化工学报 ›› 2020, Vol. 71 ›› Issue (8): 3444-3451.DOI: 10.11949/0438-1157.20191577
收稿日期:2019-12-25
修回日期:2020-05-25
出版日期:2020-08-05
发布日期:2020-08-05
通讯作者:
施云海
作者简介:谈金辉(1994—),男,硕士研究生,基金资助:
Jinhui TAN( ),Jumei XU,Yunhai SHI(
),Jumei XU,Yunhai SHI( )
)
Received:2019-12-25
Revised:2020-05-25
Online:2020-08-05
Published:2020-08-05
Contact:
Yunhai SHI
摘要:
采用Ellis汽液两相双循环平衡蒸馏仪测定了常压下乙醇(1)-水(2)和乙醇(1)-水(2)-醋酸钾(3)物系的汽液平衡数据,结果表明:醋酸钾对乙醇(1)-水(2)系统具有盐析效应,乙醇对水的相对挥发度α12上升。系统中10%(质量)的醋酸钾已经消除了乙醇-水间的共沸点,可作为加盐萃取精馏的分离剂。采用已授权的Aspen Plus软件自带的eNRTL、Wilson和UNIQUAC模型,以及eNRTL模型分别对乙醇(1)-水(2)、乙醇(1)-水(2)-10%(质量)醋酸钾(3)系统实验数据进行了关联,计算结果表明:乙醇(1)-水(2)系统中,平衡温度的平均绝对偏差(AAD)为0.72℃(eNRTL)、0.78℃(Wilson)和0.71℃(UNIQUAC),气相组成计算值的平均绝对偏差为0.0083(eNRTL)、0.0077(Wilson)和0.0101(UNIQUAC)。而在乙醇(1)-水(2)-10%(质量)醋酸钾(3)系统中平衡温度和气相组成平均绝对偏差分别为0.25℃和0.0168(eNRTL)。
中图分类号:
谈金辉, 徐菊美, 施云海. 常压下乙醇-水-醋酸钾系统汽液平衡数据的测定与关联[J]. 化工学报, 2020, 71(8): 3444-3451.
Jinhui TAN, Jumei XU, Yunhai SHI. Measurement and correlation of vapor-liquid equilibria for ethanol-water-potassium acetate system under atmospheric pressure[J]. CIESC Journal, 2020, 71(8): 3444-3451.
| 原料 | 纯度/ %(质量) | 原料及纯度来源 | 沸点/℃ | Antoine常数① | 温度范围, t /℃ | |||
|---|---|---|---|---|---|---|---|---|
| 文献值 | 测定值 | A | B | C | ||||
| 无水乙醇,AR | >99.7 | 上海泰坦科技股份有限公司 | 78.29 | 78.15 | 8.0449 | 1554.3 | 222.65 | 1~197 |
| 无水醋酸钾,AR | >99.0 | 上海碧云天生物技术有限公司 | — | — | — | — | — | — |
| 超纯水 | >99.9 | 自制,气相色谱检测未见杂峰,经ICP检测,钙镁离子 总量低于0.1%(质量) | 100.00 | 99.87 | 8.0713 | 1730.6 | 233.42 | 1~100 |
表1 实验试剂及纯物质的物理性质[7]
Table 1 Chemical source and physical properties of pure components[7]
| 原料 | 纯度/ %(质量) | 原料及纯度来源 | 沸点/℃ | Antoine常数① | 温度范围, t /℃ | |||
|---|---|---|---|---|---|---|---|---|
| 文献值 | 测定值 | A | B | C | ||||
| 无水乙醇,AR | >99.7 | 上海泰坦科技股份有限公司 | 78.29 | 78.15 | 8.0449 | 1554.3 | 222.65 | 1~197 |
| 无水醋酸钾,AR | >99.0 | 上海碧云天生物技术有限公司 | — | — | — | — | — | — |
| 超纯水 | >99.9 | 自制,气相色谱检测未见杂峰,经ICP检测,钙镁离子 总量低于0.1%(质量) | 100.00 | 99.87 | 8.0713 | 1730.6 | 233.42 | 1~100 |
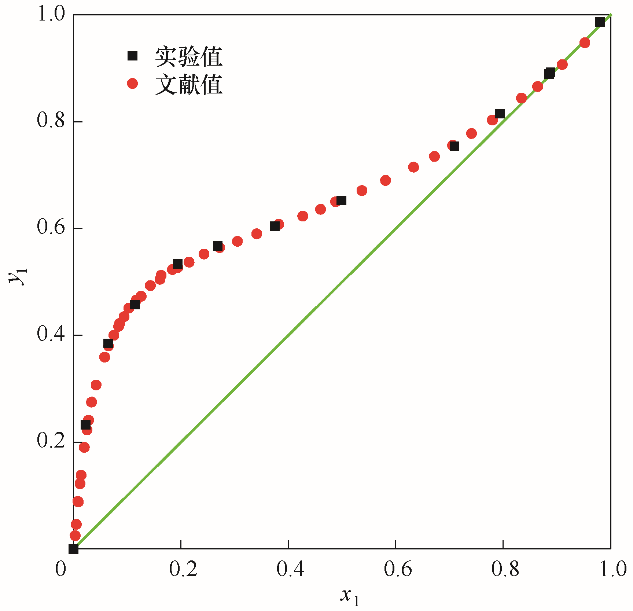
图1 101.325 kPa下乙醇(1)-水(2)系统实验值与文献值[7]的汽液平衡相图对比
