化工学报 ›› 2022, Vol. 73 ›› Issue (3): 1194-1206.DOI: 10.11949/0438-1157.20211439
王旭( ),张乐瑶,张昊轩,演嘉辉,吴玉帅,吴冬,陈汇勇(
),张乐瑶,张昊轩,演嘉辉,吴玉帅,吴冬,陈汇勇( ),马晓迅
),马晓迅
收稿日期:2021-10-21
修回日期:2021-12-03
出版日期:2022-03-15
发布日期:2022-03-14
通讯作者:
陈汇勇
作者简介:王旭(1995—),男,博士研究生,基金资助:
Xu WANG( ),Leyao ZHANG,Haoxuan ZHANG,Jiahui YAN,Yushuai WU,Dong WU,Huiyong CHEN(
),Leyao ZHANG,Haoxuan ZHANG,Jiahui YAN,Yushuai WU,Dong WU,Huiyong CHEN( ),Xiaoxun MA
),Xiaoxun MA
Received:2021-10-21
Revised:2021-12-03
Online:2022-03-15
Published:2022-03-14
Contact:
Huiyong CHEN
摘要:
吸附容量高、吸附速率快以及憎水性强是分子筛用于挥发性有机物(VOCs)高效吸附的主要性能指标。分别以纯硅(S-1)和W掺杂(WS-1)MFI分子筛为母体,通过一步水热脱硅/补钨后处理制备了具有全空腔(HWS-1_S)和多孔芯(HWS-1_W)的两种中空结构分子筛,并以典型的VOCs气体分子丙酮为探针,系统研究了中空结构形态对于分子筛吸附性能的影响。结果表明:HWS-1_S表面部分开孔,内部全空腔且与外部连通,相比于母体S-1,相对结晶度较低,微孔孔容减少;HWS-1_W表面开孔细微,内部出现不规则的大/中孔结构,相比母体WS-1,相对结晶度提高,微孔孔容增大。干气条件下,HWS-1_S与HWS-1_W相比母体S-1和WS-1对丙酮具有更快的吸附速率;HWS-1_S微孔孔容损失严重,导致吸附容量有限(27.4 mg·g-1);HWS-1_W由于重结晶修复了部分结构缺陷,提高了丙酮吸附容量(51.2 mg·g-1)。通过吸附动力学拟合,HWS-1_S和HWS-1_W符合典型的孔扩散机理,对丙酮主要以物理吸附为主。湿气条件下,W掺杂可有效中和中空分子筛表面硅醇基团,在一定程度上提高了W掺杂中空分子筛抗水汽竞争吸附能力。
中图分类号:
王旭, 张乐瑶, 张昊轩, 演嘉辉, 吴玉帅, 吴冬, 陈汇勇, 马晓迅. 中空孔结构对W掺杂MFI分子筛丙酮吸附行为的研究[J]. 化工学报, 2022, 73(3): 1194-1206.
Xu WANG, Leyao ZHANG, Haoxuan ZHANG, Jiahui YAN, Yushuai WU, Dong WU, Huiyong CHEN, Xiaoxun MA. Effect of hollow structure on the acetone adsorption property of tungsten-substituted MFI zeolite[J]. CIESC Journal, 2022, 73(3): 1194-1206.
