化工学报 ›› 2021, Vol. 72 ›› Issue (6): 3411-3420.DOI: 10.11949/0438-1157.20201424
收稿日期:2020-10-12
修回日期:2021-03-09
出版日期:2021-06-05
发布日期:2021-06-05
通讯作者:
刘杰
作者简介:于瑞广(1992—),男,博士研究生,基金资助:
YU Ruiguang1,2( ),LIU Jie1,2(
),LIU Jie1,2( ),MA Biao1,2
),MA Biao1,2
Received:2020-10-12
Revised:2021-03-09
Online:2021-06-05
Published:2021-06-05
Contact:
LIU Jie
摘要:
为探究替代燃料丙烷/甲醇混合气的氧化反应特性,利用爆炸极限开展了甲醇对丙烷/氧气混合气的负温度系数(NTC)响应特性的研究。结果表明:在NTC区域,下拐点的压力随着甲醇摩尔分数的增加而升高,但下拐点的温度几乎保持不变。然而上拐点的温度与压力随着甲醇摩尔分数的增加没有明显变化。整体而言,随着甲醇摩尔分数的增加,丙烷/氧气混合气的NTC区域不断减小并向高压区域移动。对比分析了不同爆炸状态下,即无爆炸、冷焰以及热焰状态,混合气的温度、压力以及主要组分变化,并获得了影响温度变化的主要基元反应。此外,对爆炸极限曲线的NTC区域上、下拐点进行了敏感性分析,确定影响拐点状态的主要基元反应。
中图分类号:
于瑞广, 刘杰, 马彪. 甲醇对丙烷/氧气混合气爆炸极限的影响[J]. 化工学报, 2021, 72(6): 3411-3420.
YU Ruiguang, LIU Jie, MA Biao. Effect of methanol on explosion limits of propane-oxygen mixture[J]. CIESC Journal, 2021, 72(6): 3411-3420.
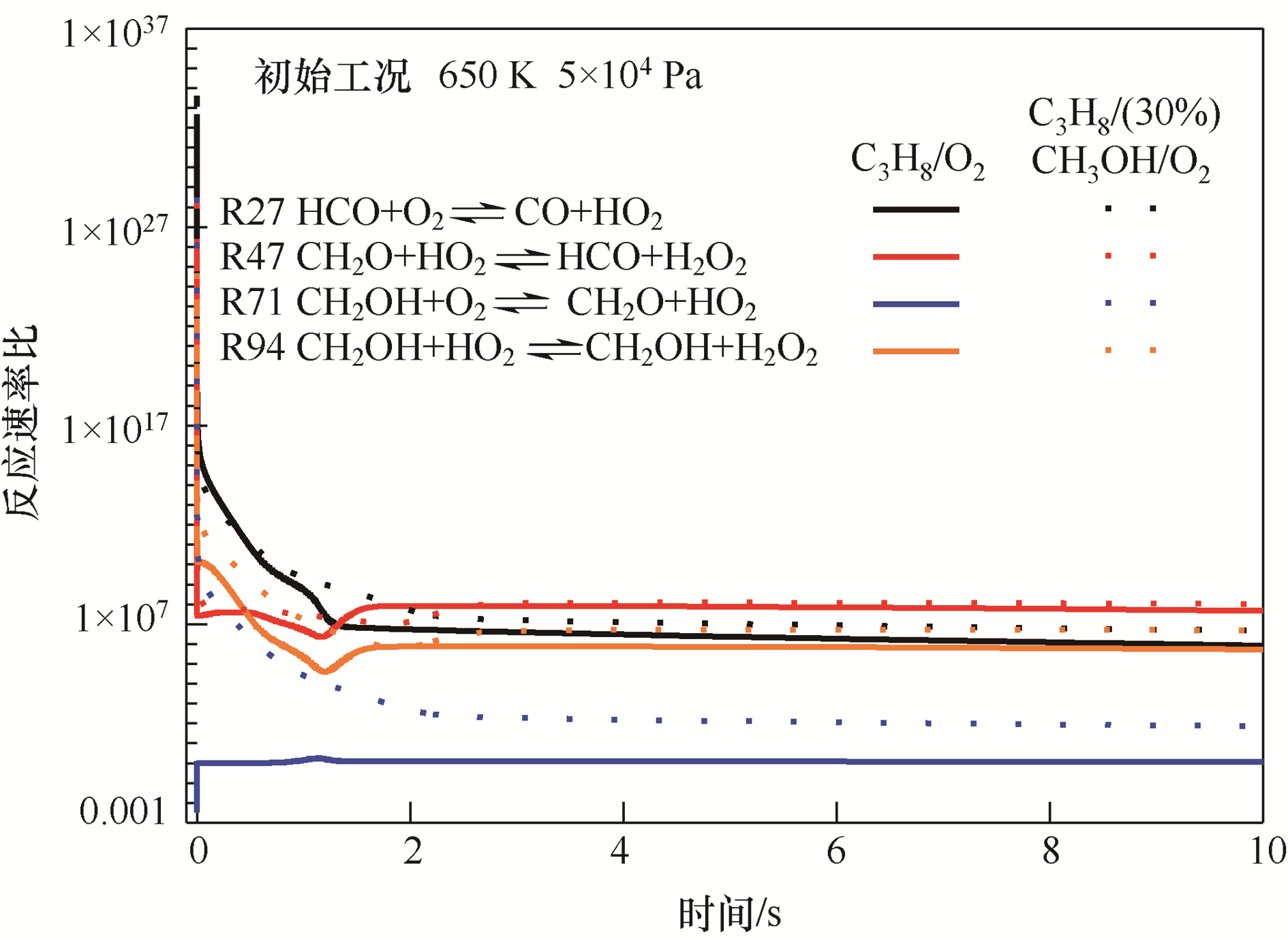
图7 650 K、5×104 Pa 下C3H8/O2和C3H8/(30%)CH3OH/O2混合气中HO2主要反应速率比
Fig.7 The forward and backward reactions ratios of the HO2 main reactions at 650 K,5×104 Pa under C3H8/O2 and C3H8/(30%)CH3OH/O2 mixtures
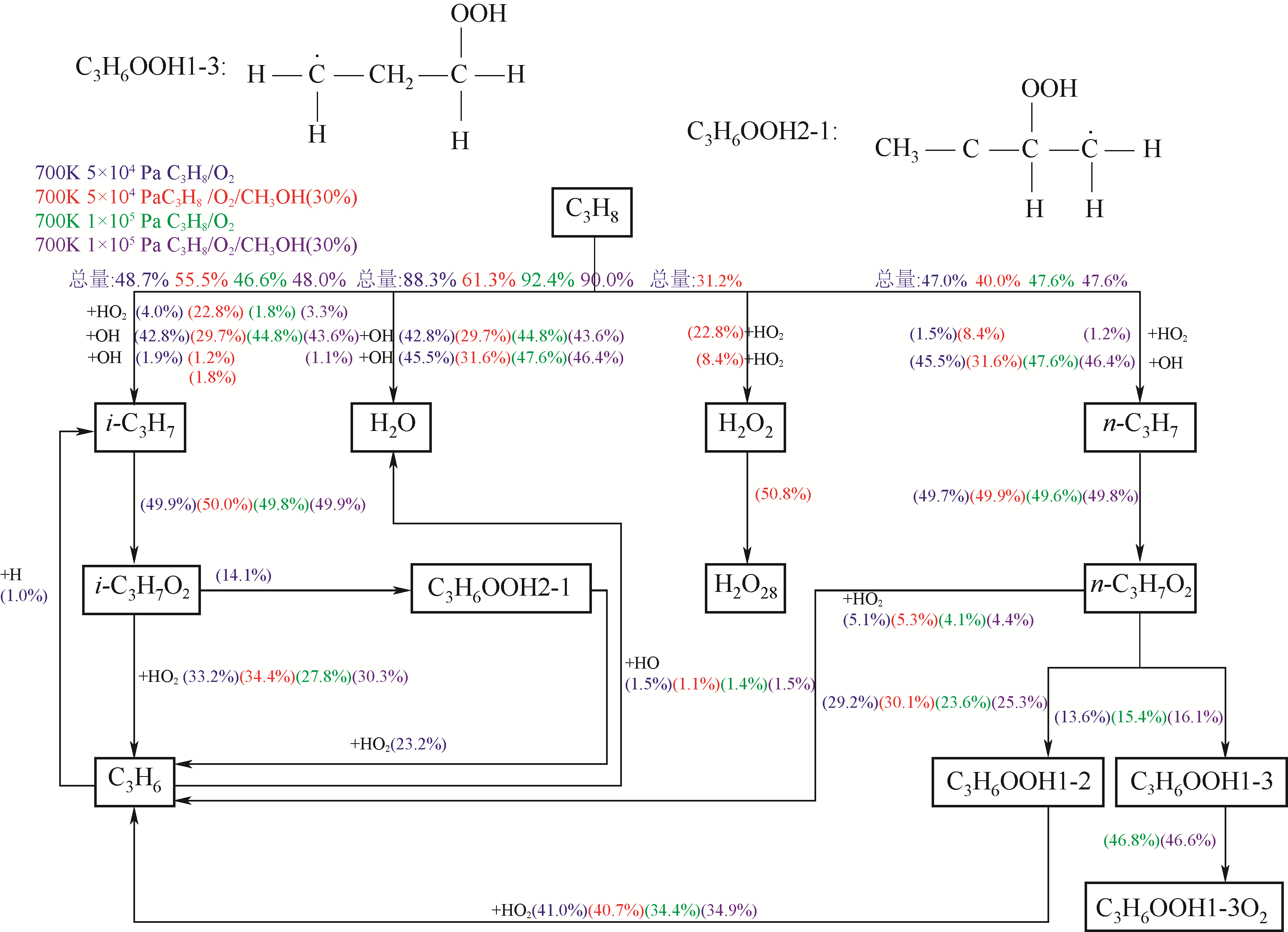
图8 反应路径分析:(700 K, 5×104 Pa)工况点下,蓝色为C3H8/O2混合气,红色为C3H8/(30%)CH3OH/O2混合气; (700 K, 1×105 Pa)工况点下,绿色为C3H8/O2混合气,紫色为C3H8/(30%)CH3OH/O2混合气
Fig.8 The reaction pathway analysis of C3H8/O2 mixtures (Blue symbols) and with 30% CH3OH addition of C3H8/O2 mixtures (Red symbols) at (700 K, 5×104 Pa) condition and, C3H8/O2 mixtures (Green symbols) and with 30% CH3OH addition of C3H8/O2 mixtures (Violet symbols) at (700 K, 1×105 Pa) condition
| 1 | Bae C, Kim J. Alternative fuels for internal combustion engines[J]. Proceedings of the Combustion Institute, 2017, 36(3): 3389-3413. |
| 2 | 姚春德, 韩国鹏, 银增辉, 等. 柴油/甲醇高温富燃动力学机理的简化[J]. 天津大学学报(自然科学与工程技术版), 2015, 48(9): 784-790. |
| Yao C D, Han G P, Yin Z H, et al. Reduction of diesel/methanol kinetic mechanism in high temperature fuel-enriched conditions[J]. Journal of Tianjin University (Science and Technology), 2015, 48(9): 784-790. | |
