化工学报 ›› 2021, Vol. 72 ›› Issue (11): 5643-5652.DOI: 10.11949/0438-1157.20211030
梁瑜1( ),赵彤1,赵斌彬1,刘雷1(
),赵彤1,赵斌彬1,刘雷1( ),董晋湘1,唐明兴2,李学宽2
),董晋湘1,唐明兴2,李学宽2
收稿日期:2021-07-23
修回日期:2021-09-07
出版日期:2021-11-05
发布日期:2021-11-12
通讯作者:
刘雷
作者简介:梁瑜(1993—),女,博士研究生,基金资助:
Yu LIANG1( ),Tong ZHAO1,Binbin ZHAO1,Lei LIU1(
),Tong ZHAO1,Binbin ZHAO1,Lei LIU1( ),Jinxiang DONG1,Mingxing TANG2,Xuekuan LI2
),Jinxiang DONG1,Mingxing TANG2,Xuekuan LI2
Received:2021-07-23
Revised:2021-09-07
Online:2021-11-05
Published:2021-11-12
Contact:
Lei LIU
摘要:
采用无酸性的α-Al2O3为载体,预先沉积WO3然后浸渍法负载Pt物种,合成了系列Pt-WO3/α-Al2O3催化剂用于萘深度加氢反应,系统地研究了氧化钨物种在萘加氢反应中的作用。通过XRD、Raman、HRTEM、XPS和H2-TPR技术表征了Pt和WO3物种在载体表面的分散情况和状态,并利用Py-IR研究了载体负载氧化钨和Pt前后的酸性质变化。在温和的反应条件下(70℃、3 MPa、1 h)Pt-WO3/α-Al2O3催化剂表现出优异的萘深度加氢活性,萘的转化率和十氢萘的选择性均达到100%。结果表明,预先在载体表面引入的WO3和Pt产生了强相互作用,WO3提高了Pt物种的分散程度,催化剂的酸性来源于氧化钨物种的引入且和负载量成正比关系。催化剂较强的酸性和较高的Pt分散程度是Pt-WO3/α-Al2O3在低温条件下能够使萘深度加氢的关键因素,对于十氢萘作为储氢介质工艺具有重要的意义。
中图分类号:
梁瑜, 赵彤, 赵斌彬, 刘雷, 董晋湘, 唐明兴, 李学宽. WO3对Pt/α-Al2O3催化萘深度加氢的促进作用[J]. 化工学报, 2021, 72(11): 5643-5652.
Yu LIANG, Tong ZHAO, Binbin ZHAO, Lei LIU, Jinxiang DONG, Mingxing TANG, Xuekuan LI. Promotion of WO3 species on Pt/α-Al2O3 for the deep hydrogenation of naphthalene[J]. CIESC Journal, 2021, 72(11): 5643-5652.
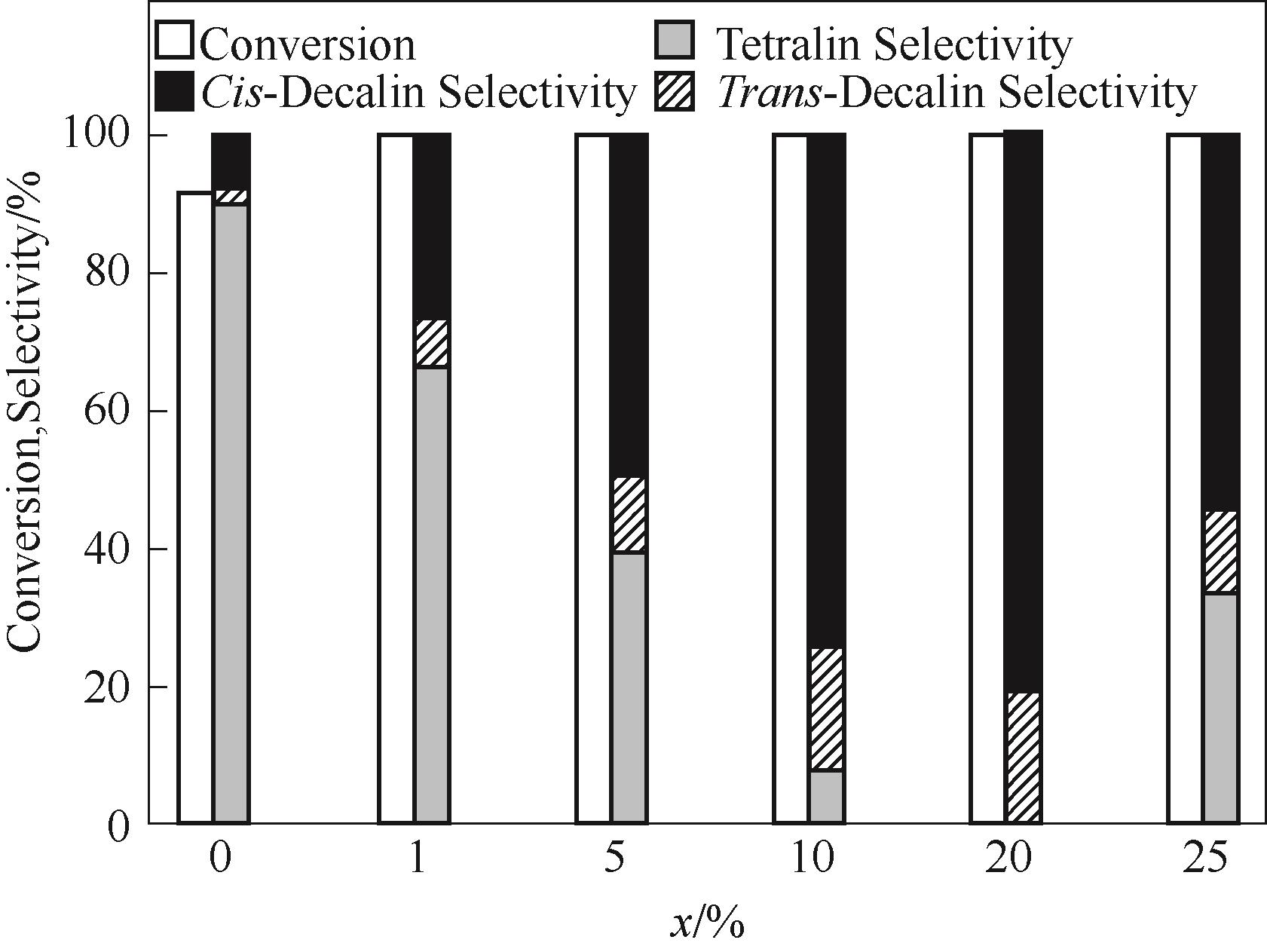
图7 萘在1%Pt-xWO3/α-Al2O3催化剂上加氢反应结果(反应条件:0.05 mol/L萘溶液5 ml,催化剂0.02 g,初始H2压力3 MPa,反应温度70℃,时间1 h)
Fig.7 The reaction results for naphthalene hydrogenation over 1%Pt-xWO3/α-Al2O3 catalyst (reaction conditions: 5 ml naphthalene solution of 0.05 mol/L, 0.02 g catalyst, 3 MPa initial H2 pressure, temperature of 70℃, and time of 1 h)

图8 yPt-20%WO3/α-Al2O3催化剂上萘加氢反应结果(反应条件:0.05 mol/L萘溶液5 ml,催化剂0.02 g,初始H2压力3 MPa,温度70℃,时间1 h)
Fig. 8 The reaction results for naphthalene hydrogenation over yPt-20%WO3/α-Al2O3 catalyst (reaction conditions: 5 ml naphthalene solution of 0.05 mol/L, 0.02 g catalyst, 3 MPa initial H2 pressure, temperature of 70℃, and time of 1 h)