| 样品 | 金属含量/ %(质量) | 产率/% | 相对结晶度/% | 比表面积/(m2·g-1) | 外表面积/(m2·g-1) | S外表面积/S比表面积 | 微孔孔容/(cm3·g-1) | 总孔容/(cm3·g-1) | V微孔孔容/V总孔容 |
|---|---|---|---|---|---|---|---|---|---|
| S-1 | — | 100 | 100 | 427 | 63 | 0.15 | 0.17 | 0.24 | 0.71 |
| WS-1 | 0.01 | 100 | 100 | 352 | 68 | 0.19 | 0.10 | 0.23 | 0.43 |
| HWS-1_S | 0.09 | 70 | 78 | 253 | 51 | 0.20 | 0.08 | 0.29 | 0.28 |
| HWS-1_W | 0.13 | 75 | 106 | 385 | 91 | 0.24 | 0.12 | 0.34 | 0.35 |
表1 微孔和中空分子筛的金属含量、合成收率、相对结晶度及织构参数
Table 1 Metal (W) contents, synthesis yields, crystallinities and textual properties of various zeolite samples
| 样品 | 金属含量/ %(质量) | 产率/% | 相对结晶度/% | 比表面积/(m2·g-1) | 外表面积/(m2·g-1) | S外表面积/S比表面积 | 微孔孔容/(cm3·g-1) | 总孔容/(cm3·g-1) | V微孔孔容/V总孔容 |
|---|---|---|---|---|---|---|---|---|---|
| S-1 | — | 100 | 100 | 427 | 63 | 0.15 | 0.17 | 0.24 | 0.71 |
| WS-1 | 0.01 | 100 | 100 | 352 | 68 | 0.19 | 0.10 | 0.23 | 0.43 |
| HWS-1_S | 0.09 | 70 | 78 | 253 | 51 | 0.20 | 0.08 | 0.29 | 0.28 |