| 3 | 姚春德, 代乾, 许汉君, 等. 柴油在甲醇/空气高温热氛围中的着火和燃烧特性[J]. 燃烧科学与技术, 2012, 18(3): 193-198. |
| Yao C D, Dai Q, Xu H J, et al. Ignition and combustion characteristics of diesel spray in premixed methanol/air mixture with high temperature[J]. Journal of Combustion Science and Technology, 2012, 18(3): 193-198. | |
| 4 | 夏琦, 姚春德, 魏立江, 等. 柴油/甲醇组合发动机燃烧压力特性研究[J]. 农业机械学报, 2014, 45(2): 6-10, 15. |
| Xia Q, Yao C D, Wei L J, et al. Investigation of combustion pressure characteristics from diesel engine with DMCC mode[J]. Transactions of the Chinese Society for Agricultural Machinery, 2014, 45(2): 6-10, 15. | |
| 5 | 姚春德, 胡江涛, 臧儒振, 等. 柴油/甲烷和柴油/甲醇二元燃料着火反应机理比较研究[J]. 工程热物理学报, 2017, 38(2): 392-398. |
| Yao C D, Hu J T, Zang R Z, et al. Comparison of ignition reaction mechanism of diesel/methane and diesel/methanol dual fuel[J]. Journal of Engineering Thermophysics, 2017, 38(2): 392-398. | |
| 6 | 徐建楠, 蒋新生, 张昌华, 等. 不同工况下汽油蒸气爆炸着火延迟与机理分析[J]. 化工学报, 2019, 70(1): 398-407. |
| Xu J N, Jiang X S, Zhang C H, et al. Ignition delay measurements and analysis of kinetic mechanisms for gasoline-air explosion under different conditions[J]. CIESC Journal, 2019, 70(1): 398-407. | |
| 7 | Pease R N. Characteristics of the non-explosive oxidation of propane and the butanes1[J]. Journal of the American Chemical Society, 1929, 51(6): 1839-1856. |
| 8 | 刘杰, 张欣, 杨福源, 等. 引燃油量对柴油引燃天然气发动机燃烧特性的影响[J]. 汽车工程, 2015, 37(4): 375-379, 386. |
| Liu J, Zhang X, Yang F Y, et al. Effects of pilot fuel quantity on the combustion characteristics of a diesel pilot ignition CNG engine[J]. Automotive Engineering, 2015, 37(4): 375-379, 386. | |
| 9 | Davy H. Some new experiments and observations on the combustion of gaseous mixtures; with an account of a method of preserving a continued light in mixtures of inflammable gases and air without flame[J]. Proc Royal Soc London, 2016, 2(1815):61-62. |
