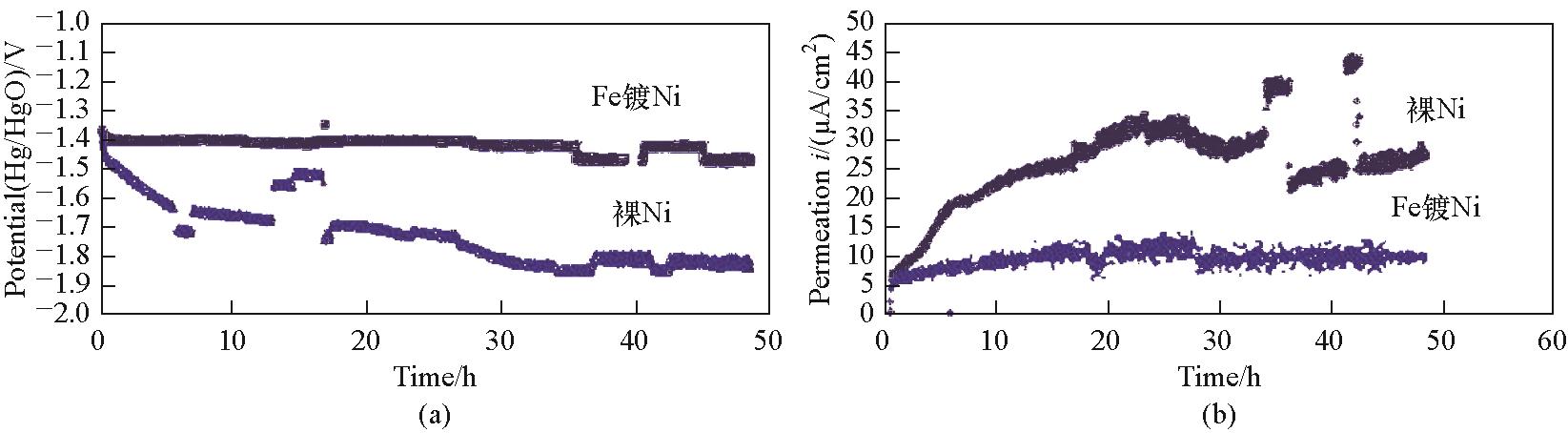
Fig.13 (a) Cathode polarization potentials during longer term experiments; (b) Hydrogen permeation currents during longer term experiments; T = 70℃, charging current = 100 mA/cm2, 100 s averaged currents from three replicate experiments for bare Ni and Ni with Fe coating[67]
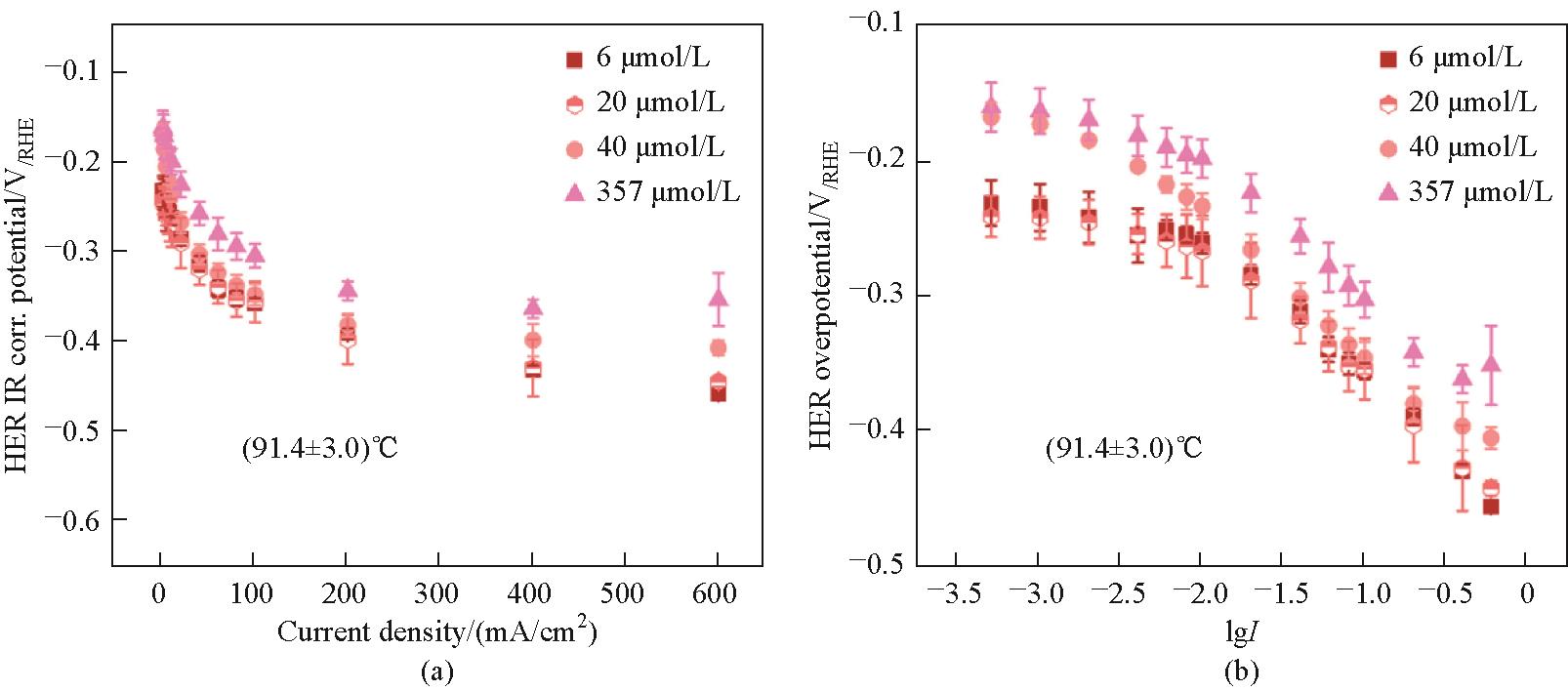
Fig.14 I-V curves for internal resistance corrected HER potential (a) and Tafel plots (b) for HER at 91℃ for Fe electrolyte concentrations of 6, 20, 40, and 357 μmol/L; Note that for the Tafel plots the logarithmic value of the current density was used in A/cm2; Data are represented as mean ± standard deviations[68]
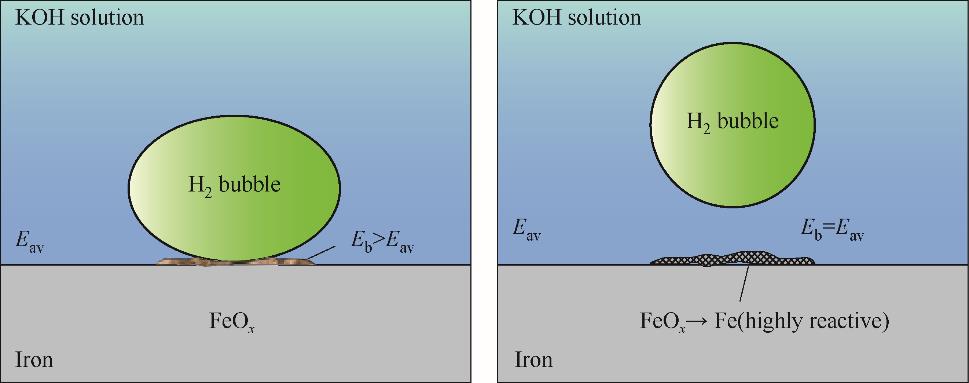
Fig.15 Schematic diagram of the formation of corrosion products at/under hydrogen bubble and their reduction to metal upon detachment of the bubble[69]
| 1 | Johnston B, Mayo M C, Khare A. Hydrogen: the energy source for the 21st century[J]. Technovation, 2005, 25(6): 569-585. |
| 2 | Hassan Q, Sameen A Z, Salman H M, et al. Hydrogen energy future: advancements in storage technologies and implications for sustainability[J]. Journal of Energy Storage, 2023, 72: 108404. |
| 3 | Thomas J M, Edwards P P, Dobson P J, et al. Decarbonising energy: the developing international activity in hydrogen technologies and fuel cells[J]. Journal of Energy Chemistry, 2020, 51: 405-415. |
| 4 | Rissman J, Bataille C, Masanet E, et al. Technologies and policies to decarbonize global industry: review and assessment of mitigation drivers through 2070[J]. Applied Energy, 2020, 266: 114848. |
| 5 | Sikiru S, Oladosu T L, Amosa T I, et al. Hydrogen-powered horizons: transformative technologies in clean energy generation, distribution, and storage for sustainable innovation[J]. International Journal of Hydrogen Energy, 2024, 56: 1152-1182. |
| 6 | Zhou Q, Liao L, Zhou H, et al. Innovative strategies in design of transition metal-based catalysts for large-current-density alkaline water/seawater electrolysis[J]. Materials Today Physics, 2022, 26: 100727. |
| 7 | Zeng K, Zhang D K. Recent progress in alkaline water electrolysis for hydrogen production and applications[J]. Progress in Energy and Combustion Science, 2010, 36(3): 307-326. |
| 8 | Smolinka T, Bergmann H, Garche J, et al. The history of water electrolysis from its beginnings to the present[M]//Electrochemical Power Sources: Fundamentals, Systems, and Applications. Amsterdam: Elsevier, 2022: 83-164. |
| 9 | Santos D M F, Sequeira C A C, Figueiredo J L. Hydrogen production by alkaline water electrolysis[J]. Química Nova, 2013, 36(8): 1176-1193. |
