化工学报 ›› 2021, Vol. 72 ›› Issue (2): 984-992.DOI: 10.11949/0438-1157.20200741
收稿日期:2020-06-11
修回日期:2020-07-06
出版日期:2021-02-05
发布日期:2021-02-05
通讯作者:
徐建鸿
作者简介:王法军(1995—),男,博士研究生,基金资助:
WANG Fajun( ),HUANG Jinpei,XU Jianhong(
),HUANG Jinpei,XU Jianhong( )
)
Received:2020-06-11
Revised:2020-07-06
Online:2021-02-05
Published:2021-02-05
Contact:
XU Jianhong
摘要:
重氮化反应是合成重氮盐中间体的传统方法,重氮盐中间体因其巨大的合成潜力,被广泛地应用于医药、农药、染颜料工业等精细化工领域。首先,利用重氮盐中间体的偶合反应以及紫外可见分光光度法建立了重氮盐中间体的定量方法,并且建立了用于红色基KD重氮化反应动力学参数测定的微反应器系统。在红色基KD低浓度以及远过量的盐酸浓度条件下,确定了该反应为二级反应,得到了反应的指前因子为1.57×1014 L/(mol·s),活化能为 72.88 kJ/mol。在实验研究范围内建立了表观反应动力学模型,并且通过验证实验表明,模拟计算值与实验值吻合较好。
中图分类号:
王法军, 黄晋培, 徐建鸿. 微反应器内红色基KD重氮化反应动力学研究[J]. 化工学报, 2021, 72(2): 984-992.
WANG Fajun, HUANG Jinpei, XU Jianhong. Kinetics of red base KD diazotization in microreactor system[J]. CIESC Journal, 2021, 72(2): 984-992.
| 样品编号 | 吸光度/A | 浓度/(mg/L) | RSD/% |
|---|---|---|---|
| 1 | 1.287 | 43.14 | 0.12 |
| 2 | 1.287 | 43.14 | |
| 3 | 1.289 | 43.21 | |
| 4 | 1.286 | 43.11 | |
| 5 | 1.285 | 43.07 |
表1 偶氮染料浓度测定方法的精密度实验结果
Table 1 Precision test results of concentration measurement of azo dye
| 样品编号 | 吸光度/A | 浓度/(mg/L) | RSD/% |
|---|---|---|---|
| 1 | 1.287 | 43.14 | 0.12 |
| 2 | 1.287 | 43.14 | |
| 3 | 1.289 | 43.21 | |
| 4 | 1.286 | 43.11 | |
| 5 | 1.285 | 43.07 |
| 样品编号 | 吸光度/A | 浓度/(mg/L) | RSD/% |
|---|---|---|---|
| 1 | 1.286 | 43.11 | 0.69 |
| 2 | 1.286 | 43.11 | |
| 3 | 1.279 | 42.87 | |
| 4 | 1.302 | 43.65 | |
| 5 | 1.283 | 43.01 |
表2 偶氮染料浓度测定方法的重现性实验结果
Table 2 Reproducible test results of concentration measurement of azo dye
| 样品编号 | 吸光度/A | 浓度/(mg/L) | RSD/% |
|---|---|---|---|
| 1 | 1.286 | 43.11 | 0.69 |
| 2 | 1.286 | 43.11 | |
| 3 | 1.279 | 42.87 | |
| 4 | 1.302 | 43.65 | |
| 5 | 1.283 | 43.01 |
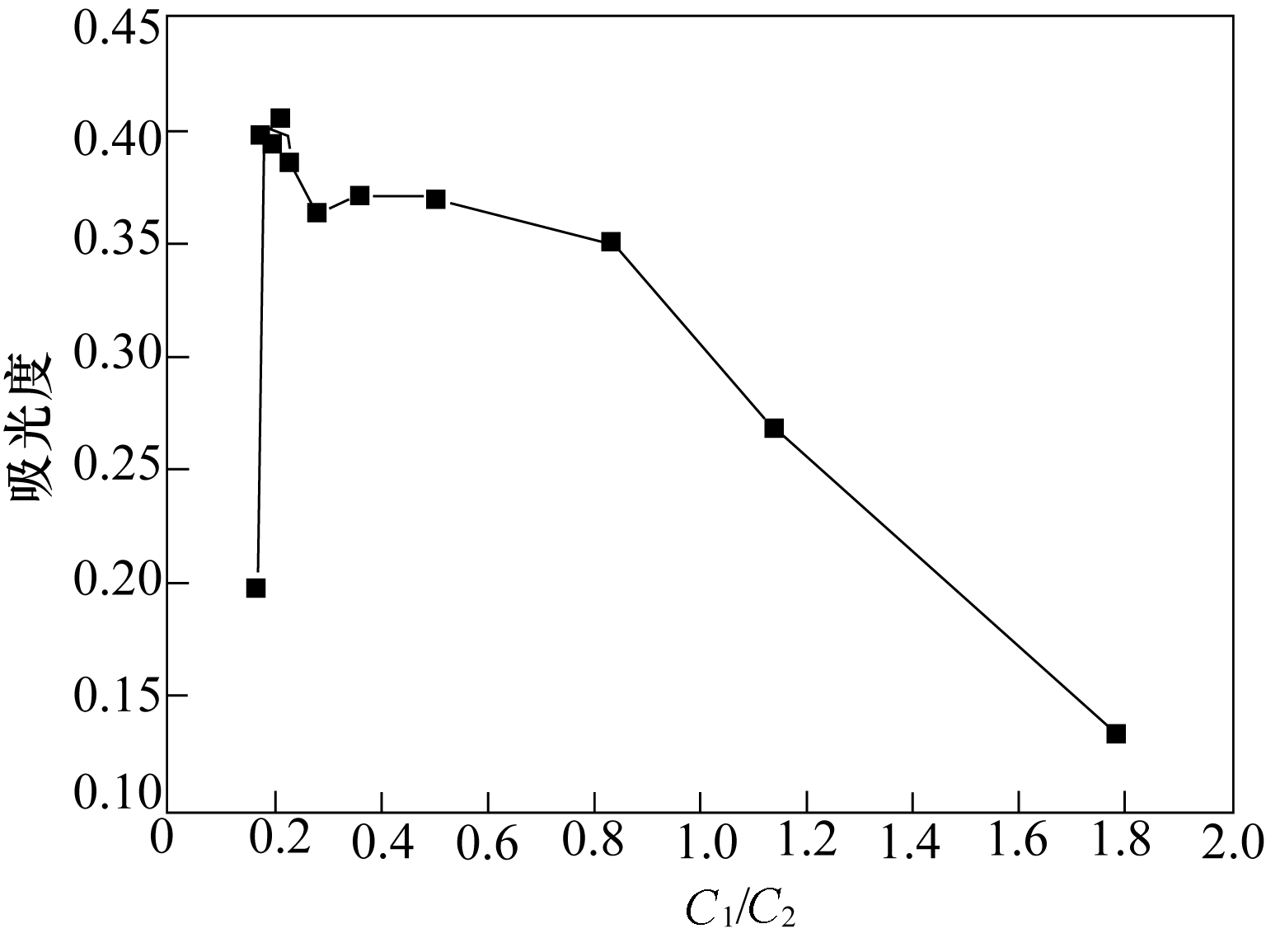
图8 不同偶合组分与氢氧化钠的摩尔比下偶氮染料A的最大吸光度反应条件:反应温度(T)=20℃;重氮化反应停留时间(t)=167 s;溶液A与溶液B流量VA=VB=2.5 ml/min;溶液C流量VC=8 ml/min
Fig.8 Different absorbance at different molar ratio of coupling component to sodium hydroxide