Fig.1 Comparison of the VLE diagrams from experimental data and literature data[7] for the system of EtOH(1)-H2O(2) at 101.325 kPa
| t /℃ | x1 | y1 | γ1 | γ2 |
|---|---|---|---|---|
| 99.48 | 0.0000 | 0.0000 | — | 1.0000 |
| 93.71 | 0.0225 | 0.2326 | 5.8912 | 0.9859 |
| 87.75 | 0.0646 | 0.3850 | 4.1914 | 1.0329 |
| 84.17 | 0.1146 | 0.4576 | 3.2006 | 1.1056 |
| 81.99 | 0.1942 | 0.5334 | 2.3880 | 1.1390 |
| 80.80 | 0.2684 | 0.5675 | 1.9227 | 1.2194 |
| 80.04 | 0.3751 | 0.6041 | 1.5074 | 1.3474 |
| 79.04 | 0.4987 | 0.6524 | 1.2722 | 1.5357 |
| 77.82 | 0.7093 | 0.7541 | 1.0836 | 1.9692 |
| 77.60 | 0.7936 | 0.8149 | 1.0555 | 2.1069 |
| 77.48 | 0.8858 | 0.8889 | 1.0363 | 2.2973 |
| 77.40 | 0.8882 | 0.8925 | 1.0410 | 2.2781 |
| 77.25 | 0.9803 | 0.9868 | 1.0489 | 1.5977 |
表2 101.325 kPa下乙醇(1)-水(2)系统的汽液平衡数据
Table 2 VLE data for the system of EtOH(1)-H2O(2) at 101.325 kPa
| t /℃ | x1 | y1 | γ1 | γ2 |
|---|---|---|---|---|
| 99.48 | 0.0000 | 0.0000 | — | 1.0000 |
| 93.71 | 0.0225 | 0.2326 | 5.8912 | 0.9859 |
| 87.75 | 0.0646 | 0.3850 | 4.1914 | 1.0329 |
| 84.17 | 0.1146 | 0.4576 | 3.2006 | 1.1056 |
| 81.99 | 0.1942 | 0.5334 | 2.3880 | 1.1390 |
| 80.80 | 0.2684 | 0.5675 | 1.9227 | 1.2194 |
| 80.04 | 0.3751 | 0.6041 | 1.5074 | 1.3474 |
| 79.04 | 0.4987 | 0.6524 | 1.2722 | 1.5357 |
| 77.82 | 0.7093 | 0.7541 | 1.0836 | 1.9692 |
| 77.60 | 0.7936 | 0.8149 | 1.0555 | 2.1069 |
| 77.48 | 0.8858 | 0.8889 | 1.0363 | 2.2973 |
| 77.40 | 0.8882 | 0.8925 | 1.0410 | 2.2781 |
| 77.25 | 0.9803 | 0.9868 | 1.0489 | 1.5977 |
| t /℃ | x1 | y1 | γ1 | γ2 |
|---|---|---|---|---|
| 99.85 | 0.0000 | 0.0000 | — | 1.0000 |
| 90.66 | 0.0451 | 0.3968 | 5.7746 | 0.9196 |
| 88.05 | 0.0974 | 0.5331 | 4.2918 | 0.9096 |
| 83.06 | 0.1530 | 0.5947 | 3.5628 | 0.9924 |
| 81.34 | 0.2564 | 0.6204 | 2.3333 | 1.1173 |
| 80.33 | 0.3376 | 0.6575 | 1.9050 | 1.1489 |
| 79.66 | 0.4124 | 0.6835 | 1.6729 | 1.2364 |
| 78.83 | 0.5168 | 0.7425 | 1.4935 | 1.2619 |
| 78.38 | 0.6456 | 0.7960 | 1.3099 | 1.3942 |
| 78.01 | 0.7989 | 0.8676 | 1.1723 | 1.6214 |
| 77.73 | 0.8768 | 0.9200 | 1.1304 | 1.5948 |
| 77.84 | 0.9427 | 0.9655 | 1.1007 | 1.4772 |
| 78.14 | 0.9946 | 0.9974 | 1.0751 | 1.1993 |