| 10 | Kreuter W, Hofmann H. Electrolysis: the important energy transformer in a world of sustainable energy[J]. International Journal of Hydrogen Energy, 1998, 23(8): 661-666. |
| 11 | Wan L, Xu Z, Xu Q, et al. Key components and design strategy of the membrane electrode assembly for alkaline water electrolysis[J]. Energy & Environmental Science, 2023, 16(4): 1384-1430. |
| 12 | Chen Y, Min L, Zhang W, et al. Crown ether as a bifunctional booster in electrochemical water splitting[J]. International Journal of Hydrogen Energy, 2024, 51: 1534-1543. |
| 13 | Song C, Min L, Zhang W, et al. A benzimidazole-linked polymer membrane in alkaline water electrolysis[J]. Journal of Membrane Science, 2023, 683: 121883. |
| 14 | Abbasi R, Setzler B P, Lin S S, et al. A roadmap to low-cost hydrogen with hydroxide exchange membrane electrolyzers[J]. Advanced Materials, 2019, 31(31): 1805876. |
| 15 | Ursúa A, Gandía L M, Sanchis P. Hydrogen production from water electrolysis: current status and future trends[C]//Proceedings of the IEEE. IEEE, 2012: 410-426. |
| 16 | El-Shafie M. Hydrogen production by water electrolysis technologies: a review[J]. Results in Engineering, 2023, 20: 101426. |
| 17 | Liu R T, Xu Z L, Li F M, et al. Recent advances in proton exchange membrane water electrolysis[J]. Chemical Society Reviews, 2023, 52(16): 5652-5683. |
| 18 | Zhang W, Liu M, Gu X, et al. Water electrolysis toward elevated temperature: advances, challenges and frontiers[J]. Chemical Reviews, 2023, 123(11): 7119-7192. |
| 19 | Baratov S, Filonova E, Ivanova A, et al. Current and further trajectories in designing functional materials for solid oxide electrochemical cells: a review of other reviews[J]. Journal of Energy Chemistry, 2024, 94: 302-331. |
| 20 | Santoro C, Lavacchi A, Mustarelli P, et al. What is next in anion-exchange membrane water electrolyzers? Bottlenecks, benefits, and future[J]. ChemSusChem, 2022, 15(8): e202200027. |
| 21 | Du N Y, Roy C, Peach R, et al. Anion-exchange membrane water electrolyzers[J]. Chemical Reviews, 2022, 122(13): 11830-11895. |
| 22 | de Groot M T. Alkaline water electrolysis: with or without iron in the electrolyte?[J]. Current Opinion in Chemical Engineering, 2023, 42: 100981. |
| 23 | Bailleux C, Damien A, Montet A. Alkaline electrolysis of water-EGF activity in electrochemical engineering from 1975 to 1982[J]. International Journal of Hydrogen Energy, 1983, 8(7): 529-538. |
| 24 | Davalos Monteiro R, van de Wetering J, Krawczyk B, et al. Corrosion behaviour of type 316L stainless steel in hot caustic aqueous environments[J]. Metals and Materials International, 2020, 26(5): 630-640. |