| HWS-1_W | 0.13 | 75 | 106 | 385 | 91 | 0.24 | 0.12 | 0.34 | 0.35 |
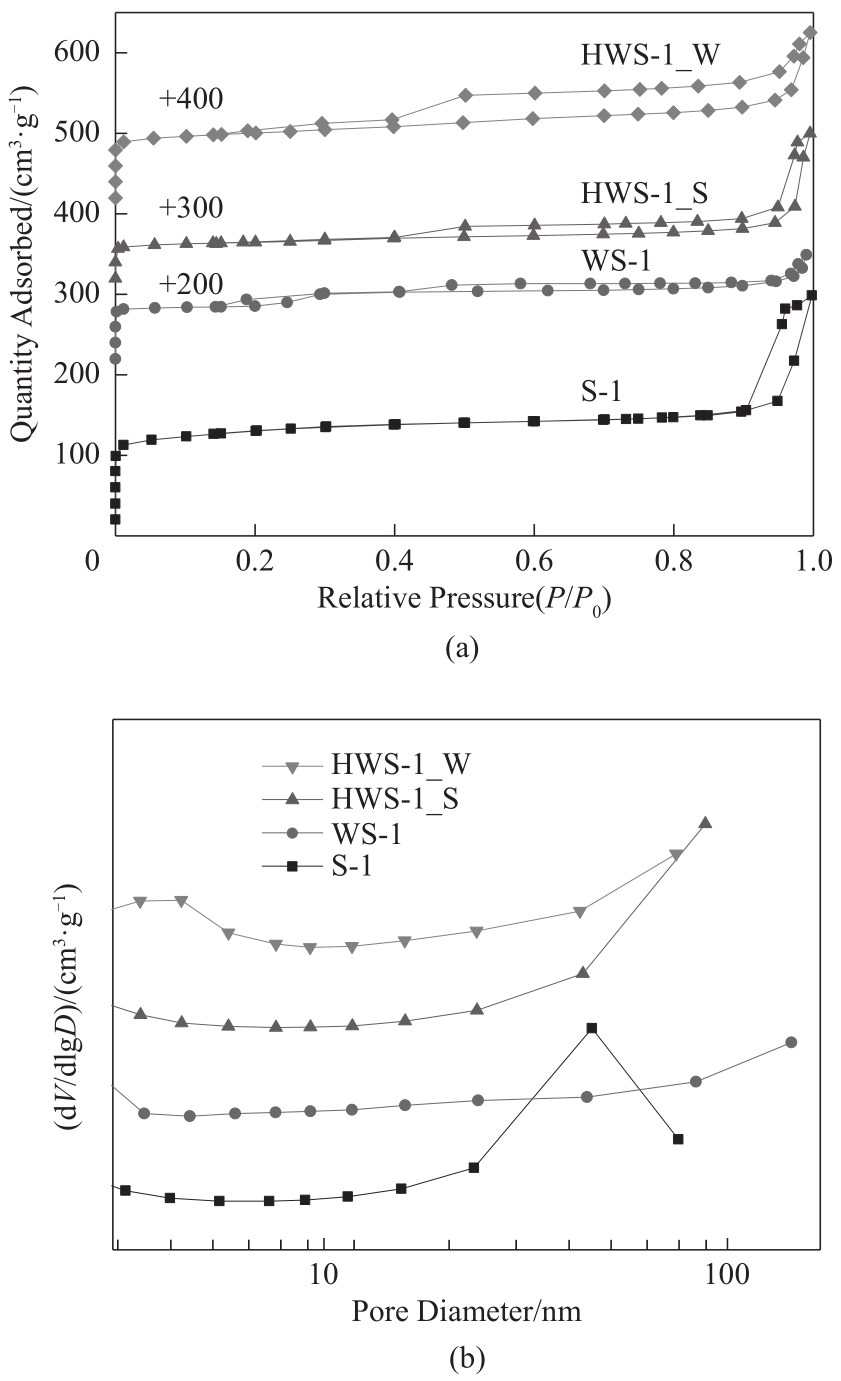
图3 S-1、WS-1、HWS-1_S和HWS-1_W的N2吸-脱附等温线(a); BJH孔径分布(b)
Fig.3 N2 physisorption isotherms (a), and BJH pore size distribution (b) of S-1, WS-1, HWS-1_S and HWS-1_W zeolites
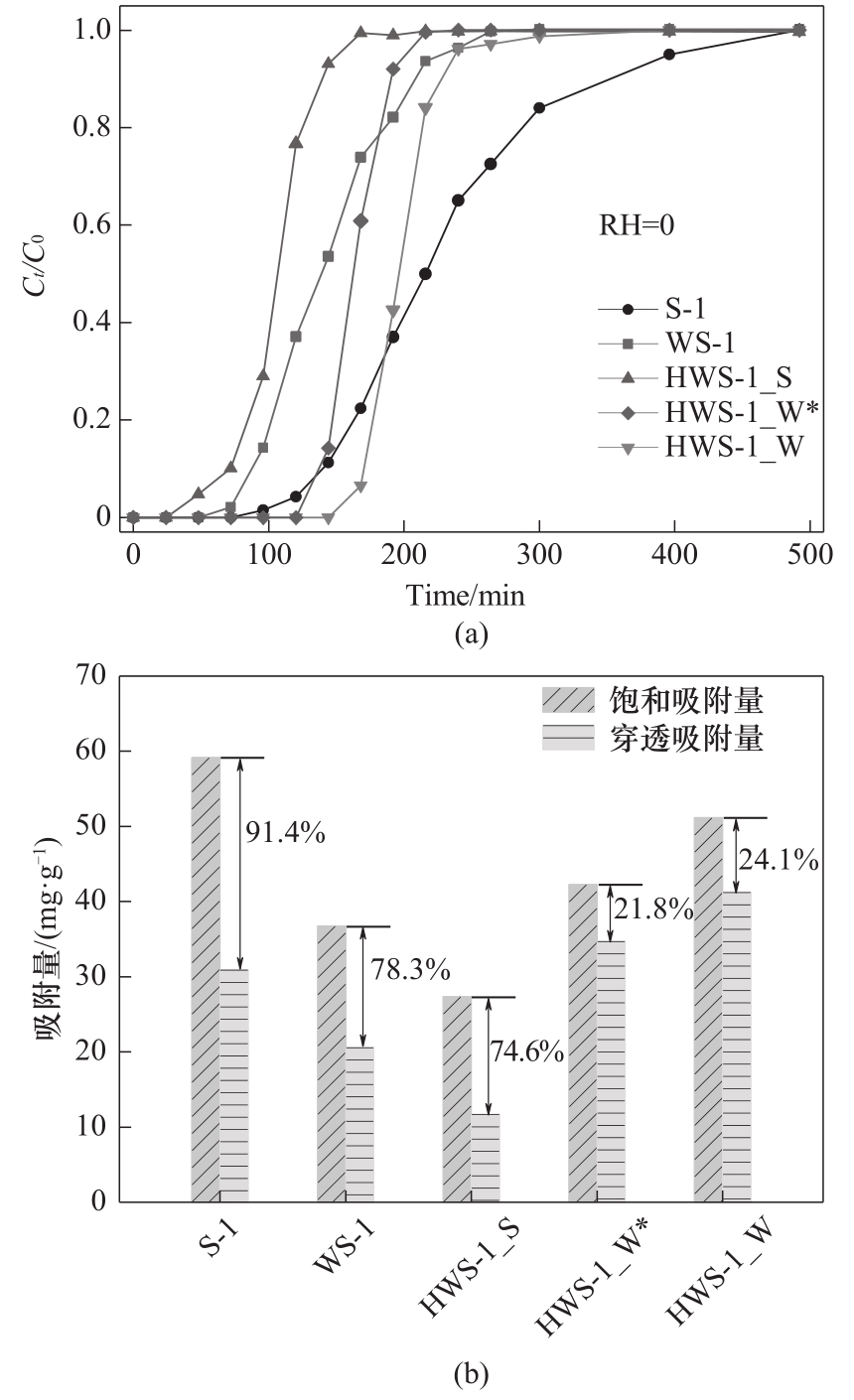
图6 母体及中空分子筛吸附干气丙酮的穿透曲线(a);穿透吸附与饱和吸附的接近程度(b)
Fig.6 Breakthrough curves of acetone adsorption on parent and corresponding hollow zeolites (a), and differences between saturation capacities and breakthrough capacities on parent and corresponding hollow zeolite adsorbents (b)
| 样品 | Yoon-Nelson模型拟合值 | 实验值 | ||
|---|---|---|---|---|
| R2 | ||||
| S-1 | 218 | 0.024 | 0.995 | 216 |
| HWS-1_S | 106 | 0.076 | 0.996 | 102 |
| WS-1 | 140 | 0.036 | 0.998 | 141 |
| HWS-1_W*③ | 163 | 0.092 | 0.999 | 162 |
| HWS-1_W | 196 | 0.085 | 0.998 | 197 |
表2 Yoon-Nelson模型拟合吸附穿透曲线
Table 2 Fitting of breakthrough curves by using Yoon-Nelson model