表3 101.325 kPa下乙醇(1)-水(2)-10%(质量)醋酸钾(3)系统的汽液平衡数据
Table 3 VLE data for the system of EtOH(1)-H2O(2)-10%(mass) KAc(3) at 101.325 kPa
| t /℃ | x1 | y1 | γ1 | γ2 |
|---|---|---|---|---|
| 99.85 | 0.0000 | 0.0000 | — | 1.0000 |
| 90.66 | 0.0451 | 0.3968 | 5.7746 | 0.9196 |
| 88.05 | 0.0974 | 0.5331 | 4.2918 | 0.9096 |
| 83.06 | 0.1530 | 0.5947 | 3.5628 | 0.9924 |
| 81.34 | 0.2564 | 0.6204 | 2.3333 | 1.1173 |
| 80.33 | 0.3376 | 0.6575 | 1.9050 | 1.1489 |
| 79.66 | 0.4124 | 0.6835 | 1.6729 | 1.2364 |
| 78.83 | 0.5168 | 0.7425 | 1.4935 | 1.2619 |
| 78.38 | 0.6456 | 0.7960 | 1.3099 | 1.3942 |
| 78.01 | 0.7989 | 0.8676 | 1.1723 | 1.6214 |
| 77.73 | 0.8768 | 0.9200 | 1.1304 | 1.5948 |
| 77.84 | 0.9427 | 0.9655 | 1.1007 | 1.4772 |
| 78.14 | 0.9946 | 0.9974 | 1.0751 | 1.1993 |
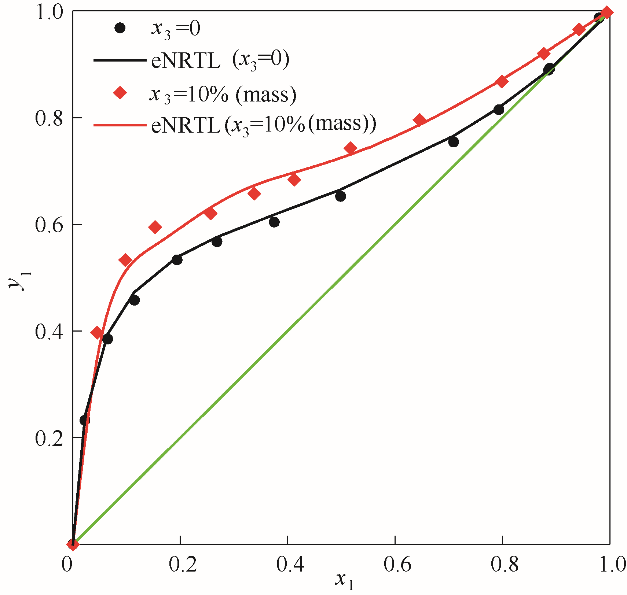
图3 101.325 kPa下乙醇(1)-水(2)加入醋酸钾后对y1-x1相图的影响
Fig.3 Influence of solution with potassium acetate on the y1-x1 diagram for the system of EtOH(1)-H2O(2) at 101.325 kPa
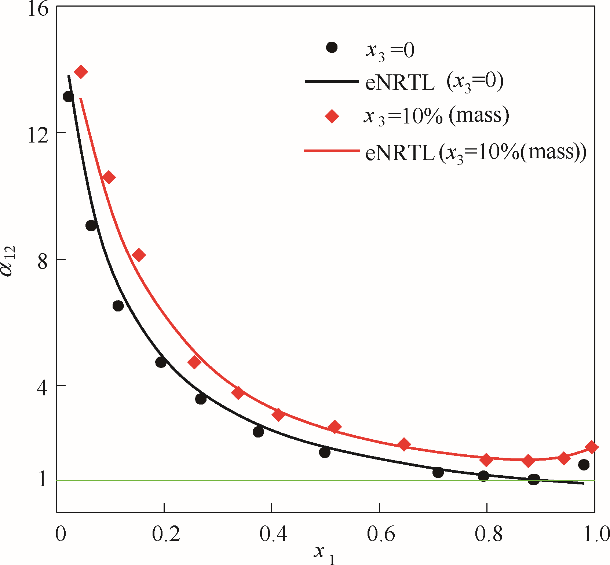
图4 101.325 kPa下乙醇(1)-水(2)加入醋酸钾后对其相对挥发度的影响
Fig.4 Influence of solution with potassium acetate on the relative volatilities for the system of EtOH(1)-H2O(2) at 101.325 kPa
| 热力学模型 | 二元系统的方程特点 | 适用范围 | 优势 | 文献来源 |
|---|---|---|---|---|
| Wilson | 双参数方程 | 互溶系统 | 精度高、计算简单 | [ |
| NRTL | 三参数方程 | 互溶系统、部分互溶系统 | 应用范围广、精度接近Wilson | [ |
| UNIQUAC | 双参数方程、算式复杂 | 互溶系统、部分互溶系统、大分子的聚合溶液 | 应用范围广,且适用于特殊系统 | [ |
表4 模型特点
Table 4 Characteristics of the models
| 热力学模型 | 二元系统的方程特点 | 适用范围 | 优势 | 文献来源 |
|---|---|---|---|---|
| Wilson | 双参数方程 | 互溶系统 | 精度高、计算简单 | [ |
| NRTL | 三参数方程 | 互溶系统、部分互溶系统 | 应用范围广、精度接近Wilson | [ |
| UNIQUAC | 双参数方程、算式复杂 | 互溶系统、部分互溶系统、大分子的聚合溶液 | 应用范围广,且适用于特殊系统 | [ |
| 模型 | 模型参数 | RMSD | AAD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A12 | A21 | B12/K | B21/K | C12 | |?t|/ ℃ | |?p|/kPa | |?x1| | |?y1| | |?t|/ ℃ | |?p|/kPa | |?x1| | |?y1| | |
| eNRTL | 237.5640 | 14.5201 | -28855.48 | -4589.96 | 0.3 | 0.75 | 0.20 | 4.84×10-5 | 0.0095 | 0.72 | 0.29 | 3.73×10-5 | 0.0083 |
| Wilson | 6.4947 | -0.5533 | -3115.36 | 188.88 | 0.82 | 0.22 | 9.59×10-5 | 0.0098 | 0.78 | 0.21 | 6.43×10-5 | 0.0077 | |
