化工学报 ›› 2020, Vol. 71 ›› Issue (7): 3213-3219.DOI: 10.11949/0438-1157.20200058
收稿日期:2020-01-15
修回日期:2020-04-13
出版日期:2020-07-05
发布日期:2020-07-05
通讯作者:
左然
作者简介:孙巍(1993—),男,硕士研究生,基金资助:Received:2020-01-15
Revised:2020-04-13
Online:2020-07-05
Published:2020-07-05
Contact:
Ran ZUO
摘要:
利用量子化学的密度泛函理论(DFT),对AlN的MOCVD生长中表面反应前体AlCH3(简称MMAl)在NH2和H混合覆盖AlN(0001)-Al面的吸附与扩散进行计算分析。通过分析表面吸附能、扩散能垒及Mulliken数量比例等,确定可能的稳定吸附结构和扩散路径。研究发现:在NH2与H混合覆盖的AlN(0001)-Al面,随着NH2与H的覆盖度变化,MMAl均稳定吸附在T4位和H3位,吸附概率相近。随着NH2比例增多、H比例减少,MMAl吸附后都向AlN表面转移电荷,同时其吸附变得相对容易,扩散变得逐渐困难。与吸附前相比,吸附后MMAl中的Al-C键长缩短,键能增强,不利于CH3的脱离,导致引入C杂质的概率增高,表明MMAl既可能是生长中主要反应物质之一,同时也是引入C杂质的主要来源之一。若AlN表面存在覆盖H,吸附后的MMAl会促使表面覆盖的H原子倾向于脱离AlN表面,有利于后续生长。
中图分类号:
孙巍, 左然. MMAl在NH2与H混合覆盖的AlN(0001)-Al表面的吸附与扩散研究[J]. 化工学报, 2020, 71(7): 3213-3219.
Wei SUN, Ran ZUO. Study on adsorption and diffusion of MMAl on AlN(0001)-Al surface covered with NH2/H[J]. CIESC Journal, 2020, 71(7): 3213-3219.
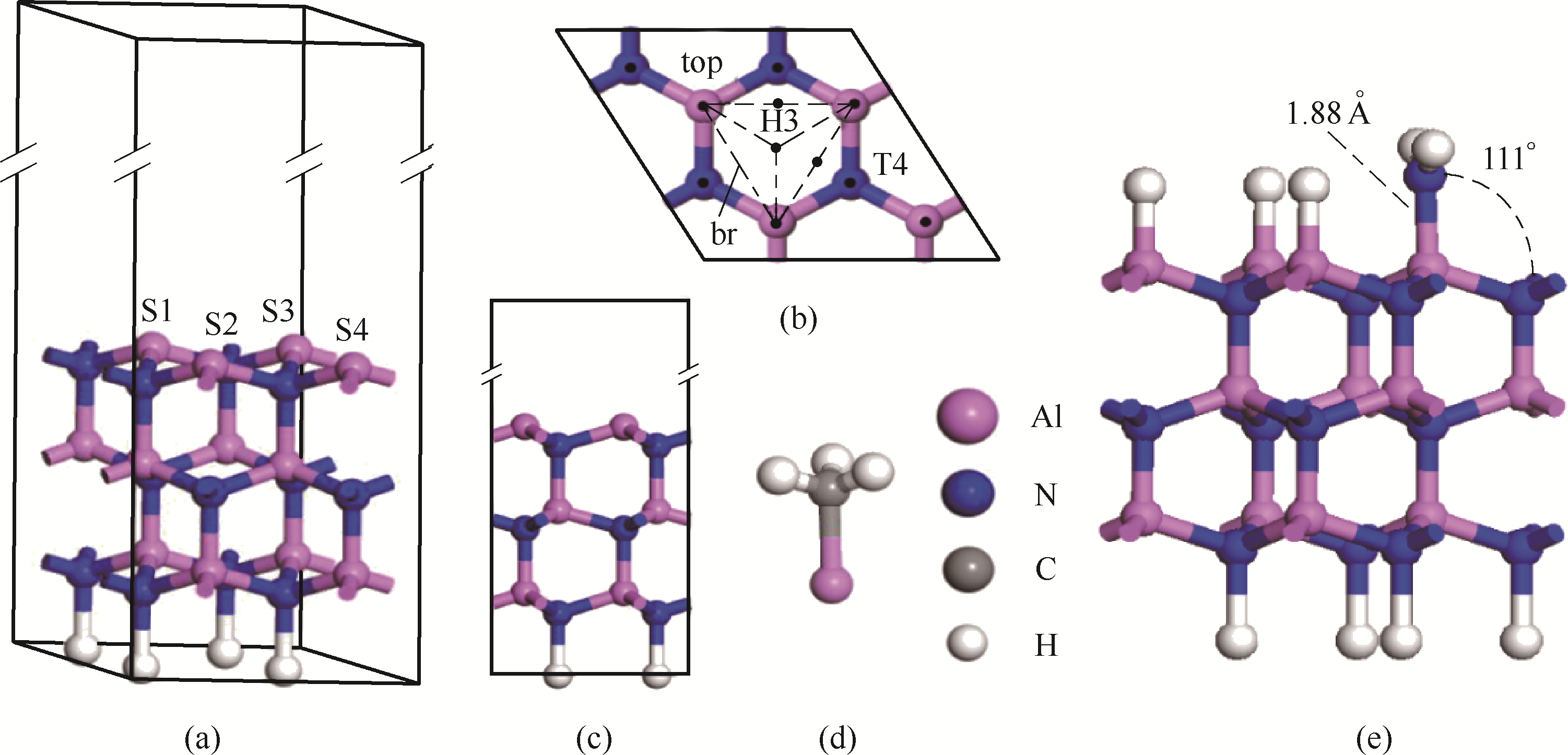
图1 AlN(0001)-Al面2×2周期超晶胞模型的三维视图(a)、俯视图(b)和主视图 (c);MMAl的分子模型(d);NH2/H=(0.25,0.75)的AlN(0001)表面(e)
Fig.1 3D view(a), top view(b) and main view(c) of 2×2 periodic supercell model on AlN (0001) - Al plane; molecular model ofMMAl(d); AlN (0001) surface with NH2/H = (0.25,0.75) (e)
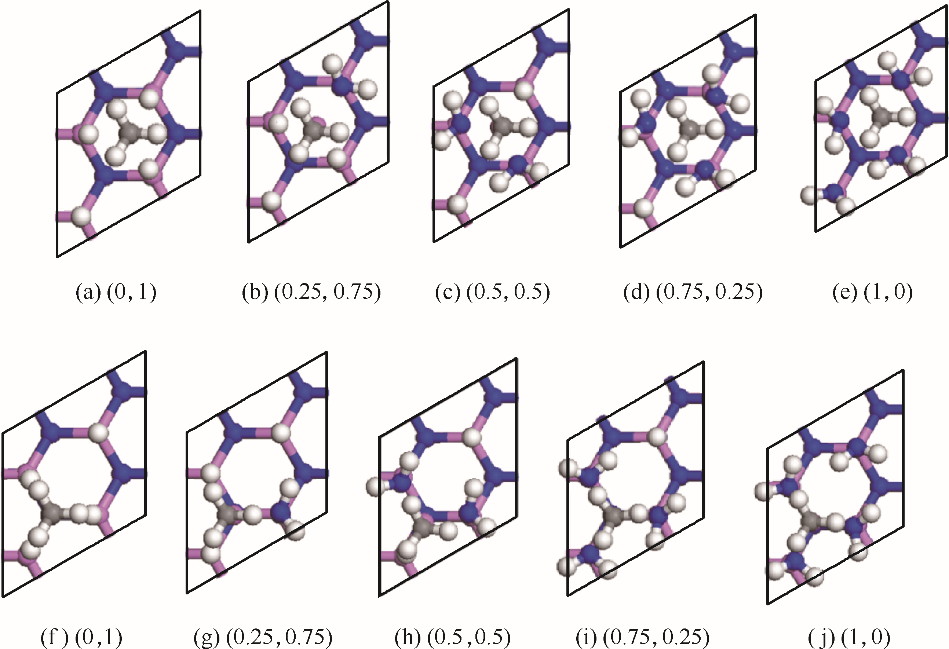
图3 MMAl在各种混合覆盖表面(不同覆盖度的AlN表面)上H3位[(a)~(e)]和T4位[(f)~(j)]的稳定吸附结构
Fig.3 Stable adsorption structures of MMAl on the H3[(a)—(e)] and T4 [(f)—(j)] sites on various mixed coating surfaces(AlN surface with different adsorption degrees)
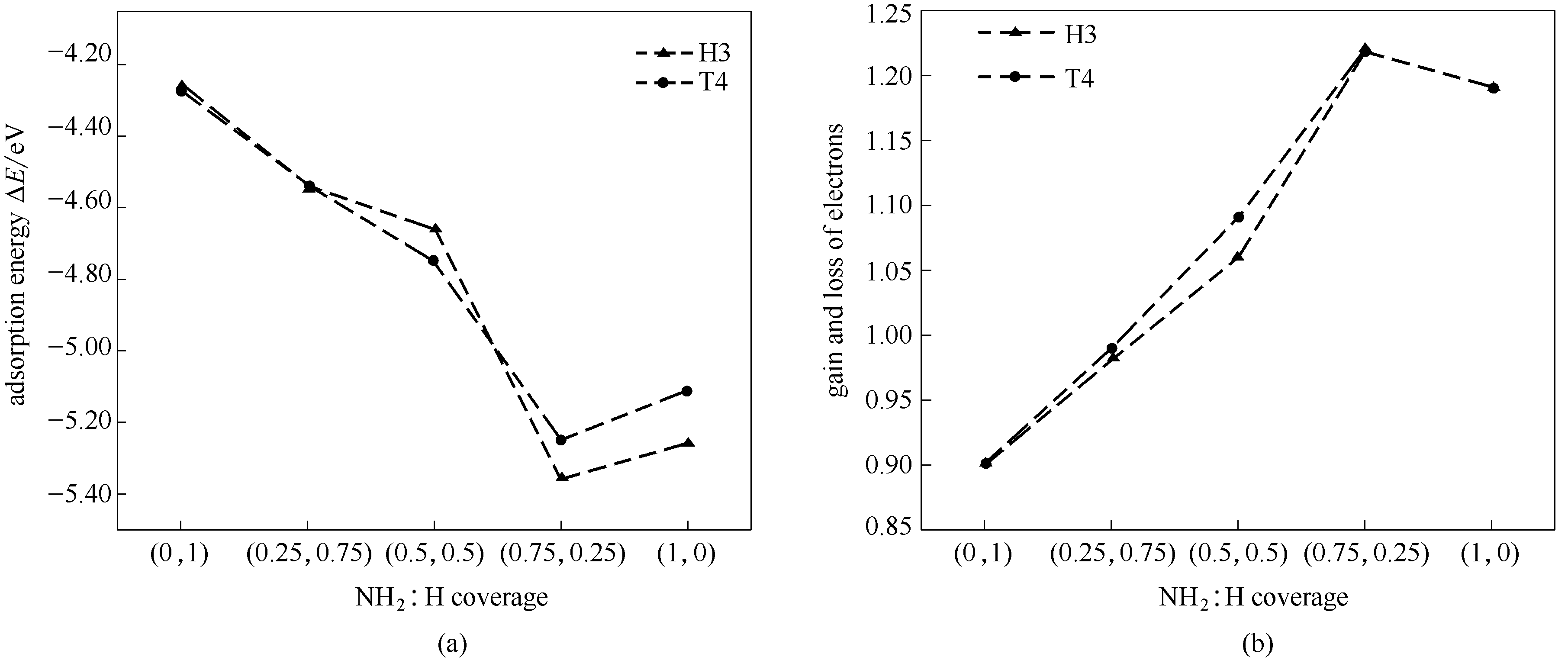
图4 MMAl在不同覆盖度AlN表面的吸附能(a)和 MMAl在不同覆盖度AlN表面吸附后的电子转移数目(b)
Fig.4 Adsorption energy of MMAl on AlN surfaces with different degrees of coverage(a); number of electron transfers after adsorption of MMAl with different coverage on AlN surface(b)
