CIESC Journal ›› 2025, Vol. 76 ›› Issue (12): 6376-6386.DOI: 10.11949/0438-1157.20250288
• Catalysis, kinetics and reactors • Previous Articles Next Articles
Zhizhong PENG( ), Xuelei LANG, Qiang FANG, Huifang JING, dazhong ZHONG(
), Xuelei LANG, Qiang FANG, Huifang JING, dazhong ZHONG( ), Jinping LI, Qiang ZHAO(
), Jinping LI, Qiang ZHAO( )
)
Received:2025-03-24
Revised:2025-04-25
Online:2026-01-23
Published:2025-12-31
Contact:
dazhong ZHONG, Qiang ZHAO
彭郅众( ), 郎学磊, 房强, 井慧芳, 钟达忠(
), 郎学磊, 房强, 井慧芳, 钟达忠( ), 李晋平, 赵强(
), 李晋平, 赵强( )
)
通讯作者:
钟达忠,赵强
作者简介:彭郅众(1998—),男,硕士研究生,pengzhizhong1054@link.tyut.edu.cn
基金资助:CLC Number:
Zhizhong PENG, Xuelei LANG, Qiang FANG, Huifang JING, dazhong ZHONG, Jinping LI, Qiang ZHAO. Ag-Sn interfacial electronic structure modulation for high-efficiency CO2 electroreduction at 1 A/cm2 under acidic conditions[J]. CIESC Journal, 2025, 76(12): 6376-6386.
彭郅众, 郎学磊, 房强, 井慧芳, 钟达忠, 李晋平, 赵强. Ag-Sn间电子结构调控在酸性环境中实现1 A/cm2下高效CO2还原[J]. 化工学报, 2025, 76(12): 6376-6386.
Add to citation manager EndNote|Ris|BibTeX
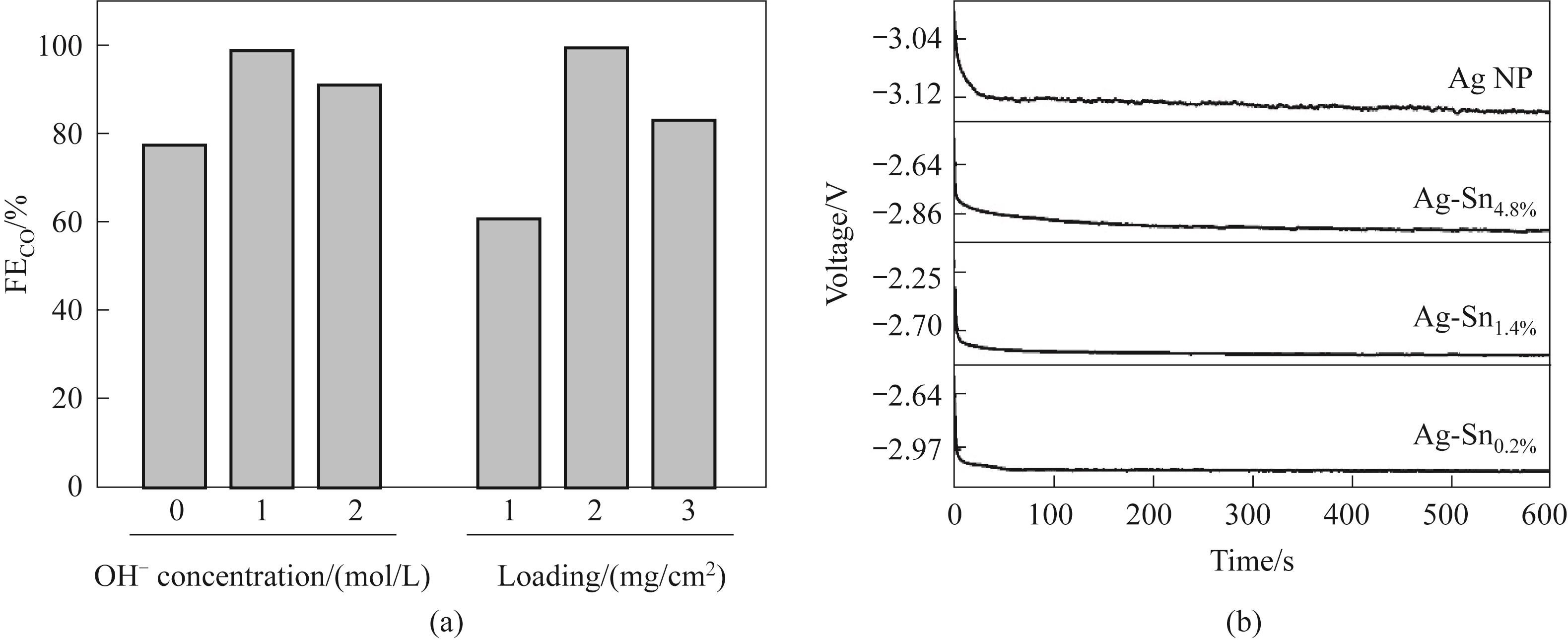
Fig.9 (a) Effect of pH of reaction solution during synthesis and loading of Ag-Sn catalyst on catalytic performance; (b) Pre-reduction process of Ag-Sn at 50 mA/cm2
| Catalyst | JTotal/(mA/cm2) | Electrolyte | FECO/% | Ref. |
|---|---|---|---|---|
| Ag-Sn1.4% | 1000 | K2SO4 + H2SO4 | 83.5 | this work |
| Ag-Sn1.4% | 400 | K2SO4 + H2SO4 | 99.4 | this work |
| Ni-N-C | 500 | K2SO4 + H2SO4 | 约80 | [ |
| Ag | 60 | Cs2SO4 + H2SO4 | 约80 | [ |
| Ag@C-d | 200 | K2SO4 + H2SO4 | 91.6 | [ |
| Ag∶Cs-DC | 400 | KHCO3 | 81.6 | [ |
| Ni1-NSC | 225 | KOH | 约100 | [ |
Table 1 Comparison of catalytic activity in MEA
| Catalyst | JTotal/(mA/cm2) | Electrolyte | FECO/% | Ref. |
|---|---|---|---|---|
| Ag-Sn1.4% | 1000 | K2SO4 + H2SO4 | 83.5 | this work |
| Ag-Sn1.4% | 400 | K2SO4 + H2SO4 | 99.4 | this work |
| Ni-N-C | 500 | K2SO4 + H2SO4 | 约80 | [ |
| Ag | 60 | Cs2SO4 + H2SO4 | 约80 | [ |
| Ag@C-d | 200 | K2SO4 + H2SO4 | 91.6 | [ |
| Ag∶Cs-DC | 400 | KHCO3 | 81.6 | [ |
| Ni1-NSC | 225 | KOH | 约100 | [ |
| [1] | Leveni M, Bielicki J M. A potential for climate benign direct air CO2 capture with CO2-driven geothermal utilization and storage (DACCUS)[J]. Environmental Research Letters, 2023, 19(1): 014007. |
| [2] | Li C, Zhang T F, Qiu Z, et al. Plasma-assisted fabrication of multiscale materials for electrochemical energy conversion and storage[J]. Carbon Energy, 2025, 7(2): e641. |
| [3] | Yun H, Yoo S, Son J, et al. Strong cation concentration effect of Ni-N-C electrocatalysts in accelerating acidic CO2 reduction reaction[J]. Chem, 2025. DOI: 10.10161j.Chempr.2025.102461 |
| [4] | Terholsen D H, Huerta-Zerón H D, Möller C, et al. Photocatalytic CO2 reduction using CO2-binding enzymes[J]. Angewandte Chemie International Edition, 2024, 63(16): e202319313. |