| UNIQUAC | -18.0414 | 13.3354 | 6409.59 | -4905.56 | 0.80 | 0.23 | 6.58×10-5 | 0.0141 | 0.71 | 0.18 | 5.50×10-5 | 0.0101 | |
表5 101.325 kPa下乙醇(1)-水(2)系统的活度系数模型参数和回归偏差
Table 5 Parameters and deviations of activity coefficient models for the system of EtOH(1)-H2O(2) at 101.325 kPa
| 模型 | 模型参数 | RMSD | AAD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A12 | A21 | B12/K | B21/K | C12 | |?t|/ ℃ | |?p|/kPa | |?x1| | |?y1| | |?t|/ ℃ | |?p|/kPa | |?x1| | |?y1| | |
| eNRTL | 237.5640 | 14.5201 | -28855.48 | -4589.96 | 0.3 | 0.75 | 0.20 | 4.84×10-5 | 0.0095 | 0.72 | 0.29 | 3.73×10-5 | 0.0083 |
| Wilson | 6.4947 | -0.5533 | -3115.36 | 188.88 | 0.82 | 0.22 | 9.59×10-5 | 0.0098 | 0.78 | 0.21 | 6.43×10-5 | 0.0077 | |
| UNIQUAC | -18.0414 | 13.3354 | 6409.59 | -4905.56 | 0.80 | 0.23 | 6.58×10-5 | 0.0141 | 0.71 | 0.18 | 5.50×10-5 | 0.0101 | |
| 组分 | 模型参数 | RMSD | AAD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| i | j | Aij | Aji | Bij/K | Bji/K | Cij | |?t|/ ℃ | |?p|/ kPa | |?x1| | |?y1| | |?t|/ ℃ | |?p|/ kPa | |?x1| | |?y1| |
| 水 | [K+][Ac-] [K+][Ac-] | 5.4421 | -0.2720 | -0.7767 | -0.1618 | 0.2 | 0.30 | 0.073 | 6.23×10-5 | 0.0199 | 0.25 | 0.060 | 4.02×10-5 | 0.0168 |
| 乙醇 | 10.2812 | -2.5372 | 0.0866 | 179.4947 | 0.2 | |||||||||
表6 101.325 kPa下乙醇(1)-水(2)-10%(质量)醋酸钾(3)系统的eNRTL活度系数模型参数和回归偏差
Table 6 Parameters and deviations of eNRTL activity coefficient model for the system of EtOH(1)-H2O(2)-10%(mass) KAc(3) at 101.325 kPa
| 组分 | 模型参数 | RMSD | AAD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| i | j | Aij | Aji | Bij/K | Bji/K | Cij | |?t|/ ℃ | |?p|/ kPa | |?x1| | |?y1| | |?t|/ ℃ | |?p|/ kPa | |?x1| | |?y1| |
| 水 | [K+][Ac-] [K+][Ac-] | 5.4421 | -0.2720 | -0.7767 | -0.1618 | 0.2 | 0.30 | 0.073 | 6.23×10-5 | 0.0199 | 0.25 | 0.060 | 4.02×10-5 | 0.0168 |
| 乙醇 | 10.2812 | -2.5372 | 0.0866 | 179.4947 | 0.2 | |||||||||

图5 101.325 kPa下乙醇(1)-水(2)加入10%(质量)醋酸钾(3)前、后的t-x1-y1相图
Fig.5 t-x1-y1 diagram for the system of EtOH(1)-H2O(2) before and after adding 10%(mass) potassium acetate(3) at 101.325 kPa
| 1 | 杨亚鸣, 范章豪, 吴淑晶, 等. 加盐萃取精馏分离乙醇-水体系的研究进展[J]. 河南化工, 2014, 31(10): 21-23. |
