化工学报 ›› 2020, Vol. 71 ›› Issue (5): 2088-2098.DOI: 10.11949/0438-1157.20191357
尚华1( ),白洪灏1,刘佳奇1,杨江峰1,2(
),白洪灏1,刘佳奇1,杨江峰1,2( ),李晋平1,2
),李晋平1,2
收稿日期:2019-11-11
修回日期:2020-02-22
出版日期:2020-05-05
发布日期:2020-05-05
通讯作者:
杨江峰
作者简介:尚华(1992—),男,博士研究生,基金资助:
Hua SHANG1( ),Honghao BAI1,Jiaqi LIU1,Jiangfeng YANG1,2(
),Honghao BAI1,Jiaqi LIU1,Jiangfeng YANG1,2( ),Jinping LI1,2
),Jinping LI1,2
Received:2019-11-11
Revised:2020-02-22
Online:2020-05-05
Published:2020-05-05
Contact:
Jiangfeng YANG
摘要:
纯硅分子筛Silicalite-1原粉(Si/Al>470)在6 MPa压力下制成自支撑颗粒状吸附剂,经XRD和77 K氮气吸脱附表征表明自支撑颗粒型的Silicalite-1保留了原粉的晶体结构和比表面。静态重量法测试了273/298/313 K下CH4和N2在其上的吸附等温线,利用理想吸附溶液理论(IAST)法计算了吸附剂对CH4/N2的选择性。动态气体穿透实验测试了颗粒型Silicalite-1吸附剂对不同浓度CH4/N2混合气的分离效果,结果表明该吸附剂更适合于低浓度甲烷(20%/80% CH4/N2)的富集脱氮。通过总传质模型利用数值模拟预测了颗粒型Silicalite-1吸附剂的变压吸附分离(PSA)富集低浓度煤层气中甲烷的效果。模拟结果显示20%/80%的CH4/N2混合气经一次提浓,CH4浓度可以提升至37%~41%,回收率达到60%~92%;30%/70%的CH4/N2混合气经一次提浓,CH4浓度可以提升至50%~53%,回收率达到58%~92%。
中图分类号:
尚华, 白洪灏, 刘佳奇, 杨江峰, 李晋平. CH4-N2在自支撑颗粒型Silicalite-1上的吸附分离及PSA模拟[J]. 化工学报, 2020, 71(5): 2088-2098.
Hua SHANG, Honghao BAI, Jiaqi LIU, Jiangfeng YANG, Jinping LI. PSA simulation and adsorption separation of CH4-N2 by self-supporting pellets Silicalite-1[J]. CIESC Journal, 2020, 71(5): 2088-2098.
| Parameter | Expression |
|---|---|
| mass balance | |
| energy balance | |
| gas phase momentum | |
| Langmuir isotherm | |
| linear driving force model |
表1 模拟穿透实验中涉及到的方程
Table 1 Equations used in breakthrough simulation
| Parameter | Expression |
|---|---|
| mass balance | |
| energy balance | |
| gas phase momentum | |
| Langmuir isotherm | |
| linear driving force model |
| Parameter | Value |
|---|---|
| bed height, z/m | 0.15 |
| bed radius, Db/m | 9×10-3 |
| bed void fraction, εb | 0.43 |
| particle void fraction, εp | 0.35 |
| adsorbent particle radius, rp/m | 4×10-4 |
| adsorbent particle density, ρp/(kg/m3) | 1.21 |
| CH4 mass transfer coefficient, | 0.66 |
| N2 mass transfer coefficient, | 0.31 |
表2 吸附床和吸附剂参数