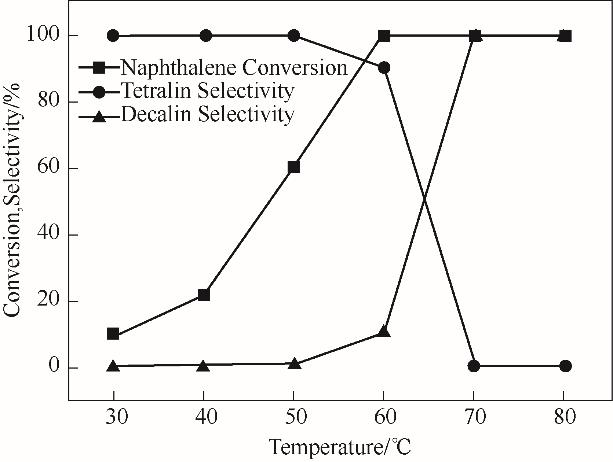
图9 1%Pt-20%WO3/α-Al2O3催化剂在不同反应温度下萘加氢的反应结果(反应条件:0.05 mol/L萘溶液5 ml,催化剂0.02 g,初始H2压力3 MPa,时间1 h)
Fig.9 Catalytic results for naphthalene hydrogenation over 1%Pt-20%WO3/α-Al2O3 at various reaction temperature (reaction conditions: 5 ml naphthalene solution of 0.05 mol/L, 0.02 g catalyst, 3 MPa initial H2 pressure, and time of 1 h)
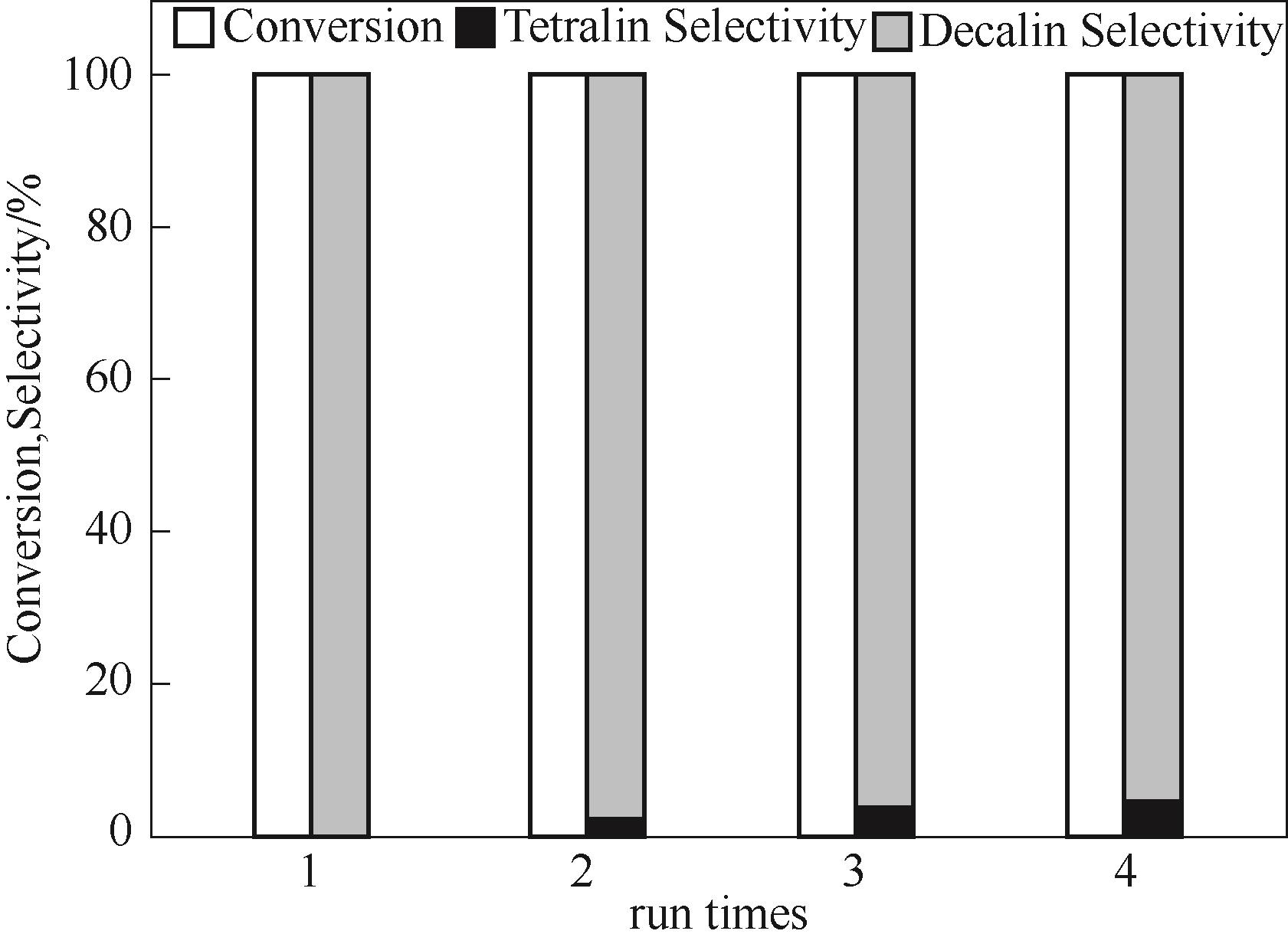
图10 1%Pt-20%WO3/α-Al2O3催化剂4次循环使用反应结果(反应条件:0.05 mol/L萘溶液5 ml,催化剂0.02 g,初始H2压力3 MPa,时间1 h)
Fig.10 Reusability of the 1%Pt-20%WO3/α-Al2O3 catalyst for naphthalene hydrogenation (reaction conditions: 5 ml naphthalene solution of 0.05 mol/L, 0.02 g catalyst, 3 MPa initial H2 pressure, and time of 1 h)
| 1 | 史晓斐, 杨思宇, 钱宇. 化学链技术在煤炭清洁高效利用中的研究进展[J]. 化工学报, 2018, 69(12): 4931-4946. |
| Shi X F, Yang S Y, Qian Y. Chemical looping technology for clean and highly efficient coal processes[J]. CIESC Journal, 2018, 69(12): 4931-4946. | |
| 2 | 蔡达理, 熊昊, 张晨曦, 等. 从分子筛上纳尺度离散行为控制到宏观煤化工过程[J]. 化工学报, 2020, 71(9): 3849-3865. |
| Cai D L, Xiong H, Zhang C X, et al. From nanoscale discrete diffusion behavior control to macroscale coal chemical process[J]. CIESC Journal, 2020, 71(9): 3849-3865. | |
| 3 | 宋会. 工业萘两步催化加氢制十氢萘的研究[D]. 大连: 大连理工大学, 2015. |
| Song H. Two-step catalytic hydrogenation of industrial naphthalene to decalin[D]. Dalian: Dalian University of Technology, 2015. | |
| 4 | Pang M, Liu C Y, Xia W, et al. Activated carbon supported molybdenum carbides as cheap and highly efficient catalyst in the selective hydrogenation of naphthalene to tetralin[J]. Green Chemistry, 2012, 14(5): 1272. |
| 5 | 谭凤宜. 固定床法萘催化加氢合成十氢萘工艺研究[D]. 南京: 南京工业大学, 2006. |