| 10 | Glassman I, Richard A Y, Nick G G. Combustion[M]. New York: Elsevier, 2015: 79. |
| 11 | 陈锐, 张鹏飞, 潘家营, 等. 丙烷自燃特性及爆震机理的试验研究[J]. 天津大学学报(自然科学与工程技术版), 2018, 51(12): 1217-1222. |
| Chen R, Zhang P F, Pan J Y, et al. Experimental study of spontaneous combustion characteristics and knocking mechanism of propane[J]. Journal of Tianjin University (Science and Technology), 2018, 51(12): 1217-1222. | |
| 12 | Yeom K, Jang J, Bae C. Homogeneous charge compression ignition of LPG and gasoline using variable valve timing in an engine[J]. Fuel, 2007, 86(4): 494-503. |
| 13 | Pease R N. The negative temperature coefficient in the rate of propane oxidation[J]. Journal of the American Chemical Society, 1938, 60(9): 2244-2246. |
| 14 | Minkoff G J, Tipper C F H. Chemistry of Combustion Reactions[M]. London: Butterworth, 1962: 3-12. |
| 15 | Curran H J, Gaffuri P, Pitz W J, et al. A comprehensive modeling study of n-heptane oxidation[J]. Combustion and Flame, 1998, 114(1/2): 149-177. |
| 16 | Lewis B, von Elbe G. Detonation waves in gases[M]. Combustion, Flames and Explosions of Gases, Amsterdam: Elsevier, 1987: 532-575. |
| 17 | Koert D N, Miller D L, Cernansky N P. Experimental studies of propane oxidation through the negative temperature coefficient region at 10 and 15 atmospheres[J]. Combustion and Flame, 1994, 96(1/2): 34-49. |
| 18 | Koert D N, Pit W J, Boelli J W, et al. Chemical kinetic modeling of high-pressure propane oxidation and comparison to experimental results[J]. Symposium (International) on Combustion, 1996, 26(1): 633-640. |
| 19 | Gallagher S M, Curran H J, Metcalfe W K, et al. A rapid compression machine study of the oxidation of propane in the negative temperature coefficient regime[J]. Combustion and Flame, 2008, 153(1/2): 316-333. |
| 20 | Zhukov V P, Sechenov V A, Starikovskii A Y. Autoignition of a lean propane-air mixture at high pressures[J]. Kinetics and Catalysis, 2005, 46(3): 319-327. |
