化工学报 ›› 2021, Vol. 72 ›› Issue (5): 2763-2772.DOI: 10.11949/0438-1157.20201484
收稿日期:2020-10-26
修回日期:2020-12-02
出版日期:2021-05-05
发布日期:2021-05-05
通讯作者:
曾敏
作者简介:李威(1993—),男,博士研究生,基金资助:
LI Wei( ),WANG Qiuwang,ZENG Min(
),WANG Qiuwang,ZENG Min( )
)
Received:2020-10-26
Revised:2020-12-02
Online:2021-05-05
Published:2021-05-05
Contact:
ZENG Min
摘要:
以水合盐K2CO3·1.5H2O和膨胀石墨(EG)分别作为化学蓄热材料和多孔基质,研制了复合储热吸附剂K2CO3@EG。对该复合吸附剂和未掺杂膨胀石墨的纯水合盐就脱附储热、吸附性能、循环稳定性等方面进行了对比分析。结果表明,复合吸附剂所需的脱附温度降低,对吸附质的吸附动力学性能也有明显提升且可有效避免潮解现象。经过连续15次的脱附-水合循环实验后,纯盐和复合吸附剂的储热密度分别下降27.6%和10.9%。此外,对储热单元的数值研究结果初步验证了该蓄热体系的可行性。
中图分类号:
李威, 王秋旺, 曾敏. 水合盐基中低温热化学储热材料性能测试及数值研究[J]. 化工学报, 2021, 72(5): 2763-2772.
LI Wei, WANG Qiuwang, ZENG Min. Performance test and numerical study of salt hydrate-based thermochemical heat storage materials at middle-low temperature[J]. CIESC Journal, 2021, 72(5): 2763-2772.
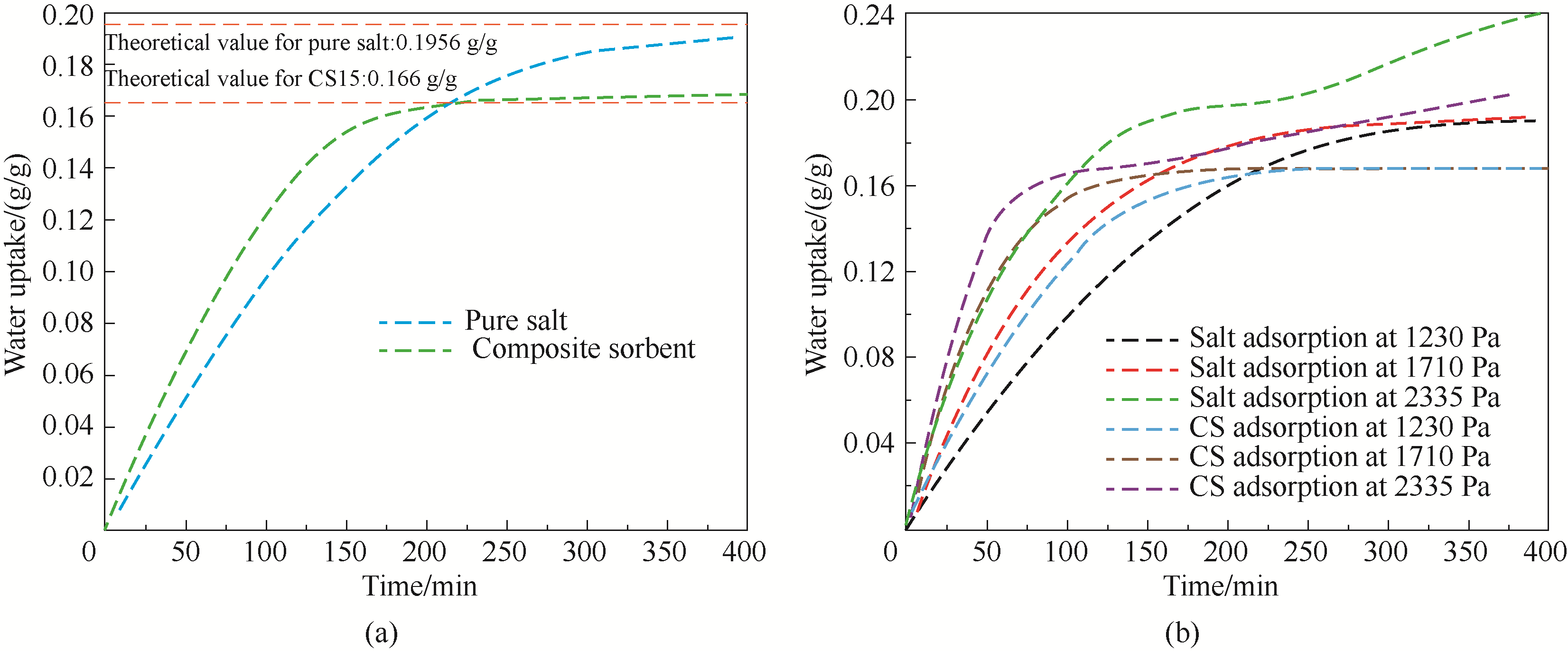
图3 纯盐与复合吸附剂在30℃,(a)29% RH 和(b)不同相对湿度条件下的吸附情况
Fig.3 Adsorption of pure salt and composite sorbent at 30℃ with (a) 29% RH and (b) various vapor pressures
| 工况 | 纯K2CO3 | CS15 | ||
|---|---|---|---|---|
平衡吸附 量/(g/g) | 所需 时间/min | 平衡吸 附量/(g/g) | 所需 时间/min | |
| 30℃,29% RH | 0.1906 | 360 | 0.168 | 250 |
| 30℃,40% RH | 0.1924 | 350 | 0.169 | 230 |
| 30℃,55% RH | 0.24 | 400 | 0.2031 | 360 |
表1 两种吸附剂不同工况下平衡吸附量及所需时间
Table 1 Equilibrium adsorption capacities and corresponding times of sorbents
| 工况 | 纯K2CO3 | CS15 | ||
|---|---|---|---|---|
平衡吸附 量/(g/g) | 所需 时间/min | 平衡吸 附量/(g/g) | 所需 时间/min | |
| 30℃,29% RH | 0.1906 | 360 | 0.168 | 250 |
| 30℃,40% RH | 0.1924 | 350 | 0.169 | 230 |
| 30℃,55% RH | 0.24 | 400 | 0.2031 | 360 |

图6 整体式储热管道(a)和储热单元对称结构一半及边界条件示意图(b),网格独立性验证(c)
Fig.6 Schematic of the integral heat storage pipe (a) and a half of the symmetrical structure and boundary conditions (b), grid independence verification (c)
| 控制方程 | 描述 | |
|---|---|---|
| 反应动力学 | α为转化率;pv, peq分别为蒸汽动态压力和平衡压力;Af为指前因子;Ea为反应活化能;R为通用气体常数 | |
| 克劳修斯-克拉贝隆方程 | pref为参考水平压力;ΔHr为反应焓;ΔSr 为熵 | |
| 质量守恒及质量输运 | ε为吸附剂反应床孔隙率;Dg为蒸汽在多孔吸附剂内扩散系数 | |
| 质量源项Sw | ρs 为储热吸附剂密度;Mv/Ms 为蒸汽摩尔质量和储热吸附剂摩尔质量比值 | |
| 湿空气混合物 | ρm 为湿空气密度;u 为气体速度 | |
| 多孔储热材料内流体流动 | k为反应床渗透率;μm为水蒸气黏度 | |
| 能量守恒 | 热源 |
表2 控制方程及描述
Table 2 Governing equations and descriptions
| 控制方程 | 描述 | |
|---|---|---|
| 反应动力学 | α为转化率;pv, peq分别为蒸汽动态压力和平衡压力;Af为指前因子;Ea为反应活化能;R为通用气体常数 | |
| 克劳修斯-克拉贝隆方程 | pref为参考水平压力;ΔHr为反应焓;ΔSr 为熵 | |
| 质量守恒及质量输运 | ε为吸附剂反应床孔隙率;Dg为蒸汽在多孔吸附剂内扩散系数 | |
| 质量源项Sw | ρs 为储热吸附剂密度;Mv/Ms 为蒸汽摩尔质量和储热吸附剂摩尔质量比值 | |
| 湿空气混合物 | ρm 为湿空气密度;u 为气体速度 | |
| 多孔储热材料内流体流动 | k为反应床渗透率;μm为水蒸气黏度 | |
| 能量守恒 | 热源 |
| 1 | Wang F, Gu J, Wu J. Perspective taking, energy policy involvement, and public acceptance of nuclear energy: evidence from China [J]. Energy Policy, 2020, 145: 111716. |