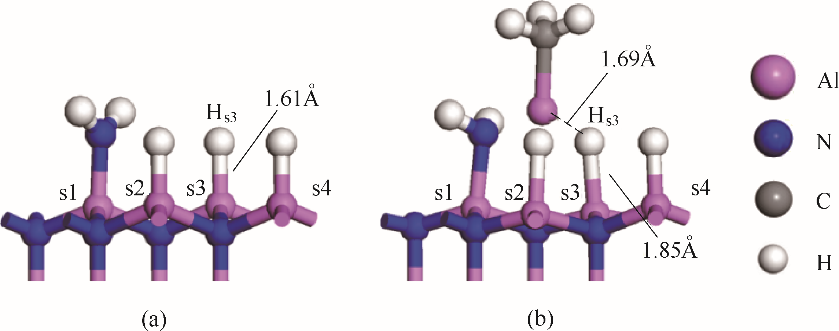
图6 MMAl吸附前(0.25,0.75)AlN表面中Al-H键的键长(a) MMAl吸附后(0.25,0.75)AlN表面中Al-H键的键长(b)
Fig.6 Bond length of Al-H bond in (0.25, 0.75) AlN surface before MMAl adsorption (a) Al-H bond length of (0.25, 0.75) AlN surface after MMAl adsorption (b)
| NH2∶H coverage | Bond | After adsorption(H3) | Bond | After adsorption(T4) | ||
|---|---|---|---|---|---|---|
Length/ ? | Population | Length/? | Population | |||
| (0,1) | Ala-Hs2 | 1.69 | 0.48 | Ala-Hs2 | 1.70 | 0.49 |
| Als-Hs2 | 1.80 | 0.32 | Als-Hs2 | 1.80 | 0.33 | |
| (0.25,075) | Ala-Hs3 | 1.69 | 0.56 | Ala-Hs3 | 1.69 | 0.54 |
| Als-Hs3 | 1.86 | 0.26 | Als-Hs3 | 1.85 | 0.27 | |
| (0.5,0.5) | Ala-Hs1 | 1.71 | 0.59 | Ala-Hs4 | 1.68 | 0.56 |
| Als-Hs1 | 1.97 | 0.23 | Als-Hs4 | 1.89 | 0.24 | |
| (0.75,0.25) | Als-Hs4 | 1.70 | 0.74 | Als-Hs1 | 1.71 | 0.74 |
表1 MMAl吸附前后Al—H键的键长变化及化学键数量比例变化
Table 1 Al—H bond length changes and chemical bond population changes before and after MMAl adsorption
| NH2∶H coverage | Bond | After adsorption(H3) | Bond | After adsorption(T4) | ||
|---|---|---|---|---|---|---|
Length/ ? | Population | Length/? | Population | |||
| (0,1) | Ala-Hs2 | 1.69 | 0.48 | Ala-Hs2 | 1.70 | 0.49 |
| Als-Hs2 | 1.80 | 0.32 | Als-Hs2 | 1.80 | 0.33 | |
| (0.25,075) | Ala-Hs3 | 1.69 | 0.56 | Ala-Hs3 | 1.69 | 0.54 |
| Als-Hs3 | 1.86 | 0.26 | Als-Hs3 | 1.85 | 0.27 | |
| (0.5,0.5) | Ala-Hs1 | 1.71 | 0.59 | Ala-Hs4 | 1.68 | 0.56 |
| Als-Hs1 | 1.97 | 0.23 | Als-Hs4 | 1.89 | 0.24 | |
| (0.75,0.25) | Als-Hs4 | 1.70 | 0.74 | Als-Hs1 | 1.71 | 0.74 |
| NH2∶H coverage | Diffusion energy barriers/eV | |
|---|---|---|
| H3→T4 | T4→H3 | |
| (0,1) | 0.67 | 0.70 |
| (0.25,075) | 0.95 | 0.98 |
| (0.5,0.5) | 1.00 | 1.10 |