| 反应温度/℃ | 线性方程 | R2 | K/(L/(mol·s)) |
|---|---|---|---|
| 18 | y= 0.0992x+0.0133 | 0.9918 | 13.22 |
| 14 | y= 0.0653x+0.0459 | 0.9974 | 8.71 |
| 5 | y= 0.0243x+0.0235 | 0.9976 | 3.24 |
表3 不同温度下的反应速率常数
Table 3 Reaction rate constants at different temperatures
| 反应温度/℃ | 线性方程 | R2 | K/(L/(mol·s)) |
|---|---|---|---|
| 18 | y= 0.0992x+0.0133 | 0.9918 | 13.22 |
| 14 | y= 0.0653x+0.0459 | 0.9974 | 8.71 |
| 5 | y= 0.0243x+0.0235 | 0.9976 | 3.24 |
| 1 | Mo F Y, Dong G B, Zhang Y, et al. Recent applications of arene diazonium salts in organic synthesis[J]. Org. Biomol. Chem., 2013, 11(10): 1582-1593. |
| 2 | 丁云成, 王法军, 艾宁, 等. 微反应器内连续重氮化/偶合反应进展[J]. 化工学报, 2018, 69(11): 4542-4552. |
| Ding Y C, Wang F J, Ai N, et al. Research progress on continuous diazotization/azo-coupling reaction in microreactors[J]. CIESC Journal, 2018, 69(11): 4542-4552. | |
| 3 | 崔永涛, 李晓亮, 何立. 3, 4, 5-三氯溴苯的合成研究[J]. 化工与医药工程, 2019, 40(6): 1-5. |
| Cui Y T, Li X L, He L. Research of 3, 4, 5-trichlorobromobenzene synthesis[J]. Chemical and Pharmaceutical Engineering, 2019, 40(6): 1-5. | |
| 4 | 范龙涛, 郭庆春, 徐莉, 等. 吡唑解草酯的合成工艺[J]. 农药, 2020, 59(5): 328-331, 346. |
| Fan L T, Guo Q C, Xu L, et al. The synthesis of mefenpyr-diethyl[J]. Agrochemicals, 2020, 59(5): 328-331, 346. | |
| 5 | Yu Z Q, Ye X, Xu Q, et al. A fully continuous-flow process for the synthesis of p-cresol: impurity analysis and process optimization[J]. Org. Process Res. Dev., 2017, 21(10): 1644-1652. |
| 6 | 王亮. 重氮化水解法合成愈创木酚生产工艺优化研究[D]. 上海: 华东理工大学, 2012. |
| Wang L. Process optimization of guaiacol sythesized by diazotization-hydrolyzation process[D]. Shanghai: East China University of Science and Technology, 2012. | |
| 7 | 杨园园. 重氮盐管道化水解制酚工艺的研究[D]. 杭州: 浙江大学, 2005. |
| Yang Y Y. Study on the synthesis of phenolic compounds by diazotization and tubular hydrolysis[D]. Hangzhou: Zhejiang University, 2005. | |
| 8 | 余武斌, 郑明明, 叶青, 等. 微通道反应器内偶氮活性染料连续偶合工艺研究[J]. 染料与染色, 2015, 52(1): 1-4. |