| 2 | Allen-Dumas M R, Rose A N, New J R, et al. Impacts of the morphology of new neighborhoods on microclimate and building energy [J]. Renewable and Sustainable Energy Reviews, 2020, 133: 110030. |
| 3 | 马坤茹, 李雪峰, 李思琦, 等. 新型太阳能/空气能直膨式热泵与空气源热泵供热性能对比[J]. 化工学报, 2020, 71: 375-381. |
| Ma K R, Li X F, Li S Q. Contrastive research of heating performance of direct expansion solar/air assisted heat pump system and air-source heat pump [J]. CIESC Journal, 2020, 71: 375-381. | |
| 4 | Gaeini M, Rouws A L, Salari J W O, et al. Characterization of microencapsulated and impregnated porous host materials based on calcium chloride for thermochemical energy storage [J]. Applied Energy, 2018, 212(15): 1165-1177. |
| 5 | Xu M, Zhao P, Huo Y, et al. Thermodynamic analysis of a novel liquid carbon dioxide energy storage system and comparison to a liquid air energy storage system[J]. Journal of Cleaner Production, 2020, 242: 118437. |
| 6 | 刘华, 彭佳杰, 余凯, 等. 活性氧化铝基质新型复合吸附剂的制备和储热性能[J].化工学报, 2020, 71(7): 3354-3361. |
| Liu H, Peng J J, Yu K, et al. Preparation and thermal storage performance of novel composite sorbent with activated alumina matrix [J]. CIESC Journal, 2020, 71(7): 3354-3361. | |
| 7 | Bennici S, Polimann T, Ondarts M, et al. Long-term impact of air pollutants on thermochemical heat storage materials [J]. Renewable and Sustainable Energy Reviews, 2020, 117: 109473. |
| 8 | 徐凯迪, 谢涛, 王升, 等. 太阳能甲烷干重整复杂反应体系的热化学储能特性[J]. 化工进展, 2019, 38(11): 4921-4929. |
| Xu K D, Xie T, Wang S, et al. Thermochemical energy storage characteristics of complex reaction system for solar methane dry reforming system [J]. Chemical Industry and Engineering Progress, 2019, 38(11): 4921-4929. | |
| 9 | Zhang Y, Wang R. Sorption thermal energy storage: concept, process, applications and perspectives[J]. Energy Storage Materials, 2020, 27: 352-369. |
| 10 | Clark R J, Mehrabadi A, Farid M. State of the art on salt hydrate thermochemical energy storage systems for use in building applications [J]. Journal of Energy Storage, 2020, 27: 101145. |
| 11 | 李威, 陈威, 王丹丹. 基于水合盐热化学储能的技术研究与进展[J]. 制冷与空调, 2017, 17(8): 14-21. |
| Li W, Chen W, Wang D D. Research and development of thermochemical energy storage based on hydrated salt [J]. Refrigeration and Air-Conditioning, 2017, 17(8): 14-21. | |
| 12 | 郝茂森, 刘洪芝, 王婉桐, 等. 水合盐热化学储热材料的研究进展[J]. 储能科学与技术, 2020, 9(3): 791-796. |
| Hao M S, Liu H Z, Wang W T, et al. Research progress of thermochemical heat storage materials of hydrated salts [J]. Energy Storage Science and Technology, 2020, 9(3): 791-796. | |