| (0.75,0.25) | 2.35 | 2.41 |
| (1,0) | 3.11 | 3.10 |
表2 MMAl在不同覆盖度AlN表面的扩散能垒
Table 2 Diffusion energy barriers of MMAl on AlN surfaces with different coverage
| NH2∶H coverage | Diffusion energy barriers/eV | |
|---|---|---|
| H3→T4 | T4→H3 | |
| (0,1) | 0.67 | 0.70 |
| (0.25,075) | 0.95 | 0.98 |
| (0.5,0.5) | 1.00 | 1.10 |
| (0.75,0.25) | 2.35 | 2.41 |
| (1,0) | 3.11 | 3.10 |
| 1 | 张红, 唐留. GaN-MOVPE寄生反应的密度泛函理论研究[J]. 化工学报, 2019, 70(9): 3275-3282. |
| Zhang H, Tang L. Density functional theory study on parasitic reactions of GaN-MOVPE[J]. CIESC Journal, 2019, 70(9): 3275-3282. | |
| 2 | 徐谦, 左然, 张红. MOCVD生长GaN的反应动力学分析与数值模拟[J]. 化工学报, 2009, 60(2): 384-388. |
| Xu Q, Zuo R, Zhang H. Analysis of reaction kinetics and numerical simulation of GaN growth by MOCVD[J]. CIESC Journal, 2009, 60(2): 384-388. | |
| 3 | 张红. 金属有机化学气相沉淀反应器结构的模拟优化[J]. 化工进展, 2009, 28(8): 1328-1332. |
| Zhang H. Simulation and optimization design in MOCVD reactor[J]. Chemical Industry and Engineering Progress, 2009, 28(8): 1328-1332. | |
| 4 | Akiyama T. Abinitio-based study for adatom kinetics on AlN(0001) surfaces during metal-organic vapor-phase epitaxy growth[J]. Applied Physics Letters, 2012, 100(25): 499-502. |
| 5 | Jindal V, Shahedipour-sandvik F. Density functional theoretical study of surface structure and adatom kinetics for wurtzite AlN[J]. Journal of Applied Physics, 2009, 105(8): 1148-1153. |
| 6 | 牛楠楠, 左然. AlN的MOCVD生长中表面吸附的量子化学研究[J]. 人工晶体学报, 2019, 48(7): 1268-1274. |
| Niu N N, Zuo R. Quantum chemistry study of surface adsorption during AlN MOCVD growth [J]. Journal of Artificial Crystals, 2019, 48(7): 1268-1274. | |
| 7 | González-Hernández R, González-Garcia A, López-Perez W. Density functional theory study of the adsorption and incorporation of Sc and Y on the AlN(0001) surface[J]. Journal of Crystal Growth, 2016, 403(1): 1-7. |