Table 2 Parameters of adsorption bed and adsorbent
| Parameter | Value |
|---|---|
| bed height, z/m | 0.15 |
| bed radius, Db/m | 9×10-3 |
| bed void fraction, εb | 0.43 |
| particle void fraction, εp | 0.35 |
| adsorbent particle radius, rp/m | 4×10-4 |
| adsorbent particle density, ρp/(kg/m3) | 1.21 |
| CH4 mass transfer coefficient, | 0.66 |
| N2 mass transfer coefficient, | 0.31 |
| Form | Applied pressure/MPa | BET surface area/(m2/g) | Langmuir surface area/(m2/g) | Total pore volume/(cm3/g) | Micropore volume/(cm3/g) | Crystal density/(g/cm3) | Bulk density/ (g/cm3) |
|---|---|---|---|---|---|---|---|
| powder | — | 384.69 | 565.77 | 0.20 | 0.12 | 1.81 | 0.62 |
| pellet | 6 | 390.25 | 577.18 | 0.21 | 0.12 | 1.21 |
表3 Silicalite-1粉末和颗粒的孔结构参数及相关密度
Table 3 Porosity data and related density of Silicalite-1 powder and particle
| Form | Applied pressure/MPa | BET surface area/(m2/g) | Langmuir surface area/(m2/g) | Total pore volume/(cm3/g) | Micropore volume/(cm3/g) | Crystal density/(g/cm3) | Bulk density/ (g/cm3) |
|---|---|---|---|---|---|---|---|
| powder | — | 384.69 | 565.77 | 0.20 | 0.12 | 1.81 | 0.62 |
| pellet | 6 | 390.25 | 577.18 | 0.21 | 0.12 | 1.21 |
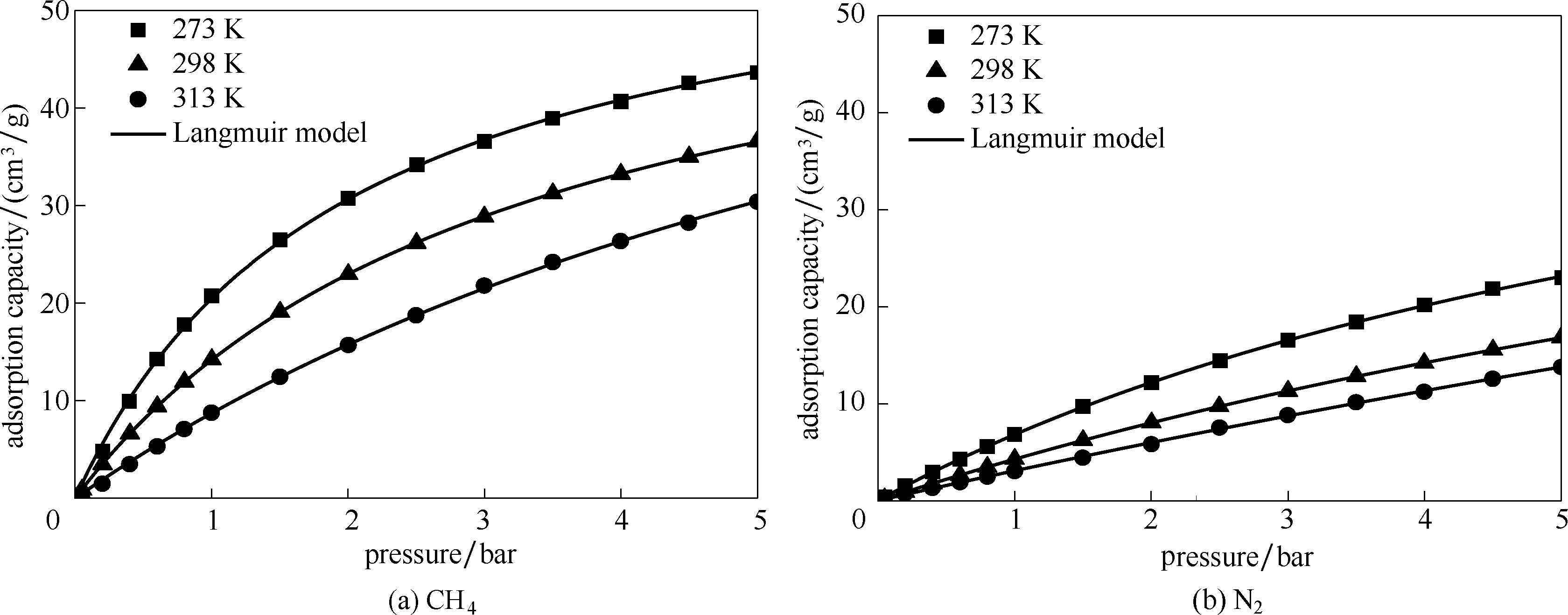
图7 不同温度下CH4和N2在颗粒型Silicalite-1吸附剂上的吸附等温线及Langmuir模拟
Fig.7 Pure gas adsorption isotherms of CH4 and N2 on particle-shaped Silicalite-1 at different temperature
| Gas | T/K | Langmuir model | ||
|---|---|---|---|---|
| qm,L/(cm3/g) | BL/bar-1 | R2 | ||
| CH4 | 273 | 61.11 | 0.50 | 0.9999 |
| 298 | 60.24 | 0.31 | 0.9999 | |
| 313 | 80.69 | 0.12 | 0.9999 | |
| N2 | 273 | 57.41 | 0.13 | 0.9999 |
| 298 | 62.00 | 0.07 | 0.9999 | |
| 313 | 101.68 | 0.03 | 0.9999 | |
表4 不同温度下Langmuir模型拟合的CH4和N2吸附等温线参数
Table 4 Langmuir model fitting parameters based on CH4 and N2 adsorption isotherm at different temperature
| Gas | T/K | Langmuir model | ||
|---|---|---|---|---|
| qm,L/(cm3/g) | BL/bar-1 | R2 | ||
| CH4 | 273 | 61.11 | 0.50 | 0.9999 |
| 298 | 60.24 | 0.31 | 0.9999 | |