| 21 | Yu R G, Liu J, Ma B. The dependence of NTC behavior on the equivalence ratio and nitrogen fraction in cool flame region[J]. Fuel, 2020, 271: 117623. |
| 22 | Liu J, Yu R G, Ma B, et al. On the second explosion limits of hydrogen, methane, ethane, and propane[J]. ACS Omega, 2020, 5(30): 19268-19276. |
| 23 | Liu J, Yu R G, Ma B. Effect of ozone addition on the cool flame and negative temperature coefficient regions of propane-oxygen mixtures[J]. ACS Omega, 2020, 5(27): 16448-16454. |
| 24 | 焦清介, Griffiths John F. 甲醇自发点火特性研究[J]. 北京理工大学学报, 1994, 14(4): 347-354. |
| Jiao Q J, Griffiths J. On the spontaneous ignition of methanol[J]. Journal of Beijing Institute of Technology, 1994, 14(4): 347-354. | |
| 25 | 杨协和, 沈文锋, 张扬, 等. 甲醇-空气层流火焰速度的数值研究和预测模型[J]. 化工学报, 2019, 70(8): 3011-3020. |
| Yang X H, Shen W F, Zhang Y, et al. Numerical investigation and prediction models for methanol-air laminar flame speed[J]. CIESC Journal, 2019, 70(8): 3011-3020. | |
| 26 | Lee H, Lim O. A computational study of DME-methanol fractions with controlling several factors on HCCI combustion[J]. Journal of Mechanical Science and Technology, 2016, 30(4): 1931-1941. |
| 27 | Lutz A E, Kee R J, Miller J A. Senkin: a Fortran program for predicting homogeneous gas phase chemical kinetics with sensitivity analysis[R]. New Mexico: Sandia National Laboratories, 1988. |
| 28 | Liu J, Wang J L, Zhang N, et al. On the explosion limit of syngas with CO2 and H2O additions[J]. International Journal of Hydrogen Energy, 2018, 43(6): 3317-3329. |
| 29 | Atef N, Kukkadapu G, Mohamed S Y, et al. A comprehensive iso-octane combustion model with improved thermochemistry and chemical kinetics[J]. Combustion and Flame, 2017, 178: 111-134. |
| 30 | Li Y, Zhou C W, Somers K P, et al. The oxidation of 2-butene: a high pressure ignition delay, kinetic modeling study and reactivity comparison with isobutene and 1-butene[J]. Proceedings of the Combustion Institute, 2017, 36(1): 403-411. |