| 25 | Todoroki N, Wadayama T. Dissolution of constituent elements from various austenitic stainless steel oxygen evolution electrodes under potential cycle loadings[J]. International Journal of Hydrogen Energy, 2022, 47(77): 32753-32762. |
| 26 | Chung D Y, Lopes P P, Farinazzo Bergamo Dias Martins P, et al. Dynamic stability of active sites in hydr(oxy)oxides for the oxygen evolution reaction[J]. Nature Energy, 2020, 5: 222-230. |
| 27 | Tyndall D, Craig M J, Gannon L, et al. Demonstrating the source of inherent instability in NiFe LDH-based OER electrocatalysts[J]. Journal of Materials Chemistry A, 2023, 11(8): 4067-4077. |
| 28 | Liu W, Ding X Q, Cheng J J, et al. Inhibiting dissolution of active sites in 80℃ alkaline water electrolysis by oxyanion engineering[J]. Angewandte Chemie International Edition, 2024, 63(32): e202406082. |
| 29 | Trotochaud L, Young S L, Ranney J K, et al. Nickel-iron oxyhydroxide oxygen-evolution electrocatalysts: the role of intentional and incidental iron incorporation[J]. Journal of the American Chemical Society, 2014, 136(18): 6744-6753. |
| 30 | Cameron D S, Phillips R L, Willis P M. Poison tolerant platinum catalysed cathodes for membrane cells[M]//Modern Chlor-Alkali Technology. Dordrecht: Springer Netherlands, 1990: 95-107. |
| 31 | Rodríguez J, Palmas S, Sánchez-Molina M, et al. Simple and precise approach for determination of ohmic contribution of diaphragms in alkaline water electrolysis[J]. Membranes, 2019, 9(10): 129. |
| 32 | O'Brien T F, Bommaraju T V, Hine F. Handbook of Chlor-Alkali Technology[M]. Boston, MA: Springer US, 2005. |
| 33 | Spanos I, Masa J, Zeradjanin A, et al. The effect of iron impurities on transition metal catalysts for the oxygen evolution reaction in alkaline environment: activity mediators or active sites?[J]. Catalysis Letters, 2021, 151(7): 1843-1856. |
| 34 | Corrigan D A. The catalysis of the oxygen evolution reaction by iron impurities in thin film nickel oxide electrodes[J]. Journal of the Electrochemical Society, 1987, 134(2): 377. |
| 35 | Lyons M E G, Brandon M P. The oxygen evolution reaction on passive oxide covered transition metal electrodes in aqueous alkaline solution(Part 1): Nickel[J]. International Journal of Electrochemical Science, 2008, 3(12): 1386-1424. |
| 36 | Kostecki R, McLarnon F. Electrochemical and in situ Raman spectroscopic characterization of nickel hydroxide electrodes(Ⅰ): Pure nickel hydroxide[J]. Journal of the Electrochemical Society, 1997, 144(2): 485-493. |
| 37 | Yeo B S, Bell A T. In situ Raman study of nickel oxide and gold-supported nickel oxide catalysts for the electrochemical evolution of oxygen[J]. The Journal of Physical Chemistry C, 2012, 116(15): 8394-8400. |