| Tan F Y. Study on hydrogenation of naphthalene to decalin in a fixed bed reactor[D]. Nanjing: Nanjing University of Technology, 2006. | |
| 6 | 张媛媛, 赵静, 鲁锡兰, 等. 有机液体储氢材料的研究进展[J]. 化工进展, 2016, 35(9): 2869-2874. |
| Zhang Y Y, Zhao J, Lu X L, et al. Progress in liquid organic hydrogen storage materials[J]. Chemical Industry and Engineering Progress, 2016, 35(9): 2869-2874. | |
| 7 | 杨平, 辛靖, 李明丰, 等. 四氢萘加氢转化研究进展[J]. 石油炼制与化工, 2011, 42(8): 1-6. |
| Yang P, Xin J, Li M F, et al. Research advances in the hydroconversion of tetralin[J]. Petroleum Processing and Petrochemicals, 2011, 42(8): 1-6. | |
| 8 | 郑修新, 赵甲, 孙国方, 等. 萘加氢催化剂的研究进展[J]. 化工进展, 2015, 34(5): 1295-1299. |
| Zheng X X, Zhao J, Sun G F, et al. Research progress in catalysts for the hydrogenation of naphthalene[J]. Chemical Industry and Engineering Progress, 2015, 34(5): 1295-1299. | |
| 9 | Egorova M, Prins R. Competitive hydrodesulfurization of 4,6-dimethyldibenzothiophene, hydrodenitrogenation of 2-methylpyridine, and hydrogenation of naphthalene over sulfided NiMo/γ- Al2O3[J]. Journal of Catalysis, 2004, 224(2): 278-287. |
| 10 | Albertazzi S, Busca G, Finocchio E, et al. New Pd/Pt on Mg/Al basic mixed oxides for the hydrogenation and hydrogenolysis of naphthalene[J]. Journal of Catalysis, 2004, 223(2): 372-381. |
| 11 | Chen H L, Yang H, Omotoso O, et al. Contribution of hydrogen spillover to the hydrogenation of naphthalene over diluted Pt/RHO catalysts[J]. Applied Catalysis A: General, 2009, 358(2): 103-109. |
| 12 | Albertazzi S, Ganzerla R, Gobbi C, et al. Hydrogenation of naphthalene on noble-metal-containing mesoporous MCM-41 aluminosilicates[J]. Journal of Molecular Catalysis A: Chemical, 2003, 200(1/2): 261-270. |
| 13 | Liu J J, Zhang H F, Lu N Y, et al. Influence of acidity of mesoporous ZSM-5-supported Pt on naphthalene hydrogenation[J]. Industrial & Engineering Chemistry Research, 2020, 59(3): 1056-1064. |