| Yang Y M, Fan Z H, Wu S J, et al. Research progress of separation of ethanol by water system extractive distillation with salt[J]. Henan Chemical Industry, 2014, 31(10): 21-23. | |
| 2 | 黄文瀛. 乙醇水系统的加盐萃取蒸馏[J]. 江西工业大学学报, 1991, 13(3): 9-12. |
| Huang W Y. Salt-adding extractive distillation of ethanol-water system[J]. Journal of Jiangxi Polytechnic University, 1991, 13(3): 9-12. | |
| 3 | 方凯, 杨亚鸣, 吴淑晶. 利用溶盐精馏法分离乙醇-水体系的研究[J]. 河南化工, 2013, 30(10): 24-26. |
| Fang K, Yang Y M, Wu S J. Research progress of industry preparation of anhydrous ethanol[J]. Henan Chemical Industry, 2013, 30(10): 24-26. | |
| 4 | Vercher E, Munoz R, Martinez-Andreu A. Isobaric vapor-liquid equilibrium data for the ethanol-water-potassium acetate and ethanol-water-(potassium acetate/sodium acetate) systems[J]. Journal of Chemical & Engineering Data, 1991, 36(3): 274-277. |
| 5 | 武文良, 张雅明, 陆小华,等. 乙醇-水-盐体系汽液平衡[J]. 南京化工大学学报, 1997, 19(3): 63-67. |
| Wu W L, Zhang Y M, Lu X H, et al. Study of vapor-liquid equilibrium for the ethanol-water system in the presence of salts[J]. Journal of Nanjing University of Chemical Technology, 1997, 19(3): 63-67. | |
| 6 | 鲍静, 张雅明, 张蕾. 乙醇-水-复合溶剂体系汽液平衡[J]. 南京工业大学学报, 2004, 26(5): 42-47. |
| Bao J, Zhang Y M, Zhang L. Vapour-liquid equilibrium data for the ethanol-water-mixed solvent systems[J]. Journal of Nanjing University of Technology, 2004, 26(5): 42-47. | |
| 7 | Gmehling J, Onken U, Arlt W. Vapor-Liquid Equilibrium Data Collection Aqueous-Organic Systeme[M]. Germany: Dechema, 1977: 152-155. |
| 8 | 彭思瑶. 含甲醛、乙醛的乙二醇相关系统的汽液平衡研究[D]. 上海: 华东理工大学, 2017. |
| Peng S Y. Study on the vapor-liquid equilibrium systems of ethylene glycol and formaldehyde/acetaldehyde[D]. Shanghai: East China University of Science and Technology, 2017. | |
| 9 | 辛华, 李青松. 含离子液体乙醇-水物系等压汽液平衡数据的测定[J]. 化工学报, 2012, 63(6): 1678-1683. |
| Xin H, Li Q S. Isobaric vapor-liquid equilibria for ethanol-water system containing ionic liquids at atmospheric aressure[J]. CIESC Journal, 2012, 63(6): 1678-1683. | |
| 10 | 吴金芝, 王琳琳, 陈小鹏, 等. 莰烯+(+)-3-蒈烯体系汽液平衡数据的测定与关联[J]. 化工学报, 2019, 70(6): 2092-2101. |