| [5] | Bai S T, De Smet G, Liao Y H, et al. Homogeneous and heterogeneous catalysts for hydrogenation of CO2 to methanol under mild conditions[J]. Chemical Society Reviews, 2021, 50(7): 4259-4298. |
| [6] | Diehl T, Lanzerath P, Franciò D G, et al. A self-separating multiphasic system for catalytic hydrogenation of CO2 and CO2-derivatives to methanol[J]. ChemSusChem, 2022, 15(22): e202201250. |
| [7] | Pang Q Q, Fan X Z, Sun K H, et al. Nickel-nitrogen-carbon (Ni-N-C) electrocatalysts toward CO2 electroreduction to CO: advances, optimizations, challenges, and prospects[J]. Energy & Environmental Materials, 2024, 7(5): e12731. |
| [8] | Weng C C, Wang C, Song Y, et al. In-situ reconstruction of active bismuth for enhanced CO2 electroreduction to formate[J]. Chemical Engineering Journal, 2025, 505: 159732. |
| [9] | Zhang N, Zhang Y L. Recent advances in electrocatalytic conversion of CO2-to-ethylene: from reaction mechanisms to tuning strategies[J]. Applied Catalysis B: Environment and Energy, 2025, 363: 124822. |
| [10] | Zhou J, He B L, Huang P, et al. Regulating interfacial hydrogen-bonding networks by implanting Cu sites with perfluorooctane to accelerate CO2 electroreduction to ethanol[J]. Angewandte Chemie International Edition, 2024, 137(6): e202418459. |
| [11] | Gong S H, Han X, Li W S, et al. Paired electrolysis for efficient coproduction of CO and S8 with techno-economic analysis[J]. Chemical Engineering Journal, 2025, 507: 160286. |
| [12] | Huang J E, Li F W, Ozden A, et al. CO2 electrolysis to multicarbon products in strong acid[J]. Science, 2021, 372(6546): 1074-1078. |
| [13] | Gu J, Liu S, Ni W Y, et al. Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium[J]. Nature Catalysis, 2022, 5: 268-276. |
| [14] | Su L N, Hua Q F, Yang Y N, et al. Regulating competing reaction pathways for efficient CO2 electroreduction in acidic conditions[J]. Journal of Energy Chemistry, 2025, 105: 326-351. |
| [15] | Hernandez-Aldave S, Andreoli E. Fundamentals of gas diffusion electrodes and electrolysers for carbon dioxide utilisation: challenges and opportunities[J]. Catalysts, 2020, 10(6): 713. |
| [16] | Jouny M, Luc W, Jiao F,et al. General techno-economic analysis of CO2 electrolysis systems[J]. Industrial & Engineering Chemistry Research, 2018, 57(6): 2165-2177. |
| [17] | Gabardo C M, O'Brien C P, Edwards J P, et al. Continuous carbon dioxide electroreduction to concentrated multi-carbon products using a membrane electrode assembly[J]. Joule, 2019, 3(11): 2777-2791. |
| [18] | De Luna P, Hahn C, Higgins D, et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes?[J]. Science, 2019, 364(6438): eaav3506. |
| [19] | Jiang Y L, Huang L S, Chen C J, et al. Catalyst-electrolyte interface engineering propels progress in acidic CO2 electroreduction[J]. Energy & Environmental Science, 2025, 18(5): 2025-2049. |