| 38 | Doyle R L, Lyons M E G. Kinetics and mechanistic aspects of the oxygen evolution reaction at hydrous iron oxide films in base[J]. Journal of the Electrochemical Society, 2013, 160(2): H142-H154. |
| 39 | Oliva P, Leonardi J, Laurent J F, et al. Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides[J]. Journal of Power Sources, 1982, 8(2): 229-255. |
| 40 | Wehrens-Dijksma M, Notten P H L. Electrochemical quartz microbalance characterization of Ni(OH)2-based thin film electrodes[J]. Electrochimica Acta, 2006, 51(18): 3609-3621. |
| 41 | Godwin I J, Lyons M E G. Enhanced oxygen evolution at hydrous nickel oxide electrodes via electrochemical ageing in alkaline solution[J]. Electrochemistry Communications, 2013, 32: 39-42. |
| 42 | Lu P W T, Srinivasan S. Electrochemical-ellipsometric studies of oxide film formed on nickel during oxygen evolution[J]. Journal of the Electrochemical Society, 1978, 125(9): 1416. |
| 43 | Bernard M C, Bernard P, Keddam M, et al. Characterisation of new nickel hydroxides during the transformation of α-Ni(OH)2 to β-Ni(OH)2 by ageing[J]. Electrochimica Acta, 1996, 41(1): 91-93. |
| 44 | Kim M S, Kim K B. A study on the phase transformation of electrochemically precipitated nickel hydroxides using an electrochemical quartz crystal microbalance[J]. Journal of the Electrochemical Society, 1998, 145(2): 507-511. |
| 45 | Louie M W, Bell A T. An investigation of thin-film Ni-Fe oxide catalysts for the electrochemical evolution of oxygen[J]. Journal of the American Chemical Society, 2013, 135(33): 12329-12337. |
| 46 | Klaus S, Cai Y, Louie M W, et al. Effects of Fe electrolyte impurities on Ni(OH)2/NiOOH structure and oxygen evolution activity[J]. The Journal of Physical Chemistry C, 2015, 119(13): 7243-7254. |
| 47 | Deng J, Nellist M R, Stevens M B, et al. Morphology dynamics of single-layered Ni(OH)2/NiOOH nanosheets and subsequent Fe incorporation studied by in situ electrochemical atomic force microscopy[J]. Nano Letters, 2017, 17(11): 6922-6926. |
| 48 | Burke M S, Kast M G, Trotochaud L, et al. Cobalt-iron (oxy)hydroxide oxygen evolution electrocatalysts: the role of structure and composition on activity, stability, and mechanism[J]. Journal of the American Chemical Society, 2015, 137(10): 3638-3648. |
| 49 | Zhang T, Nellist M R, Enman L J, et al. Modes of Fe incorporation in Co-Fe (oxy)hydroxide oxygen evolution electrocatalysts[J]. ChemSusChem, 2019, 12(9): 2015-2021. |
| 50 | Stevens M B, Enman L J, Korkus E H, et al. Ternary Ni-Co-Fe oxyhydroxide oxygen evolution catalysts: intrinsic activity trends, electrical conductivity, and electronic band structure[J]. Nano Research, 2019, 12(9): 2288-2295. |