| Wu J Z, Wang L L, Chen X P, et al. Determination and correlation of vapor-liquid equilibrium data for camphene+(+)-3-carene system[J]. CIESC Journal, 2019, 70(6): 2092-2101. | |
| 11 | 周格, 顾正桂, 曹晓艳. 四氢呋喃-甲苯-氟苯-水体系汽液平衡数据的测定和关联[J]. 化学工程, 2018, 46(11): 25-29, 73. |
| Zhou G, Gu Z G, Cao X Y. Measurement and correlation of vapor-liquid equilibria for tetrahydrofuran-toluene-fluorobenzene-water system[J]. Chemical Engineering (China), 2018, 46(11): 25-29, 73. | |
| 12 | Malanowski S. Experimental methods for vapour-liquid equilibria(part i), Circulation methods[J]. Fluid Phase Equilibria, 1982, 8(2): 197-219. |
| 13 | Shen R C, Chen Y, Shi Y H, et al. Determination of ternary vapor-liquid equilibrium of dimethyl oxalate-methanol-1,2-butanediol under atmosphere pressure[J]. Journal of Chemical & Engineering Data, 2019, 64(4): 1349-1356. |
| 14 | 金丽萍, 邬时清, 陈大勇. 物理化学实验[M]. 2版. 上海: 华东理工大学出版社, 2007: 204. |
| Jin L P, Wu S Q, Chen D Y, et al. Physical Chemistry Experiment[M]. 2nd ed. Shanghai: East China University of Science and Technology Press, 2007: 204. | |
| 15 | Prausnitz J M, Lichtenthaler R N, Azevedo E G. Molecular Thermodynamics of Fluid-phase Equilibria[M]. Singapore: Pearson Education, 1998: 18. |
| 16 | Wisniak J. A new test for the thermodynamic consistency of vapor-liquid equilibrium[J]. Industrial & Engineering Chemistry Research, 1993, 32(7): 1531-1533. |
| 17 | Redlich O, Kiste A T. Algebraic representation of thermodynamic properties and the classification of solutions[J]. Industrial & Engineering Chemistry Research, 1948, 40(2): 345-348. |
| 18 | Ohe S. Vapor-liquid Equilibrium Data-salt Effect[M]. Tokyo: Kodansha, 1991: 129-130. |
| 19 | Mathias P M. Guidelines for the analysis of vapor-liquid equilibrium data[J]. Journal of Chemical & Engineering Data, 2017, 62(8): 2231-2233. |
| 20 | 张祝蒙, 李东风. 加盐萃取精馏技术的研究进展[J]. 石油化工, 2008, 37(9): 955-959. |
| Zhang Z M, Li D F. Advanced in research of extractive distillation with salt[J]. Petrochemical Technology, 2008, 37(9): 955-959. | |
| 21 | 雷志刚, 周荣琪. 溶剂加盐对醇水汽液平衡的影响[J]. 精细化工, 2000, 17(5): 307-309. |