| [20] | Goyal A, Marcandalli G, Mints V A, et al. Competition between CO2 reduction and hydrogen evolution on a gold electrode under well-defined mass transport conditions[J]. Journal of the American Chemical Society, 2020, 142(9): 4154-4161. |
| [21] | Liu Z K, Yan T, Shi H, et al. Acidic electrocatalytic CO2 reduction using space-confined nanoreactors[J]. ACS Applied Materials & Interfaces, 2022, 14(6): 7900-7908. |
| [22] | Sheng X D, Ge W X, Jiang H L, et al. Engineering the Ni-N-C catalyst microenvironment enabling CO2 electroreduction with nearly 100% CO selectivity in acid[J]. Advanced Materials, 2022, 34(38): 2201295. |
| [23] | Singh M R, Goodpaster J D, Weber A Z, et al. Mechanistic insights into electrochemical reduction of CO2 over Ag using density functional theory and transport models[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(42): E8812-E8821. |
| [24] | Wu X H, Guo Y N, Sun Z S, et al. Fast operando spectroscopy tracking in situ generation of rich defects in silver nanocrystals for highly selective electrochemical CO2 reduction[J]. Nature Communications, 2021, 12(1): 660. |
| [25] | Ren D W, Xu D A, Chan P K, et al. A cation concentration gradient approach to tune the selectivity and activity of CO2 electroreduction[J]. Angewandte Chemie International Edition, 2022, 134(49): e202214173. |
| [26] | Cai C, Liu B, Liu K, et al. Heteroatoms induce localization of the electric field and promote a wide potential-window selectivity towards CO in the CO2 electroreduction[J]. Angewandte Chemie International Edition, 2022, 61(44): e202212640. |
| [27] | Dai R Y, Sun K A, Shen R A, et al. Direct microenvironment modulation of CO2 electroreduction: negatively charged Ag sites going beyond catalytic surface reactions[J]. Angewandte Chemie International Edition, 2024, 63(37): e202408580. |
| [28] | Bokuniaeva A O, Vorokh A S. Estimation of particle size using the Debye equation and the Scherrer formula for polyphasic TiO2 powder[J]. Journal of Physics: Conference Series, 2019, 1410(1): 012057. |
| [29] | Sandoval M G, Walia J, Houache M S E, et al. CO2 adsorption and activation on Ag(111) surfaces in the presence of surface charge density: a static gas phase DFT study[J]. Applied Surface Science, 2023, 610: 155498. |
| [30] | Li H F, Li H B, Wei P F, et al. Tailoring acidic microenvironments for carbon-efficient CO2 electrolysis over a Ni-N-C catalyst in a membrane electrode assembly electrolyzer[J]. Energy & Environmental Science, 2023, 16(4): 1502-1510. |
| [31] | Pan B B, Fan J, Zhang J, et al. Close to 90% single-pass conversion efficiency for CO2 electroreduction in an acid-fed membrane electrode assembly[J]. ACS Energy Letters, 2022, 7(12): 4224-4231. |
| [32] | Zhang B, Zou J H, Chen Z H, et al. Defect-engineered carbon-confined silver for enhanced CO2 electrocatalytic reduction to CO in acidic media[J]. Next Nanotechnology, 2023, 2: 100014. |