| Yu W B, Zheng M M, Ye Q, et al. Study on the continuous coupling reaction for synthesis of azo reactive dyes in microreactor[J]. Dyestuffs and Coloration, 2015, 52(1): 1-4. | |
| 9 | 梁栋. 缩放螺旋混合器用于偶氮染料连续化制备的研究[D]. 大连: 大连理工大学, 2014. |
| Liang D. Contraction-expansion helical mixer and its application in continuous preparation of azo dyes[D]. Dalian: Dalian University of Technology, 2014. | |
| 10 | Pennemann H, Forster S, Kinkel J, et al. Improvement of dye properties of the Azo Pigment Yellow 12 using a micromixer-based process[J]. Org. Process Res. Dev., 2005, 9(2): 188-192. |
| 11 | D'Attoma J, Camara T, Routier S, et al. Efficient transposition of the Sandmeyer reaction from batch to continuous process[J]. Org. Process Res. Dev., 2017, 21(1): 44-51. |
| 12 | Yu Z Q, Lv Y W, Yu C M, et al. Continuous flow reactor for Balz-Schiemann reaction: a new procedure for the preparation of aromatic fluorides[J]. Tetrahedron Letters, 2013, 54(10): 1261-1263. |
| 13 | Malet-Sanz L, Madrzak J, Holveyr R S, et al. A safe and reliable procedure for the iododeamination of aromatic and heteroaromatic amines in a continuous flow reactor[J]. Tetrahedron Letters, 2009, 50(52): 7263-7267. |
| 14 | Partington S, Waldram S P. Runaway reaction during production of an azo dye intermediate [J]. Process Safety and Environmental Protection, 2002, 80: 33-39. |
| 15 | 黄郑华, 李建华. 重氮化反应火灾危险性分析与防火对策探讨[J]. 化工劳动保护, 1997, (6): 7-8. |
| Huang Z H, Li J H. Fire hazard analysis and fire prevention countermeasures for diazotization reaction [J]. Chemical Labor Protection, 1997, (6): 7-8. | |
| 16 | Wang F J, Huang J P, Xu J H. Continuous-flow synthesis of azo dyes in a microreactor system[J]. Chemical Engineering & Processing: Process Intensification, 2018, 127: 43-49. |
| 17 | Akwi F M, Watts P. The in situ generation and reactive quench of diazonium compounds in the synthesis of azo compounds in microreactors[J]. Beilstein J. Org. Chem., 2016, 12: 1987-2004. |