| 样品 | Yoon-Nelson模型拟合值 | 实验值 | ||
|---|---|---|---|---|
| R2 | ||||
| S-1 | 218 | 0.024 | 0.995 | 216 |
| HWS-1_S | 106 | 0.076 | 0.996 | 102 |
| WS-1 | 140 | 0.036 | 0.998 | 141 |
| HWS-1_W*③ | 163 | 0.092 | 0.999 | 162 |
| HWS-1_W | 196 | 0.085 | 0.998 | 197 |
| 理论模型 | 参数 | S-1 | WS-1 | HWS-1_S | HWS-1_W |
|---|---|---|---|---|---|
| 准一级模型 | k1/min-1 | 0.006 | 0.008 | 0.01 | 0.003 |
| qe/(mg·g-1) | 64.73 | 45.64 | 34.91 | 110.99 | |
| R2 | 0.983 | 0.991 | 0.988 | 0.992 | |
| 准二级模型 | k2/min-1 | 5.79×10-5 | 7.91×10-5 | 1.28×10-4 | 7.75×10-6 |
| qe/(mg·g-1) | 87.22 | 68.93 | 53.63 | 198.83 | |
| R2 | 0.968 | 0.987 | 0.985 | 0.992 | |
| 班厄姆模型 | k3/min-z | 9.80×10-4 | 1.95×10-3 | 2.20×10-3 | 1.14×10-3 |
| qe/(mg·g-1) | 59.77 | 38.33 | 28.26 | 62.25 | |
| z | 1.388 | 1.371 | 1.445 | 1.346 | |
| R2 | 0.999 | 0.999 | 0.998 | 0.997 | |
| 表观扩散系数 | (D/r02) | 5.10 | 8.63 | 12.9 | 7.56 |
| R2 | 0.993 | 0.995 | 0.996 | 0.988 |
表3 微孔和中空分子筛的动力学模型拟合参数