| 313 | 80.69 | 0.12 | 0.9999 | |
| N2 | 273 | 57.41 | 0.13 | 0.9999 |
| 298 | 62.00 | 0.07 | 0.9999 | |
| 313 | 101.68 | 0.03 | 0.9999 | |
| Adsorbate | Condition | Capacity/(cm3/g) | |
|---|---|---|---|
| Ref.[ | This work | ||
| CH4 | 298 K,1 bar | 11.42 | 16.82 |
| N2 | 298 K,1 bar | 3.58 | 4.30 |
表5 本文测试结果与文献中吸附容量比较
Table 5 Comparison of adsorption capacities with literature data
| Adsorbate | Condition | Capacity/(cm3/g) | |
|---|---|---|---|
| Ref.[ | This work | ||
| CH4 | 298 K,1 bar | 11.42 | 16.82 |
| N2 | 298 K,1 bar | 3.58 | 4.30 |
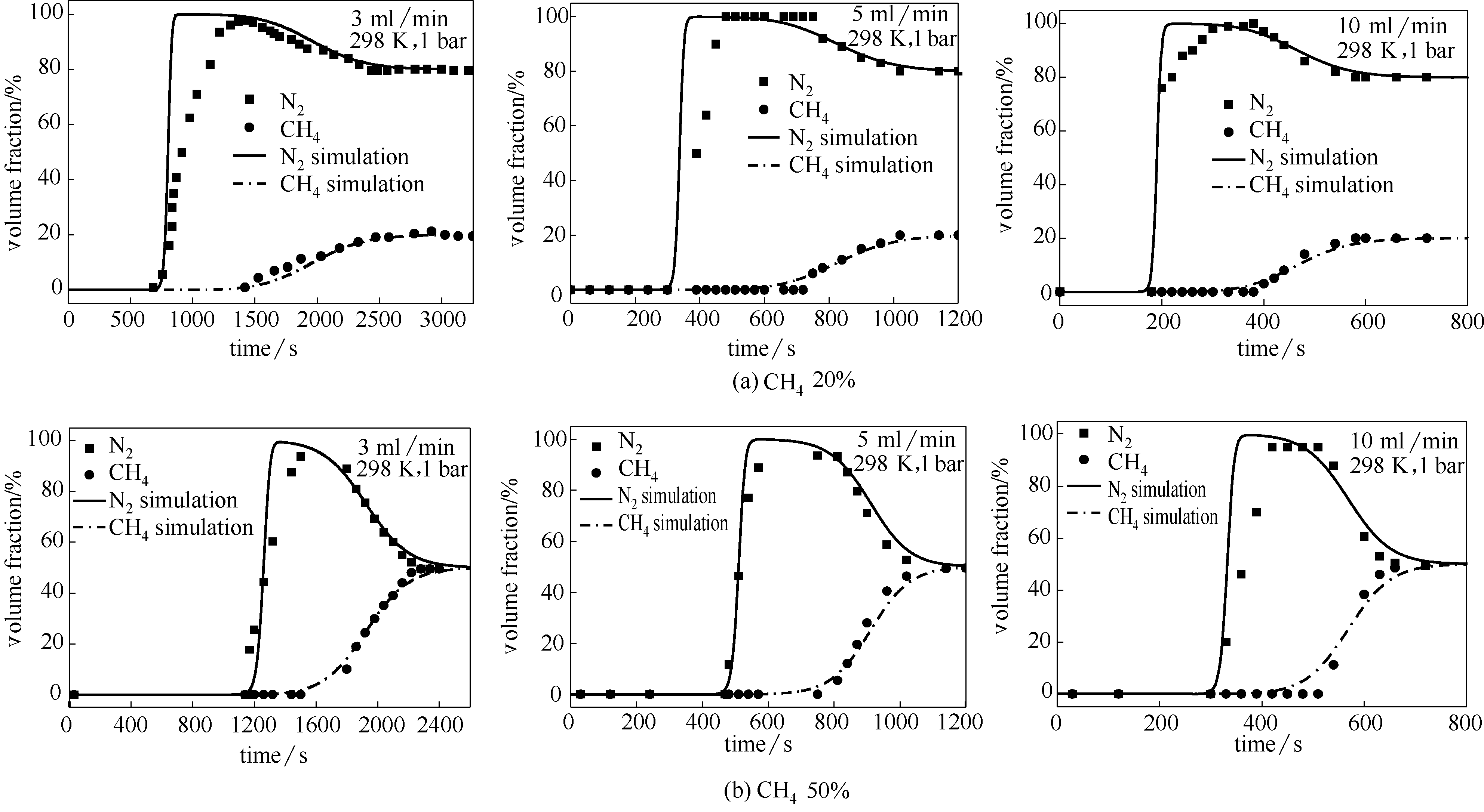
图10 颗粒型Silicalite-1吸附剂对甲烷浓度为20%和50%的CH4/N2混合气穿透实验及其模拟
Fig.10 Experimental and simulated breakthrough for 20% and 50% CH4/N2 mixtures on particle-shaped Silicalite-1
| Content/% | Flow/ (ml/min) | CH4 breakthrough time/s | N2 breakthrough time/s | Retention time/s | CH4 adsorption amount/(mmol/g) | N2 adsorption amount/(mmol/g) | CH4/N2 selectivity |
|---|---|---|---|---|---|---|---|
| 20 | 3 | 1420 | 683 | 737 | 0.154 | 0.222 | 2.77 |
| 5 | 720 | 350 | 370 | 0.124 | 0.206 | 2.41 | |
| 10 | 380 | 190 | 190 | 0.113 | 0.190 | 2.37 | |
| 50 | 3 | 1500 | 1140 | 360 | 0.397 | 0.234 | 1.70 |
| 5 | 750 | 470 | 280 | 0.389 | 0.210 | 1.85 | |
| 10 | 510 | 310 | 200 | 0.306 | 0.159 | 1.92 |
表6 原料气浓度和流量对穿透时间、保留时间、吸附量和CH4/N2吸附选择性的影响
Table 6 Effect of content and flow on breakthrough time, retention time, adsorbed amount and selectivity for CH4/N2
| Content/% | Flow/ (ml/min) | CH4 breakthrough time/s | N2 breakthrough time/s | Retention time/s | CH4 adsorption amount/(mmol/g) | N2 adsorption amount/(mmol/g) | CH4/N2 selectivity |
|---|---|---|---|---|---|---|---|
| 20 | 3 | 1420 | 683 | 737 | 0.154 | 0.222 | 2.77 |
| 5 | 720 | 350 | 370 | 0.124 | 0.206 | 2.41 | |
| 10 | 380 | 190 | 190 | 0.113 | 0.190 | 2.37 | |
| 50 | 3 | 1500 | 1140 | 360 | 0.397 | 0.234 | 1.70 |
| 5 | 750 | 470 | 280 | 0.389 | 0.210 | 1.85 | |
| 10 | 510 | 310 | 200 | 0.306 | 0.159 | 1.92 |
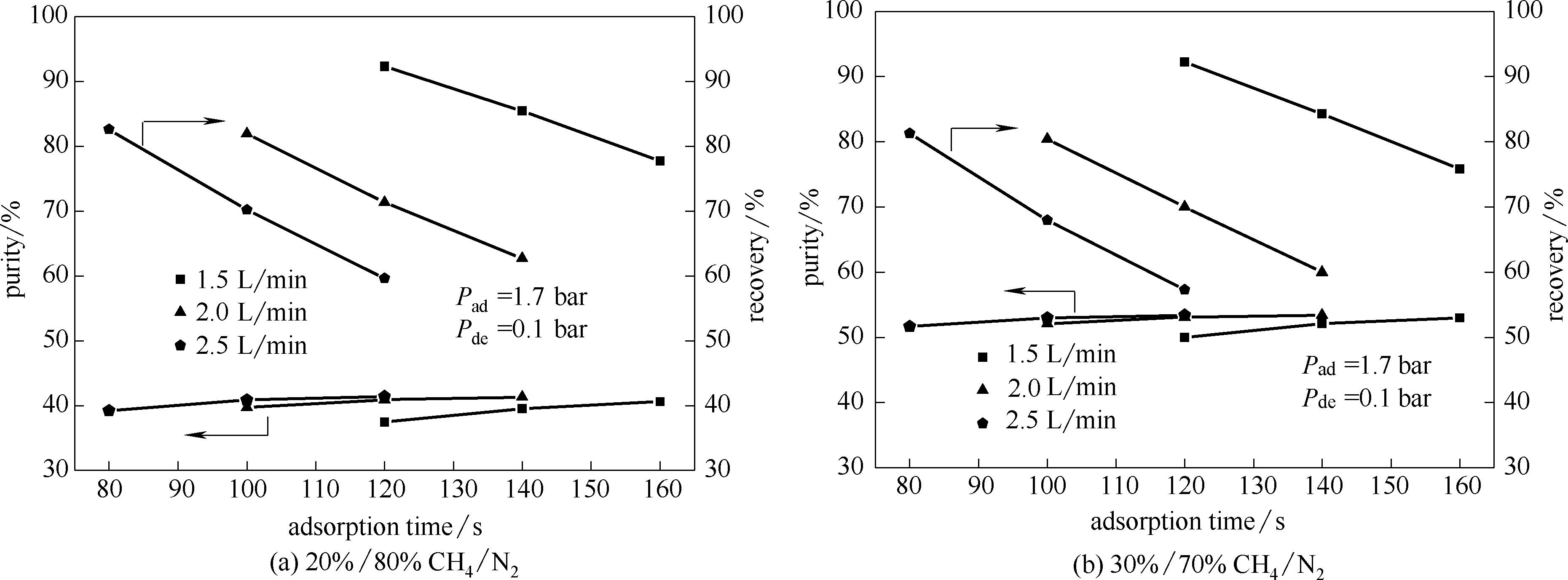