| Lei Z G, Zhou R Q. Influence of solvent with salts on the vapour-liquid equilibrium of alcohol-water systems[J]. Fine Chemicals, 2000, 17(5): 307-309. | |
| 22 | 韩莎莎, 孙晓岩, 陈玉石, 等. 电解质NRTL模型研究与开发[J]. 计算机与应用化学, 2019, 36(3): 185-194. |
| Han S S, Sun X Y, Chen Y S, et al. Reserach and development of electrolyte NRTL model[J]. Computers and Applied Chemistry, 2019, 36(3): 185-194. | |
| 23 | Chen C C, Britt H I, Boston J F, et al. Local compositions model for excess gibbs energy of electrolyte systems(part I): Single solvent, single completely dissociated electrolyte systems[J]. American Institute of Chemical Engineers Journal, 1982, 28(4): 588-596. |
| 24 | Chen C C, Evans L B. A local composition model for the excess gibbs energy of aqueous electrolyte systems[J]. American Institute of Chemical Engineers Journal, 1986, 32(3): 444-459. |
| 25 | Mock B, Evans L B, Chen C C. Thermodynamic representation of phase equilibria of mixed-solvent electrolyte systems[J]. American Institute of Chemical Engineers Journal, 1986, 32(10): 1655-1664. |
| 26 | Chen C C, Mathias P M, Orbey H. Use of hydration and dissociation chemistries with the electrolyte-NRTL model[J]. American Institute of Chemical Engineers Journal, 1999, 45(7): 1576-1586. |
| 27 | Chen C C, Bokis C P, Mathias P. Segment-based excess Gibbs energy model for aqueous organic electrolytes[J]. American Institute of Chemical Engineers Journal, 2001, 47(11): 2593-2602. |
| 28 | Chen C C, Song Y H. Generalized electrolyte-NRTL model for mixed-solvent electrolyte systems[J]. American Institute of Chemical Engineers Journal, 2004, 50(8): 1928-1941. |
| 29 | 孙剑, 夏剑忠, 施云海. MDEA-MEA混合醇胺脱硫脱碳的模拟计算[J]. 化学反应工程与工艺, 2007, 23(3): 279-283. |
| Sun J, Xia J Z, Shi Y H. Modelling of H2S and CO2 absorption in aqueous solution of MDEA-MEA[J]. Chemical Reaction Engineering and Technology, 2007, 23(3): 279-283. | |
| 30 | 施云海, 王艳莉, 彭阳峰, 等. 化工热力学[M]. 2版. 上海: 华东理工大学出版社, 2007: 104-110. |
| Shi Y H, Wang Y L, Peng Y F, et al. Chemical Engineering Thermodynamics[M]. 2nd ed. Shanghai: East China University of Science and Technology Press, 2007: 104-110. | |