| 8 | Uchida T, Kusakabe K, Ohkawa K. Influence of polymer formation on metalorganic vapor-phase epitaxial growth of AlN[J]. Journal of Crystal Growth, 2007, 304(1): 133-140. |
| 9 | Suzuki H, Panyukova U, Togashi R, et al. Theoretical investigation of the decomposition mechanism of AlN(0001) surface under a hydrogen atmosphere[J]. Physica Status Solidi, 2010, 7(7/8): 2265-2267. |
| 10 | Lü N X, Xu Y J, Chen W K, et al. A DFT study for NH3 adsorption on the GaN (0001) surface[J]. Jiegou Huaxue, 2004, 23(8): 845-849. |
| 11 | Won Y S, Lee J, Kim C S, et al. Computational study of adsorption, diffusion, and dissociation of precursor species on the GaN (0 0 0 1) surface during GaN MOCVD[J]. Surf. Sci., 2009, 603(4): L31-L34. |
| 12 | Suzuki H, Togashi R, Murakami H, et al. Ab initio calculation for an initial growth process of GaN on ( 0001) and ( 0001- ) surfaces by vapor phase epitaxy[J]. Physica Status Solidi, 2009, 6(S2): 321-329. |
| 13 | Kempisty P, Strak P, Sakowski K, et al. Adsorption of ammonia on hydrogen covered GaN(0001) surface - density functional theory study[J]. Journal of Crystal Growth, 2014, 401(9): 514-517. |
| 14 | Pignedoli C A, Di F R, Bertoni C M, et al. Surface effects in GaN growth[J]. Surface Science, 2003, 547(1): 63-70. |
| 15 | Suzuki H, Murakami H, Kumagai Y, et al. Theoretical study on the influence of surface hydrogen coverage on the initial growth process of AlN(0001) surfaces[J]. Physica Status Solidi (C), 2011, 8(5): 1577-1580. |
| 16 | Suzuki H, Ogash R, Murakami H,et al. Theoretical analysis for surface reconstruction of AlN and InN in the presence of hydrogen[J]. Japanese Journal of Applied Physics, 2007, 46(8A): 5112-5115. |