| 51 | Spanos I, Tesch M F, Yu M Q, et al. Facile protocol for alkaline electrolyte purification and its influence on a Ni-Co oxide catalyst for the oxygen evolution reaction[J]. ACS Catalysis, 2019, 9(9): 8165-8170. |
| 52 | Zhou Y C, López N. The role of Fe species on NiOOH in oxygen evolution reactions[J]. ACS Catalysis, 2020, 10(11): 6254-6261. |
| 53 | Enman L J, Burke M S, Batchellor A S, et al. Effects of intentionally incorporated metal cations on the oxygen evolution electrocatalytic activity of nickel (oxy)hydroxide in alkaline media[J]. ACS Catalysis, 2016, 6(4): 2416-2423. |
| 54 | Stevens M B, Trang C D M, Enman L J, et al. Reactive Fe-sites in Ni/Fe (oxy)hydroxide are responsible for exceptional oxygen electrocatalysis activity[J]. Journal of the American Chemical Society, 2017, 139(33): 11361-11364. |
| 55 | Marquez R A, Kalokowski E, Espinosa M, et al. Transition metal incorporation: electrochemical, structure, and chemical composition effects on nickel oxyhydroxide oxygen-evolution electrocatalysts[J]. Energy & Environmental Science, 2024, 17(5): 2028-2045. |
| 56 | Friebel D, Louie M W, Bajdich M, et al. Identification of highly active Fe sites in (Ni, Fe)OOH for electrocatalytic water splitting[J]. Journal of the American Chemical Society, 2015, 137(3): 1305-1313. |
| 57 | Farhat R, Dhainy J, Halaoui L I. OER catalysis at activated and codeposited NiFe-oxo/hydroxide thin films is due to postdeposition surface-Fe and is not sustainable without Fe in solution[J]. ACS Catalysis, 2020, 10(1): 20-35. |
| 58 | Ou Y Q, Twight L P, Samanta B, et al. Cooperative Fe sites on transition metal (oxy)hydroxides drive high oxygen evolution activity in base[J]. Nature Communications, 2023, 14(1): 7688. |
| 59 | Kuai C G, Xu Z R, Xi C, et al. Phase segregation reversibility in mixed-metal hydroxide water oxidation catalysts[J]. Nature Catalysis, 2020, 3: 743-753. |
| 60 | Zou S H, Burke M S, Kast M G, et al. Fe (oxy)hydroxide oxygen evolution reaction electrocatalysis: intrinsic activity and the roles of electrical conductivity, substrate, and dissolution[J]. Chemistry of Materials, 2015, 27(23): 8011-8020. |
| 61 | Zhang Q, Xiao W, Fu H C, et al. Unraveling the mechanism of self-repair of NiFe-based electrocatalysts by dynamic exchange of iron during the oxygen evolution reaction[J]. ACS Catalysis, 2023, 13(22): 14975-14986. |
| 62 | Bao F X, Kemppainen E, Dorbandt I, et al. Host, suppressor, and promoter-the roles of Ni and Fe on oxygen evolution reaction activity and stability of NiFe alloy thin films in alkaline media[J]. ACS Catalysis, 2021, 11(16): 10537-10552. |