| [33] | Sun Y H, Chen P J, Du X M, et al. Anchoring Cs+ ions on carbon vacancies for selective CO2 electroreduction to CO at high current densities in membrane electrode assembly electrolyzers[J]. Angewandte Chemie International Edition, 2024, 63(40): e202410802. |
| [34] | Chen Z Y, Wang C H, Zhong X, et al. Achieving efficient CO2 electrolysis to CO by local coordination manipulation of nickel single-atom catalysts[J]. Nano Letters, 2023, 23(15): 7046-7053. |
| [35] | Han J Y, Bai X, Xu X Q, et al. Advances and challenges in the electrochemical reduction of carbon dioxide[J]. Chemical Science, 2024, 15(21): 7870-7907. |
| [36] | Bohra D, Ledezma-Yanez I, Li G N, et al. Lateral adsorbate interactions inhibit HCOO- while promoting CO selectivity for CO2 electrocatalysis on silver[J]. Angewandte Chemie International Edition, 2019, 58(5): 1345-1349. |
| [37] | Chernyshova I V, Somasundaran P, Ponnurangam S. On the origin of the elusive first intermediate of CO2 electroreduction[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(40): E9261-E9270. |
| [38] | Lin Y X, Wang S C, Liu H J, et al. Regulating the electrocatalytic active centers for accelerated proton transfer towards efficient CO2 reduction[J]. National Science Review, 2025, 12(3): nwaf010. |
| [39] | Feng S J, Wang X J, Cheng D F, et al. Stabilizing *CO2 intermediates at the acidic interface using molecularly dispersed cobalt phthalocyanine as catalysts for CO2 reduction[J]. Angewandte Chemie International Edition, 2024, 63(8): e202317942. |
| [40] | Liu H M, Yan T, Tan S D, et al. Observation on microenvironment changes of dynamic catalysts in acidic CO2 reduction[J]. Journal of the American Chemical Society, 2024, 146(8): 5333-5342. |
| [41] | Zamora Zeledón J A, Stevens M B, Gunasooriya G T K K, et al. Tuning the electronic structure of Ag-Pd alloys to enhance performance for alkaline oxygen reduction[J]. Nature Communications, 2021, 12(1): 620. |
| [1] | Huihui QIAN, Wenjie WANG, Wenyao CHEN, Xinggui ZHOU, Jing ZHANG, Xuezhi DUAN. Synergistic metal-zeolite catalysis for conversion of polypropylene into aromatics [J]. CIESC Journal, 2025, 76(9): 4838-4849. |
| [2] | Ning ZENG, Zhenjiang GUO, Jianhua CHEN, Zixuan ZHANG, Yujiao ZENG, Xin XIAO, Songlin LIU, Shaoxiu XUE, Zhiwu ZHOU, Zhenming LU, Limin WANG. Molecular dynamics simulation of water-insoluble phosphorus in dihydrate wet-process phosphoric acid [J]. CIESC Journal, 2025, 76(9): 4539-4550. |
| [3] | Bing LIAO, Xinyu ZHU, Qianqian HUANG, Wen XU, Mengyao KOU, Na GUO. Performance and mechanism of enhanced Fenton system by hydroxylamine hydrochloride for removal of 2, 4-DCP under near-neutral conditions [J]. CIESC Journal, 2025, 76(8): 4273-4283. |