| 31 | Wilson G M. Vapor-liquid equilibrium(XI): A new expression for the excess free energy of mixing[J]. Journal of the American Chemical Society, 1964, 86(2): 127-130. |
| 32 | Renon H, Prausnitz J M. Local compositions in thermodynamic excess functions for liquid mixtures[J]. American Institute of Chemical Engineers Journal, 1968, 14(1): 135-144. |
| 33 | Abrams D S, Prausnitz J M. Statistical thermodynamics of liquid mixtures: a new expression for the excess Gibbs energy of partly or completely miscible systems[J]. American Institute of Chemical Engineers Journal, 1975, 21(1): 116-128. |
| 34 | Zhong Y, Wu Y, Zhu J, et al. Thermodynamics in separation for the ternary system 1,2-ethanediol + 1,2-propanediol + 2,3-butanediol[J]. Industrial & Engineering Chemistry Research, 2014, 53(30): 12143-12148. |
| 35 | 陈钟秀, 顾飞燕. 化工热力学[M]. 2版. 北京: 化学工业出版社, 2006: 93-97. |
| Chen Z X, Gu F Y. Chemical Engineering Thermodynamics[M]. 2nd ed. Beijing: Chemical Industry Press, 2006: 93-97. |
| [1] | 吴馨, 龚建英, 靳龙, 王宇涛, 黄睿宁. 超声波激励下铝板表面液滴群输运特性的研究[J]. 化工学报, 2023, 74(S1): 104-112. |
| [2] | 苏伟, 马东旭, 金旭, 刘忠彦, 张小松. 表面润湿性对霜层传递特性影响可视化实验研究[J]. 化工学报, 2023, 74(S1): 122-131. |
| [3] | 邵苛苛, 宋孟杰, 江正勇, 张旋, 张龙, 高润淼, 甄泽康. 水平方向上冰中受陷气泡形成和分布实验研究[J]. 化工学报, 2023, 74(S1): 161-164. |
| [4] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [5] | 连梦雅, 谈莹莹, 王林, 陈枫, 曹艺飞. 地下水预热新风一体化热泵空调系统制热性能研究[J]. 化工学报, 2023, 74(S1): 311-319. |
| [6] | 黄琮琪, 吴一梅, 陈建业, 邵双全. 碱性电解水制氢装置热管理系统仿真研究[J]. 化工学报, 2023, 74(S1): 320-328. |
| [7] | 吴迪, 胡斌, 王如竹, 梁俊宇. 水蒸气准饱和压缩高温热泵循环性能分析[J]. 化工学报, 2023, 74(S1): 45-52. |
| [8] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [9] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [10] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [11] | 汪尔奇, 彭书舟, 杨震, 段远源. 含HFO混合体系气液相平衡的理论模型评价[J]. 化工学报, 2023, 74(8): 3216-3225. |
| [12] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [13] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [14] | 李彬, 徐正虎, 姜爽, 张天永. 双氧水催化氧化法清洁高效合成促进剂CBS[J]. 化工学报, 2023, 74(7): 2919-2925. |
| [15] | 董明, 徐进良, 刘广林. 超临界水非均质特性分子动力学研究[J]. 化工学报, 2023, 74(7): 2836-2847. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号