图12 预测颗粒型Silicalite-1吸附剂对低浓度煤层气中甲烷含量为20%和30%的富集效果
Fig.12 Prediction of enrichment effect of particle-shaped Silicalite-1 on methane in low concentration coalbed methane
| 1 | Águeda V I, Delgado J A, Álvarez S, et al. Modeling and simulation of the efficient separation of methane/nitrogen mixtures with [Ni3(HCOO)6] MOF by PSA[J]. Chemical Engineering Journal, 2019, 361: 1007-1018. |
| 2 | Effendy S, Xu C, Farooq S. Optimization of a pressure swing adsorption process for nitrogen rejection from natural gas[J]. Industrial & Engineering Chemistry Research, 2017, 56(18): 5417-5431. |
| 3 | Kim J, Maiti A, Lin L C, et al. New materials for methane capture from dilute and medium-concentration sources[J]. Nature Communications, 2013, 4: 1694. |
| 4 | 郑德志. 我国煤层气产业政策评价研究[J]. 煤炭经济研究, 2019, 39(1): 62-65. |
| Zheng D Z. Research on China's coalbed methane industry policy evaluation[J]. Coal Economic Research, 2019, 39(1): 62-65. | |
| 5 | Bhadra S, Farooq S. Separation of methane-nitrogen mixture by pressure swing adsorption for natural gas upgrading[J]. Industrial & Engineering Chemistry Research, 2011, 50(24): 14030-14045. |
| 6 | 杨颖, 曲冬蕾, 李平, 等. 低浓度煤层气吸附浓缩技术研究与发展[J]. 化工学报, 2018, 69(11): 4518-4529. |
| Yang Y, Qu D L, Li P, et al. Research and development on enrichment of low concentration coal mine methane by adsorption technology[J]. CIESC Journal, 2018, 69(11): 4518-4529. | |
| 7 | 吕秋楠, 李小森, 徐纯刚, 等. 低浓度煤层气分离提纯的研究进展[J]. 化工进展, 2013, 32(6): 1267-1272. |
| Lyu Q N, Li X S, Xu C G, et al. Progress of purification technology for low concentration coal-bed methane[J]. Chemical Industry and Engineering Progress, 2013, 32(6): 1267-1272. | |
| 8 | 聂李红, 徐绍平, 苏艳敏, 等. 低浓度煤层气提纯的研究现状[J]. 化工进展, 2008, (10): 1505-1511+1521. |
| Nie L H, Xu S P, Su Y M, et al. Progress of recovery of low concentration coal bed methane[J]. Chemical Industry and Engineering Progress, 2008, (10): 1505-1511+1521. | |