| [4] | Xinyi CHAO, Wenyao CHEN, Jing ZHANG, Gang QIAN, Xinggui ZHOU, Xuezhi DUAN. Controlled preparation and performance regulation of catalysts for one-step synthesis of methyl propionate from methanol and methyl acetate [J]. CIESC Journal, 2025, 76(8): 4030-4041. |
| [5] | Fang WANG, Suxia MA, Ying TIAN, Zhongyuan LIU. NO x emission prediction method of CFB unit based on 1D mechanism model dynamicly corrected with LSTM [J]. CIESC Journal, 2025, 76(7): 3416-3425. |
| [6] | Tonghui LI, Tianli HUI, Tao ZHENG, Rui ZHANG, Haiyan LIU, Zhichang LIU, Chunming XU, Xianghai MENG. Synergistic palladium double active sites with hydroxide for high current density and pH-universal hydrogen evolution reaction [J]. CIESC Journal, 2025, 76(7): 3671-3685. |
| [7] | Lei WU, Zixuan HU, Yuan GAO, Changbo LIU, Husheng CAO, Tiantian LIU, Ruiyu ZHU, Jun ZHOU. Oxidation remediation of polycyclic aromatic hydrocarbons contaminated soil by microwave combined with biochar activated persulfate [J]. CIESC Journal, 2025, 76(7): 3659-3670. |
| [8] | Jun HE, Yong LI, Nan ZHAO, Xiaojun HE. Study on the properties of carbon with Se doping cobalt sulfide in lithium-sulfur batteries [J]. CIESC Journal, 2025, 76(6): 2995-3008. |
| [9] | Chang ZHANG, Qiang XIE, Yutong SHA, Bingjie WANG, Dingcheng LIANG, Jinchang LIU. Preparation of bamboo char with low ash and silicon content and electrochemical properties of its derived hard carbon [J]. CIESC Journal, 2025, 76(6): 3073-3083. |
| [10] | Qingping ZHAO, Min ZHANG, Hui ZHAO, Gang WANG, Yongfu QIU. Hydrogen bond effect and kinetic studies on hydroesterification of ethylene to methyl propionate [J]. CIESC Journal, 2025, 76(6): 2701-2713. |
| [11] | Yaohui ZHANG, Yujie BAN, Weishen YANG. Vapor-phase synthesis and post-synthetic modification of metal-organic framework membranes [J]. CIESC Journal, 2025, 76(5): 2070-2086. |
| [12] | Xiaokun WANG, Zelin LIAO, Junliang WU, Xingyu CHEN, Yifei YU, Gaohong HE, Xiujuan ZHANG. Preparation and performance evaluation of LDH-PTFPMS/PEI composite membrane for improving blood compatibility and CO2 transfer [J]. CIESC Journal, 2025, 76(4): 1800-1808. |
| [13] | Ji LI, Jiacai WANG, Yongqiang MA, Haibin YUAN, Luming JIAN, Jican JIANG, Jiahua ZHU. Thermodynamic analysis and engineering practice of high efficiently recycle of fluorine contained in tail gas from wet-process phosphoric acid plant [J]. CIESC Journal, 2025, 76(4): 1484-1492. |
| [14] | Jing ZHANG, Yue YUAN, Yanmei LIU, Zhiwen WANG, Tao CHEN. Advance on the preparation of itaconic acid by biological method [J]. CIESC Journal, 2025, 76(3): 909-921. |
| [15] | Zhongqing CHEN, Jiaxu LIU, Yanyu WANG, Hongquan JING, Cuihong HOU, Lingbo QU. Effect of K-B-Al ternary system on the melting characteristics and glass structure of tailings [J]. CIESC Journal, 2025, 76(3): 1323-1333. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||