| 14 | Kishore Kumar S A, John M, Pai S M, et al. Low temperature hydrogenation of aromatics over Pt-Pd/SiO2-Al2O3 catalyst[J]. Fuel Processing Technology, 2014, 128: 303-309. |
| 15 | Huang T C, Kang B C. Hydrogenation of naphthalene with platinum-aluminium borate catalysts[J]. The Chemical Engineering Journal and the Biochemical Engineering Journal, 1996, 63(1): 27-36. |
| 16 | Zheng J, Guo M, Song C S. Characterization of Pd catalysts supported on USY zeolites with different SiO2/Al2O3 ratios for the hydrogenation of naphthalene in the presence of benzothiophene[J]. Fuel Processing Technology, 2008, 89(4): 467-474. |
| 17 | Sun W D, Zhao Z B, Guo C, et al. Study of the alkylation of isobutane with n-butene over WO3/ZrO2 strong solid acid(Ⅰ): Effect of the preparation method, WO3 loading, and calcination temperature[J]. Industrial & Engineering Chemistry Research, 2000, 39(10): 3717-3725. |
| 18 | Papp J, Soled S, Dwight K, et al. Surface acidity and photocatalytic activity of TiO2, WO3/TiO2, and MoO3/TiO2 photocatalysts[J]. Chemistry of Materials, 1994, 6(4): 496-500. |
| 19 | Zhou M X, Yang M, Yang X F, et al. On the mechanism of H2 activation over single-atom catalyst: an understanding of Pt1/WOx in the hydrogenolysis reaction[J]. Chinese Journal of Catalysis, 2020, 41(3): 524-532. |
| 20 | Kurosaka T, Maruyama H, Naribayashi I, et al. Production of 1,3-propanediol by hydrogenolysis of glycerol catalyzed by Pt/WO3/ZrO2[J]. Catalysis Communications, 2008, 9(6): 1360-1363. |
| 21 | García-Fernández S, Gandarias I, Requies J, et al. The role of tungsten oxide in the selective hydrogenolysis of glycerol to 1,3-propanediol over Pt/WOx/Al2O3[J]. Applied Catalysis B: Environmental, 2017, 204: 260-272. |