| 9 | Kuo J C, Wang K H, Chen C. Pros and cons of different nitrogen removal unit(NRU) technology[J]. Journal of Natural Gas Science and Engineering, 2012, 7: 52-59. |
| 10 | Liu C M, Zhou Y P, Sun Y, et al. Enrichment of coal-bed methane by PSA complemented with CO2 displacement[J]. AIChE J., 2011, 57(3): 645-654. |
| 11 | 杨江峰, 赵强, 于秋红, 等. 煤层气回收及CH4/N2分离PSA材料的研究进展[J]. 化工进展, 2011, 30(4): 793-801. |
| Yang J F, Zhao Q, Yu Q H, et al. Progress of recovery of coal bed methane and adsorption materials for separation of CH4/N2 by pressure swing adsorption[J]. Chemical Industry and Engineering Progress, 2011, 30(4): 793-801. | |
| 12 | Ruthven D M. Past progress and future challenges in adsorption research[J]. Industrial & Engineering Chemistry Research, 2000, 39(7): 2127-2131. |
| 13 | Kaerger J, Ruthven D M. Diffusion in nanoporous materials: fundamental principles, insights and challenges[J]. New Journal of Chemistry, 2016, 40(5): 4027-4048. |
| 14 | Sethia G, Somani R S, Bajaj H C. Sorption of methane and nitrogen on cesium exchanged zeolite-X: structure, cation position and adsorption relationship[J]. Industrial & Engineering Chemistry Research, 2014, 53(16): 6807-6814. |
| 15 | 刘海庆, 吴一江, 杨颖, 等. 沸石ZSM-5吸附回收低浓度煤层气中CH4[J]. 化工学报, 2016, 67(5): 1931-1941. |
| Liu H Q, Wu Y J, Yang Y, et al. Adsorption and recovery of low concentration coal-bed methane by zeolite ZSM-5[J]. CIESC Journal, 2016, 67(5): 1931-1941. | |
| 16 | Mello M R, Phanon D, Silveira G Q, et al. Amine-modified MCM-41 mesoporous silica for carbon dioxide capture[J]. Microporous and Mesoporous Materials, 2011, 143(1): 174-179. |
| 17 | Pan H Y, Zhao J Y, Lin Q, et al. Preparation and characterization of activated carbons from bamboo sawdust and its application for CH4 selectivity adsorption from a CH4/N2 system[J]. Energy & Fuels, 2016, 30(12): 10730-10738. |
| 18 | Zhang J H, Qu S J, Li L T, et al. Preparation of carbon molecular sieves used for CH4/N2 separation[J]. Journal of Chemical & Engineering Data, 2018, 63(5): 1737-1744. |