| 18 | Müller S T R, Wirth T. Diazo compounds in continuous-flow technology[J]. ChemSusChem., 2015, 8: 245-250. |
| 19 | Deadman B J, Collins S G, Maguire A R. Taming hazardous chemistry in flow: the continuous processing of diazo and diazonium compounds[J]. Chemistry-A European Journal, 2015, 21: 2298-2308. |
| 20 | Oger N, Grognec E L, Felpin F X. Handling diazonium salts in flow for organic and material chemistry[J]. Org. Chem. Front., 2015, 2: 590-614. |
| 21 | Hartman R L, Jensen K F. Microchemical systems for continuous-flow synthesis[J]. Lab on a Chip, 2009, 9(17): 2495-2507. |
| 22 | Zhang J S, Lu Y C, Jin Q R, et al. Determination of kinetic parameters of dehydrochlorination of dichloropropanol in a microreactor[J]. Chem. Eng. J., 2012, 203: 142-147. |
| 23 | Huang J P, Sang F N, Luo G S, et al. Continuous synthesis of Gabapentin with a microreaction system[J]. Chem. Eng. Sci., 2017, 173: 507-513. |
| 24 | Fitzgerald E. Separation and quantitation of diazonium salts as heptanesulfonate ion pairs by high pressure liquid chromatography[J]. Analytical Chemistry, 1976, 48(12): 1734-1735. |
| 25 | 杜允, 刘东志, 石至平, 等. 二氯联苯二胺重氮盐浓度的定量检测方法[J]. 现代化工, 2019, 39(4): 225-227, 229. |
| Du Y, Liu D Z, Shi Z P, et al. Quantitative detection method for dichlorobenzidine diazonium salt concentration[J]. Modern Chemical Industry, 2019, 39(4): 225-227, 229. | |
| 26 | 石至平, 周雪琴, 许芳, 等. 微溶性二氯联苯二胺的连续重氮化工艺[J]. 精细化工, 2020, 37(5): 1051-1055. |
| Shi Z P, Zhou X Q, Xu F, et al. Continuous-flow diazotization of slightly soluble dichlorobenzidine[J]. Fine Chemicals, 2020, 37(5): 1051-1055. | |
| 27 | 杨玉宇, 陈冰冰, 张元平. 无冷却螺旋管式苯胺重氮化反应工艺试验研究[J]. 浙江工业大学学报, 2009, 37(2): 213-216. |
| Yang Y Y, Chen B B, Zhang Y P. Experimental study on aniline diazotization without cooling in a spiral reactor[J]. Journal of Zhejiang University of Technology, 2009, 37(2): 213-216. | |
| 28 | Wang F J, Ding Y C, Xu J H. Continuous-flow synthesis of Pigment Red 146 in a microreactor system[J]. Ind. Eng. Chem. Res., 2019, 58(36): 16338-16347. |