| 22 | García-Fernández S, Gandarias I, Requies J, et al. New approaches to the Pt/WOx/Al2O3 catalytic system behavior for the selective glycerol hydrogenolysis to 1,3-propanediol[J]. Journal of Catalysis, 2015, 323: 65-75. |
| 23 | Barton D G, Shtein M, Wilson R D, et al. Structure and electronic properties of solid acids based on tungsten oxide nanostructures[J]. The Journal of Physical Chemistry B, 1999, 103(4): 630-640. |
| 24 | Arundhathi R, Mizugaki T, Mitsudome T, et al. Highly selective hydrogenolysis of glycerol to 1,3-propanediol over a boehmite-supported platinum/tungsten catalyst[J]. ChemSusChem, 2013, 6(8): 1345-1347. |
| 25 | Kim T, Burrows A, Kiely C J, et al. Molecular/electronic structure-surface acidity relationships of model-supported tungsten oxide catalysts[J]. Journal of Catalysis, 2007, 246(2): 370-381. |
| 26 | 谭训彦, 王昕, 尹衍升, 等. α-Al2O3的晶体结构与价电子结构[J]. 中国有色金属学报, 2002, 12(z1): 18-23. |
| Tan X Y, Wang X, Yin Y S, et al. Crystal structure and valence electron structure of α-Al2O3[J]. The Chinese Journal of Nonferrous Metals, 2002, 12(z1): 18-23. | |
| 27 | Lee S H, Cheong H M, Tracy C E, et al. Raman spectroscopic studies of electrochromic a-WO3[J]. Electrochimica Acta, 1999, 44(18): 3111-3115. |
| 28 | Farbotko J, Rynkowski J, Touroude R. Efekty SMSI w katalizatorach Pt/WO3/Al2O3[J]. Zeszyty Naukowe, 1998,46: 133-146. |
| 29 | Qin L Z, Song M J, Chen C L. Aqueous-phase deoxygenation of glycerol to 1,3-propanediol over Pt/WO3/ZrO2 catalysts in a fixed-bed reactor[J]. Green Chemistry, 2010, 12(8): 1466. |
| 30 | Zhu S H, Qiu Y N, Zhu Y L, et al. Hydrogenolysis of glycerol to 1,3-propanediol over bifunctional catalysts containing Pt and heteropolyacids[J]. Catalysis Today, 2013, 212: 120-126. |
| 31 | Alexeev O S, Graham G W, Shelef M, et al. γ-Al2O3-supported Pt catalysts with extremely high dispersions resulting from Pt-W interactions[J]. Journal of Catalysis, 2000, 190(1): 157-172. |