| 63 | Nidola A, Schira R. Deactivation of low hydrogen overvoltage cathodes in chlor-alkali membrane cell technology by metallic impurities[C]//Proceedings of the Symposium on Advances in the Chlor-Alkali and Chlorate Industry. Electrochemical Society, Industrial Electrolytic Division, 1984: F: 206-221. |
| 64 | Riley M A, Moran P J. The influence of iron deposition on the voltage-time behavior of nickel cathodes in alkaline water electrolysis[J]. Journal of the Electrochemical Society, 1986, 133(4): 760-761. |
| 65 | Rommal H E G, Moran P J. Time-dependent energy efficiency losses at nickel cathodes in alkaline water electrolysis systems[J]. Journal of the Electrochemical Society, 1985, 132(2): 325-329. |
| 66 | Brossard L. Electrocatalytic performance for alkaline water electrolysis of Ni electrodes electrocoated with Fe or Fe/Mo[J]. International Journal of Hydrogen Energy, 1991, 16(1): 13-21. |
| 67 | Mauer A E, Kirk D W, Thorpe S J. The role of iron in the prevention of nickel electrode deactivation in alkaline electrolysis[J]. Electrochimica Acta, 2007, 52(11): 3505-3509. |
| 68 | Demnitz M, Lamas Y M, Garcia Barros R L, et al. Effect of iron addition to the electrolyte on alkaline water electrolysis performance[J]. iScience, 2024, 27(1): 108695. |
| 69 | Flis-Kabulska I, Flis J, Sun Y, et al. Hydrogen evolution on plasma carburised nickel and effect of iron deposition from the electrolyte in alkaline water electrolysis[J]. Electrochimica Acta, 2015, 167: 61-68. |
| 70 | Flis-Kabulska I, Flis J. Hydrogen evolution and corrosion products on iron cathodes in hot alkaline solution[J]. International Journal of Hydrogen Energy, 2014, 39(8): 3597-3605. |
| [1] | Ke ZHANG, Weijie REN, Mengna WANG, Kaifeng FAN, Liping CHANG, Jiabin LI, Tao MA, Jinping TIAN. Liquid-liquid mixing characteristics of Bunsen reaction products in microchannels [J]. CIESC Journal, 2025, 76(2): 623-636. |
| [2] | Chuanchao HE, Jinghong ZHOU, Yueqiang CAO, Yao SHI, Xinggui ZHOU. Bed-particle dual scale coupled simulation on Ag/SiO2 catalyzed hydrogenation of oxalate to methyl glycolate [J]. CIESC Journal, 2025, 76(2): 654-666. |
| [3] | Mengfan YIN, Qian WANG, Tao ZHENG, Kui JI, Shaogui WANG, Hui GUO, Zhiqiang LIN, Rui ZHANG, Hui SUN, Haiyan LIU, Zhichang LIU, Chunming XU, Xianghai MENG, Yueping WANG. Process design of 10000 t industrial demonstration of hydrogen production from renewable energy electrolytic water - low temperature and low pressure ammonia synthesis [J]. CIESC Journal, 2025, 76(2): 825-834. |
| [4] | Jijun ZOU, Baohong LIU, Chengxiang SHI, Lun PAN, Xiangwen ZHANG. Research progress of heterogeneous catalysts for conversion of holocellulose derivatives into bio-aviation fuels [J]. CIESC Journal, 2025, 76(1): 1-17. |