| 13 | 翁立奎, 张叶龙, 姜琳, 等. 基于水合盐的热化学吸附储热技术研究进展[J]. 储能科学与技术, 2020, 9(6): 1729-1736. |
| Weng L K, Zhang Y L, Jiang L, et al. Research progress on thermochemical adsorption heat storage technology based on hydrate [J]. Energy Starage Science and Technology, 2020, 9(6): 1729-1736. | |
| 14 | 李琳, 黄宏宇, 邓立生, 等. 低品位能源化学储热材料研究进展[J]. 化工进展, 2020, 39(9): 3608-3616. |
| Li L, Huang H Y, Deng L S, et al. Research progress of low-grade energy chemical heat storage materials [J] Chemical Industry and Engineering Progress, 2020, 39(9): 3608-3616. | |
| 15 | Fumey B, Weber R, Baldini L. Sorption based long-term thermal energy storage - process classification and analysis of performance limitations: a review [J]. Renewable and Sustainable Energy Reviews, 2019, 111: 57-74. |
| 16 | Okhrimenko L, Favergeon L, Johannes K, et al. New kinetic model of the dehydration reaction of magnesium sulfate hexahydrate: application for heat storage [J]. Thermochimica Acta, 2020, 687: 178569. |
| 17 | Li W, Zeng M, Wang Q W. Development and performance investigation of MgSO4/SrCl2 composite salt hydrate for mid-low temperature thermochemical heat storage [J]. Solar Energy Materials and Solar Cells, 2020, 210: 110509. |
| 18 | Zhang Y N, Wang R Z, Li T X. Thermochemical characterizations of high-stable activated alumina/LiCl composites with multistage sorption process for thermal storage [J]. Energy, 2018, 156: 240-249. |
| 19 | Xu J X, Li T X, Chao J W, et al. High energy-density multi-form thermochemical energy storage based on multi-step sorption processes [J]. Energy, 2019, 185: 1131-1142. |
| 20 | Wei S, Han R, Su Y, et al. Development of pomegranate-type CaCl2@C composites via a scalable one-pot pyrolysis strategy for solar-driven thermochemical heat storage [J]. Energy Conversion and Management, 2020, 212: 112694. |
| 21 | Nonnen T, Preißler H, Kött S, et al. Salt inclusion and deliquescence in salt/zeolite X composites for thermochemical heat storage [J]. Microporous and Mesoporous Materials, 2020, 303: 110239. |
| 22 | Togawa J, Kurokawa A, Nagano K. Water sorption property and cooling performance using natural mesoporous siliceous shale impregnated with LiCl for adsorption heat pump [J]. Applied Thermal Engineering, 2020, 173: 115241. |
| 23 | Calabrese L, Brancato V, Palomba V, et al. Magnesium sulphate-silicone foam composites for thermochemical energy storage: assessment of dehydration behaviour and mechanical stability [J]. Solar Energy Materials and Solar Cells, 2019, 200: 109992. |
| 24 | Ait Ousaleh H, Said S, Zaki A, et al. Silica gel/inorganic salts composites for thermochemical heat storage: Improvement of energy storage density and assessment of cycling stability [J]. Materials Today: Proceedings, 2020, 30: 937-941. |