| 17 | Inagaki Y, Kozawa T. Chemical reaction pathways for MOCVD growth of aluminum nitride[J]. ECS Journal of Solid State Science and Technology, 2016, 5(2): 73-75. |
| 18 | Smith A R, Feenstra R M, Greve D W, et al. GaN(0001) surface structures studied using scanning tunneling microscopy and first-principles total energy calculations[J]. Surface Science, 1999, 423(1): 70-84. |
| 19 | Davis C S, Novikov S V, Cheng T S, et al. Surface reconstruction patterns of AlN grown by molecular beam epitaxy on sapphire[J]. Journal of Crystal Growth, 2001, 226(2): 203-208. |
| 20 | Xu Y N, Ching W Y. Electronic, optical, and structural properties of some wurtzite crystals[J]. Physical Review B Condensed Matter, 1993, 48(7): 4335-4351. |
| 21 | Schulz H, Thiemann K H. Crystal structure refinement of AlN and GaN[J]. Solid State Communications, 1977, 23(11): 815-819. |
| 22 | 大卫·S·肖尔, 贾妮丝·A·斯特克尔. 密度泛函理论[M]. 李健, 周勇, 译. 北京: 国防工业出版社, 2014: 10-50. |
| Shore D S, Stecker J A. Density Functional Theory [M]. Li J, Zhou Y, trans. Beijing: National Defense Industry Press, 2014: 10-50. | |
| 23 | Walle C G V D, Neugebauer J. Structure and energetics of nitride surfaces under MOCVD growth conditions[J]. Journal of Crystal Growth, 2003, 248(2): 8-13. |
| 24 | Ikeda Y, Ohmori N, Maida N, et al. Theoretical study of gallium nitride crystal growth reaction mechanism[J]. Japanese Journal of Applied Physics, 2011, 50(50): 943-949. |
| 25 | Lin T T, Xiang Y L, He C. A DFT study on poly (lactic acid) polymorphs [J]. Polymer, 2010, 51(12): 2779-2785. |
| 26 | Jordaan M, Helden P V, Sittert C G C E V, et al. Experimental and DFT investigation of the 1-octene metathesis reaction mechanism with the Grubbs 1 precatalyst[J]. Journal of Molecular Catalysis A Chemical, 2006, 254(1/2): 145-154. |