| [5] | Chen YANG, Wei MAO, Xingzong DONG, Song TIAN, Fengwei ZHAO, Jian LYU. Research progress in the synthesis of olefins by selective hydrodechlorination [J]. CIESC Journal, 2025, 76(1): 53-70. |
| [6] | Zhijiao JI, Xiaofang ZHANG, Wen GAN, Yunpeng XUE. Influence of support on the performance of single atom electrocatalyst for ammonia synthesis and the control strategy [J]. CIESC Journal, 2025, 76(1): 18-39. |
| [7] | Shan GUO, Yu TIAN, Yongbin XU, Peng WANG, Zhiming LIU. Synthesis of a high-efficacy medium-entropy alloy catalyst via the recycling of spent batteries and its subsequent performance evaluation [J]. CIESC Journal, 2025, 76(1): 231-240. |
| [8] | Fan LI, Yanjun YIN, Junchao XU, Liqiao JIANG, Xiaohan WANG, Huaqiang CHU. Enhancing the flame stability in a flat plate burner using catalytic coating of CeO2-ZrO2 [J]. CIESC Journal, 2025, 76(1): 394-404. |
| [9] | Meilin SHI, Lianda ZHAO, Xingjian DENG, Jingsong WANG, Haibin ZUO, Qingguo XUE. Research progress on catalytic methane reforming process [J]. CIESC Journal, 2024, 75(S1): 25-39. |
| [10] | Shuzhen WANG, Yuting WANG, Mengxi MA, Wei ZHANG, Jiangnan XIANG, Haiying LU, Yan WANG, Binbin FAN, Jiajun ZHENG, Weijiong DAI, Ruifeng LI. Synthesis of ZSM-22 molecular sieve by two-step crystallization and its hydroisomerization performance [J]. CIESC Journal, 2024, 75(9): 3176-3187. |
| [11] | Junfeng WANG, Junjie ZHANG, Wei ZHANG, Jiale WANG, Shuyan SHUANG, Yadong ZHANG. Liquid-phase discharge plasma decomposition of methanol for hydrogen production: optimization of electrode configuration [J]. CIESC Journal, 2024, 75(9): 3277-3286. |
| [12] | Ran WANG, Huan WANG, Xiaoyun XIONG, Huimin GUAN, Yunfeng ZHENG, Cailin CHEN, Yucai QIN, Lijuan SONG. Visual analysis of mass transfer enhanced active site utilization efficiency of FCC catalyst [J]. CIESC Journal, 2024, 75(9): 3198-3209. |
| [13] | Xinyi LUO, Qiang XU, Yonglu SHE, Tengfei NIE, Liejin GUO. Study on bubble dynamic characteristics and mass transfer mechanism in photoelectrochemical water splitting for hydrogen production [J]. CIESC Journal, 2024, 75(9): 3083-3093. |
| [14] | Yachao LIU, Xiaojie TAN, Xudong LI, Rui WANG, Hui WANG, Xuan HAN, Qingshan ZHAO. Synthesis of efficient cobalt carbonate nanosheets based on DES for oxygen evolution reaction [J]. CIESC Journal, 2024, 75(9): 3320-3328. |
| [15] | Mengting ZHANG, Shulin WANG, Xi SANG, Xinghao YUAN, Gang XU. Artificial Cu-TM1459 metalloenzyme catalyzes asymmetric Michael addition reaction [J]. CIESC Journal, 2024, 75(9): 3255-3265. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||