| 32 | Wang J, Zhao X C, Lei N, et al. Hydrogenolysis of glycerol to 1, 3-propanediol under low hydrogen pressure over WOx-supported single/pseudo-single atom Pt catalyst[J]. ChemSusChem, 2016, 9(8): 784-790. |
| 33 | Parry E P. An infrared study of pyridine adsorbed on acidic solids. Characterization of surface acidity[J]. Journal of Catalysis, 1963, 2(5): 371-379. |
| 34 | Impéror-Clerc M, Davidson P, Davidson A. Existence of a microporous corona around the mesopores of silica-based SBA-15 materials templated by triblock copolymers[J]. Journal of the American Chemical Society, 2000, 122(48): 11925-11933. |
| 35 | Zhou W, Luo J, Wang Y, et al. WOx domain size, acid properties and mechanistic aspects of glycerol hydrogenolysis over Pt/WOx/ZrO2[J]. Applied Catalysis B: Environmental, 2019, 242: 410-421. |
| [1] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [2] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 曹跃, 余冲, 李智, 杨明磊. 工业数据驱动的加氢裂化装置多工况切换过渡状态检测[J]. 化工学报, 2023, 74(9): 3841-3854. |
| [5] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [6] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [7] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [8] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [9] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [10] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [11] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [12] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [13] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [14] | 张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| [15] | 王辰, 史秀锋, 武鲜凤, 魏方佳, 张昊虹, 车寅, 吴旭. 氧化还原法制备Mn3O4催化剂及其甲苯催化氧化性能与机理研究[J]. 化工学报, 2023, 74(6): 2447-2457. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号