| 29 | 马瑛, 孟明扬, 谭立哲, 等. 红色基KD新工艺研究[J]. 精细与专用化学品, 2003, 11(6): 18-19. |
| Ma Y, Meng M Y, Tan L Z, et al. Study on new technology of Red Base KD [J]. Fine and Specialty Chemicals, 2003, 11(6): 18-19. | |
| 30 | 吕玉芝, 唐会林. 红色基KD合成新工艺探讨[J]. 染料工业, 1996, 2: 37-38. |
| Lyu Y Z, Tang H L. Discussion on new synthesis process of Red Base KD [J]. Dye Industry, 1996, 2: 37-38 | |
| 31 | Hughes E D, Ingold C K, Ridd J H. Kinetics and mechanism of diazotization[J]. Nature, 1950, 166(4224): 642-643. |
| 32 | Casado U, Castro A, Iglesias E, et al. Kinetics of acid and nucleophile catalysis of the diazotization of 1-naphthylamine[J]. Conadian Journal of Chemistry, 1985, 64: 133-137. |
| [1] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [2] | 苏伟, 马东旭, 金旭, 刘忠彦, 张小松. 表面润湿性对霜层传递特性影响可视化实验研究[J]. 化工学报, 2023, 74(S1): 122-131. |
| [3] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [4] | 王玉兵, 李杰, 詹宏波, 朱光亚, 张大林. R134a在菱形离散肋微小通道内的流动沸腾换热实验研究[J]. 化工学报, 2023, 74(9): 3797-3806. |
| [5] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [6] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [7] | 葛运通, 王玮, 李楷, 肖帆, 于志鹏, 宫敬. 多相分散体系中微油滴与改性二氧化硅表面间作用力的AFM研究[J]. 化工学报, 2023, 74(4): 1651-1659. |
| [8] | 禹进, 余彬彬, 蒋新生. 一种基于虚拟组分的燃烧调控化学作用量化及分析方法研究[J]. 化工学报, 2023, 74(3): 1303-1312. |
| [9] | 付家崴, 陈帅帅, 方凯伦, 蒋新. 微反应器共沉淀反应制备铜锰催化剂[J]. 化工学报, 2023, 74(2): 776-783. |
| [10] | 章承浩, 罗京, 张吉松. 微反应器内基于氮氧自由基催化剂连续氧气/空气氧化反应的研究进展[J]. 化工学报, 2023, 74(2): 511-524. |
| [11] | 谢煜, 张民, 胡卫国, 王玉军, 骆广生. 利用膜分散微反应器高效溶解D-7-ACA的研究[J]. 化工学报, 2023, 74(2): 748-755. |
| [12] | 杨星宇, 马优, 朱春英, 付涛涛, 马友光. 梳状并行微通道内液液分布规律研究[J]. 化工学报, 2023, 74(2): 698-706. |
| [13] | 陈晨, 杨倩, 陈云, 张睿, 刘冬. 不同氧浓度下煤挥发分燃烧的化学动力学研究[J]. 化工学报, 2022, 73(9): 4133-4146. |
| [14] | 张经纬, 周弋惟, 陈卓, 徐建鸿. 微反应器内的有机合成前沿进展[J]. 化工学报, 2022, 73(8): 3472-3482. |
| [15] | 侯跃辉, 刘璇, 廉应江, 韩梅, 尧超群, 陈光文. 超声微反应器内三硝基间苯三酚合成工艺研究[J]. 化工学报, 2022, 73(8): 3597-3607. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号