| 25 | Li W, Klemeš J J, Wang Q W, et al. Development and characteristics analysis of salt-hydrate based composite sorbent for low-grade thermochemical energy storage [J]. Renewable Energy, 2020, 157: 920-940. |
| 26 | Cammarata A, Verda V, Sciacovelli A, et al. Hybrid strontium bromide-natural graphite composites for low to medium temperature thermochemical energy storage: formulation, fabrication and performance investigation [J]. Energy Conversion and Management, 2018, 166: 233-240. |
| 27 | Ait Ousaleh H, Sair S, Mansouri S, et al. New hybrid graphene/inorganic salt composites for thermochemical energy storage: synthesis, cyclability investigation and heat exchanger metal corrosion protection performance [J]. Solar Energy Materials and Solar Cells, 2020, 215: 110601. |
| 28 | Zhou H, Zhang D. Effect of graphene oxide aerogel on dehydration temperature of graphene oxide aerogel stabilized MgCl2⋅6H2O composites [J]. Solar Energy, 2019, 184: 202-208. |
| 29 | Li W, Klemeš J J, Wang Q W, et al. Performance analysis of consolidated sorbent based closed thermochemical energy storage reactor for environmental sustainability[J]. Journal of Cleaner Production, 2020, 265: 121821. |
| 30 | Li W, Guo H, Zeng M, et al. Performance of SrBr2·6H2O based seasonal thermochemical heat storage in a novel multilayered sieve reactor [J]. Energy Conversion and Management, 2019, 198: 111843. |
| [1] | 张双星, 刘舫辰, 张义飞, 杜文静. R-134a脉动热管相变蓄放热实验研究[J]. 化工学报, 2023, 74(S1): 165-171. |
| [2] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [3] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [4] | 陈爱强, 代艳奇, 刘悦, 刘斌, 吴翰铭. 基板温度对HFE7100液滴蒸发过程的影响研究[J]. 化工学报, 2023, 74(S1): 191-197. |
| [5] | 刘明栖, 吴延鹏. 导光管直径和长度对传热影响的模拟分析[J]. 化工学报, 2023, 74(S1): 206-212. |
| [6] | 王志国, 薛孟, 董芋双, 张田震, 秦晓凯, 韩强. 基于裂隙粗糙性表征方法的地热岩体热流耦合数值模拟与分析[J]. 化工学报, 2023, 74(S1): 223-234. |
| [7] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [8] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [9] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [10] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [11] | 肖明堃, 杨光, 黄永华, 吴静怡. 浸没孔液氧气泡动力学数值研究[J]. 化工学报, 2023, 74(S1): 87-95. |
| [12] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [13] | 王玉兵, 李杰, 詹宏波, 朱光亚, 张大林. R134a在菱形离散肋微小通道内的流动沸腾换热实验研究[J]. 化工学报, 2023, 74(9): 3797-3806. |
| [14] | 李科, 文键, 忻碧平. 耦合蒸气冷却屏的真空多层绝热结构对液氢储罐自增压过程的影响机制研究[J]. 化工学报, 2023, 74(9): 3786-3796. |
| [15] | 齐聪, 丁子, 余杰, 汤茂清, 梁林. 基于选择吸收纳米薄膜的太阳能温差发电特性研究[J]. 化工学报, 2023, 74(9): 3921-3930. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号