| 19 | Yang Y, Ribeiro A M, Li P, et al. Adsorption equilibrium and kinetics of methane and nitrogen on carbon molecular sieve[J]. Industrial & Engineering Chemistry Research, 2014, 53(43): 16840-16850. |
| 20 | Majumdar B, Bhadra S, Marathe R, et al. Adsorption and diffusion of methane and nitrogen in barium exchanged ETS-4[J]. Industrial & Engineering Chemistry Research, 2011, 50(5): 3021-3034. |
| 21 | Yang J F, Yu Q H, Zhao Q, et al. Adsorption CO2, CH4 and N2 on two different spacing flexible layer MOFs[J]. Microporous and Mesoporous Materials, 2012, 161: 154-159. |
| 22 | Saha D, Bao Z B, Jia F, et al. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A[J]. Environmental Science & Technology, 2010, 44(5): 1820-1826. |
| 23 | 贾晓霞, 王丽, 元宁, 等. 二价铬/钼/镍空配位MOFs的CH4/N2吸附分离研究[J]. 化工学报, 2018, 69(9): 3896-3904. |
| Jia X X, Wang L, Yuan N, et al. CH4/N2 adsorption separation research of MOFs with divalent Cr/Mo/Ni unsaturated metal sites [J]. CIESC Journal, 2018, 69(9): 3896-3904. | |
| 24 | Ho M T, Allinson G W, Wiley D E. Reducing the cost of CO2 capture from flue gases using pressure swing adsorption[J]. Industrial & Engineering Chemistry Research, 2008, 47(14): 4883-4890. |
| 25 | Epiepang F E, Yang X, Li J, et al. Air separation sorbents: mixed-cation zeolites with minimum lithium and silver[J]. Chemical Engineering Science, 2019, 198: 43-51. |
| 26 | Fan M, Panezai H, Sun J, et al. Thermal and kinetic performance of water desorption for N2 adsorption in Li-LSX zeolite[J]. The Journal of Physical Chemistry C, 2014, 118(41): 23761-23767. |
| 27 | Bao Z B, Yu L, Dou T, et al. Adsorption equilibria of CO2, CH4, N2, O2, and Ar on high silica zeolites[J]. Journal of Chemical & Engineering Data, 2011, 56(11): 4017-4023. |
| 28 | Yang J F, Li J M, Wang W, et al. Adsorption of CO2, CH4, and N2 on 8-, 10-, and 12-membered ring hydrophobic microporous high-silica zeolites: DDR, silicalite-1, and beta[J]. Industrial & Engineering Chemistry Research, 2013, 52(50): 17856-7864. |