| 31 | University of California at San Diego. Chemical-kinetic mechanisms for combustion applications[EB/OL]. [2021-02-27].. |
| 32 | Petersen E L, Kalitan D M, Simmons S, et al. Methane/propane oxidation at high pressures: Experimental and detailed chemical kinetic modeling[J]. Proceedings of the Combustion Institute, 2007, 31(1): 447-454. |
| 33 | Wang X, Law C K. An analysis of the explosion limits of hydrogen-oxygen mixtures[J]. Journal of Chemical Physics, 2013, 138(13): 134305. |
| 34 | Newitt D M, Thornes L S. The oxidation of propane (Ⅲ). The kinetics of the oxidation[J]. Journal of the Chemical Society, 1937: 1669. |
| [1] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [2] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [3] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [4] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [5] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [6] | 李贵贤, 曹阿波, 孟文亮, 王东亮, 杨勇, 周怀荣. 耦合固体氧化物电解槽的CO2制甲醇过程设计与评价研究[J]. 化工学报, 2023, 74(7): 2999-3009. |
| [7] | 李振, 张博, 王丽伟. PEG-EG固-固相变材料的制备和性能研究[J]. 化工学报, 2023, 74(6): 2680-2688. |
| [8] | 吕阳光, 左培培, 杨正金, 徐铜文. 三嗪框架聚合物膜用于有机纳滤甲醇/正己烷分离[J]. 化工学报, 2023, 74(4): 1598-1606. |
| [9] | 何金峰, 李秀珍, 寇建耀, 陶庭杰, 余灿, 刘欢, 陈永元, 赵豪健, 江大好, 李小年. 乙醇制高级醇有序介孔氧化铝负载铜基催化剂研究[J]. 化工学报, 2023, 74(3): 1082-1091. |
| [10] | 王帅, 杨富凯, 徐新宇. 阻燃型全生物基多元醇聚氨酯泡沫的制备及性能研究[J]. 化工学报, 2023, 74(3): 1399-1408. |
| [11] | 毛元敬, 杨智, 莫松平, 郭浩, 陈颖, 罗向龙, 陈健勇, 梁颖宗. C6~C10烷醇的SAFT-VR Mie状态方程参数回归及其热物性研究[J]. 化工学报, 2023, 74(3): 1033-1041. |
| [12] | 禹进, 余彬彬, 蒋新生. 一种基于虚拟组分的燃烧调控化学作用量化及分析方法研究[J]. 化工学报, 2023, 74(3): 1303-1312. |
| [13] | 张雪婷, 胡激江, 赵晶, 李伯耿. 高分子量聚丙二醇在微通道反应器中的制备[J]. 化工学报, 2023, 74(3): 1343-1351. |
| [14] | 项望凯, 刘园园, 郑映, 潘鹏举. 基于熔融/固相缩聚制备中高分子量聚乙醇酸[J]. 化工学报, 2023, 74(2): 933-940. |
| [15] | 张家庆, 蒋榕培, 史伟康, 武博翔, 杨超, 刘朝晖. 煤基/石油基火箭煤油高参数黏温特性与组分特性研究[J]. 化工学报, 2023, 74(2): 653-665. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号