| 27 | Neugebauer J, Zywietz T, Scheffler M, et al. Theory of surfaces and interfaces of group III-nitrides[J]. Applied surface Science, 2000(159/160): 355-359. |
| 28 | Doi K, Maida N, Kimura K, et al. First-principle study on crystal growth of Ga and N layers on GaN substrate[J]. Physica Status Solidi (C), 2007, 4(7): 2293-2296. |
| 29 | 陆大成, 段树坤. 金属有机化合物气相外延基础及应用(半导体科学与技术丛书)(精)[M]. 北京: 科学出版社, 2009: 37-50. |
| Lu D C, Duan S K. The Foundation and Application of Vapor Phase Epitaxy of Metal Organic Compounds (Semiconductor Science and Technology Series) (Fine) [M]. Beijing: Science Press, 2009: 37-50. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [3] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [4] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [5] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [6] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [7] | 何晓崐, 刘锐, 薛园, 左然. MOCVD生长AlN单晶薄膜的气相和表面化学反应综述[J]. 化工学报, 2023, 74(7): 2800-2813. |
| [8] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [9] | 周继鹏, 何文军, 李涛. 异形催化剂上乙烯催化氧化失活动力学反应工程计算[J]. 化工学报, 2023, 74(6): 2416-2426. |
| [10] | 王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588. |
| [11] | 李晨曦, 刘永峰, 张璐, 刘海峰, 宋金瓯, 何旭. O2/CO2氛围下正庚烷的燃烧机理研究[J]. 化工学报, 2023, 74(5): 2157-2169. |
| [12] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [13] | 李辰鑫, 潘艳秋, 何流, 牛亚宾, 俞路. 基于碳微晶结构的炭膜模型及其气体分离模拟[J]. 化工学报, 2023, 74(5): 2057-2066. |
| [14] | 陈韶云, 徐东, 陈龙, 张禹, 张远方, 尤庆亮, 胡成龙, 陈建. 单层聚苯胺微球阵列结构的制备及其吸附性能[J]. 化工学报, 2023, 74(5): 2228-2238. |
| [15] | 王蕾, 王磊, 白云龙, 何柳柳. SA膜状锂离子筛的制备及其锂吸附性能[J]. 化工学报, 2023, 74(5): 2046-2056. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号