| 29 | 宋汉成, 焦文玲, 李娟娟, 等. 煤层气利用与输送的安全性[J]. 燃气与热力, 2006, 26(11): 8-11. |
| Song H C, Jiao W L, Li J J, et al. Safety of utilization and transmission of coalbed methane[J]. Gas & Heat, 2006, 26(11): 8-11. | |
| 30 | Myers A L, Prausnitz J M. Thermodynamics of mixed-gas adsorption[J]. AIChE J., 1965, 11(1): 121-127. |
| 31 | Rudzinski W, Everett D H. Adsorption of Gases on Heterogeneous Surfaces[M]. Cornwall: Academic Press, 2012: 59-64. |
| 32 | Yang J F, Yuan N, Xu M, et al. Enhanced mass transfer on hierarchical porous pure silica zeolite used for gas separation[J]. Microporous and Mesoporous Materials, 2018, 266: 56-63. |
| 33 | Yang J F, Shang H, Krishna R, et al. Adjusting the proportions of extra-framework K+ and Cs+ cations to construct a “molecular gate” on ZK-5 for CO2 removal[J]. Microporous and Mesoporous Materials, 2018, 268: 50-57. |
| 34 | Shang H, Li Y P, Liu J Q, et al. CH4/N2 separation on methane molecules grade diameter channel molecular sieves with a CHA-type structure[J]. Chinese Journal of Chemical Engineering, 2019, 27(5): 1044-1049. |
| 35 | Sing K S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity(Recommendations 1984)[J]. Pure and Applied Chemistry, 1985, 57(4): 603-619. |
| 36 | Lestinsky P, Vecer M, Navratil P, et al. The removal of CO2 from biogas using a laboratory PSA unit: design using breakthrough curves[J]. Clean Technologies and Environmental Policy, 2015, 17(5): 1281-1289. |
| 37 | Liu W J, Jiang H, Tian K, et al. Mesoporous carbon stabilized MgO nanoparticles synthesized by pyrolysis of MgCl2 preloaded waste biomass for highly efficient CO2 capture[J]. Environmental Science & Technology, 2013, 47(16): 9397-9403. |
| 38 | Bakhtyari A, Mofarahi M. Pure and binary adsorption equilibria of methane and nitrogen on zeolite 5A[J]. Journal of Chemical & Engineering Data, 2014, 59(3): 626-639. |
| 39 | Mofarahi M, Bakhtyari A. Experimental investigation and thermodynamic modeling of CH4/N2 adsorption on zeolite 13X[J]. Journal of Chemical & Engineering Data, 2015, 60(3): 683-696. |
| 40 | Kumar K V, Gadipelli S, Wood B, et al. Characterization of the adsorption site energies and heterogeneous surfaces of porous materials[J]. Journal of Materials Chemistry A, 2019, 7(17): 10104-10137. |
| 41 | Birkmann F, Pasel C, Luckas M, et al. Adsorption thermodynamics and kinetics of light hydrocarbons on microporous activated carbon at low temperatures[J]. Industrial & Engineering Chemistry Research, 2018, 57(23): 8023-8035. |
| [1] | 牛超, 沈胜强, 杨艳, 潘泊年, 李熠桥. 甲烷BOG喷射器流动过程计算与性能分析[J]. 化工学报, 2023, 74(7): 2858-2868. |
| [2] | 刘晓洋, 喻健良, 侯玉洁, 闫兴清, 张振华, 吕先舒. 螺旋微通道对掺氢甲烷爆轰传播的影响[J]. 化工学报, 2023, 74(7): 3139-3148. |
| [3] | 周小文, 杜杰, 张战国, 许光文. 基于甲烷脉冲法的Fe2O3-Al2O3载氧体还原特性研究[J]. 化工学报, 2023, 74(6): 2611-2623. |
| [4] | 胡晗, 杨亮, 李春晓, 刘道平. 天然烟浸滤液水合物法储甲烷动力学研究[J]. 化工学报, 2023, 74(3): 1313-1321. |
| [5] | 彭晓婉, 郭笑楠, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8浆液法分离CH4/N2的双吸收-吸附塔工艺流程建模与模拟[J]. 化工学报, 2023, 74(2): 784-795. |
| [6] | 孟金琳, 汪宇, 张群锋, 叶光华, 周兴贵. 介孔材料低温氮气吸脱附的孔道网络模型[J]. 化工学报, 2023, 74(2): 893-903. |
| [7] | 廖珊珊, 张少刚, 陶骏骏, 刘家豪, 汪金辉. 竖直射流火撞击障碍管道数值模拟分析[J]. 化工学报, 2022, 73(9): 4226-4234. |
| [8] | 沈嘉辉, 王侃宏, 郁达伟, 胡大洲, 魏源送. 游离氨调理污泥厌氧消化优化产甲烷过程与强化有机物释放[J]. 化工学报, 2022, 73(9): 4147-4155. |
| [9] | 唐翠萍, 张雅楠, 梁德青, 李祥. 聚乙烯己内酰胺链端改性及其对甲烷水合物形成的抑制作用研究[J]. 化工学报, 2022, 73(5): 2130-2139. |
| [10] | 闫帅, 杨家宝, 龚岩, 郭庆华, 于广锁. CO2稀释对甲烷反扩散火焰结构的影响研究[J]. 化工学报, 2022, 73(3): 1335-1342. |
| [11] | 韩昌亮, 辛镜青, 于广滨, 刘俊秀, 许麒澳, 姚安卡, 尹鹏. 微通道内超临界氮气三维热流场实验与数值模拟[J]. 化工学报, 2022, 73(2): 653-662. |
| [12] | 王保文, 张港, 刘同庆, 李炜光, 王梦家, 林德顺, 马晶晶. CeO2/CuFe2O4氧载体CH4化学链重整耦合CO2热催化还原研究[J]. 化工学报, 2022, 73(12): 5414-5426. |
| [13] | 杨霄, 丁锐, 李墨含, 宋正昶. 氧浓度对微通道内甲烷均相/非均相耦合反应特性的影响[J]. 化工学报, 2022, 73(12): 5427-5437. |
| [14] | 胡慧慧, 杨亮, 刘道平, 张柯. 低剂量超吸水树脂溶液微滴中甲烷水合物生成动力学[J]. 化工学报, 2022, 73(10): 4659-4667. |
| [15] | 吴一, 温小萍, 张素梅, 郭志东, 邓浩鑫, 纪文涛. 垂直圆管内掺氢甲烷燃烧不稳定性研究[J]. 化工学报, 2022, 73(10): 4780-4790. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号