Table 3 Fitting parameters of acetone adsorption kinetics of parent and corresponding hollow zeolite adsorbents
| 理论模型 | 参数 | S-1 | WS-1 | HWS-1_S | HWS-1_W |
|---|---|---|---|---|---|
| 准一级模型 | k1/min-1 | 0.006 | 0.008 | 0.01 | 0.003 |
| qe/(mg·g-1) | 64.73 | 45.64 | 34.91 | 110.99 | |
| R2 | 0.983 | 0.991 | 0.988 | 0.992 | |
| 准二级模型 | k2/min-1 | 5.79×10-5 | 7.91×10-5 | 1.28×10-4 | 7.75×10-6 |
| qe/(mg·g-1) | 87.22 | 68.93 | 53.63 | 198.83 | |
| R2 | 0.968 | 0.987 | 0.985 | 0.992 | |
| 班厄姆模型 | k3/min-z | 9.80×10-4 | 1.95×10-3 | 2.20×10-3 | 1.14×10-3 |
| qe/(mg·g-1) | 59.77 | 38.33 | 28.26 | 62.25 | |
| z | 1.388 | 1.371 | 1.445 | 1.346 | |
| R2 | 0.999 | 0.999 | 0.998 | 0.997 | |
| 表观扩散系数 | (D/r02) | 5.10 | 8.63 | 12.9 | 7.56 |
| R2 | 0.993 | 0.995 | 0.996 | 0.988 |
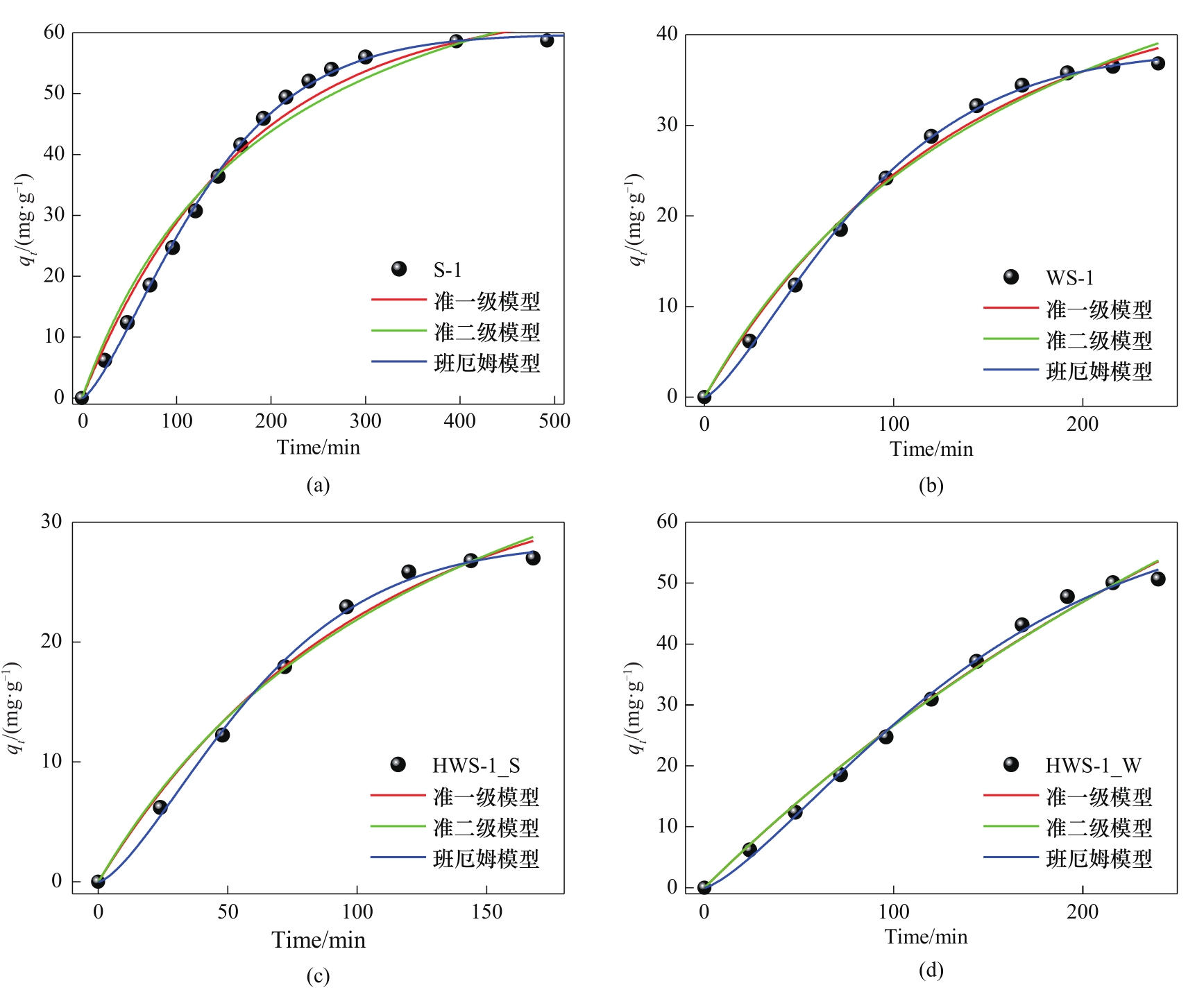
图7 S-1(a)、WS-1(b)、HWS-1_S(c)和HWS-1_W(d)上丙酮吸附动力学拟合
Fig.7 Fitting of acetone adsorption kinetics on S-1 (a), WS-1 (b), HWS-1_S (c), and HWS-1_W (d) zeolite adsorbents
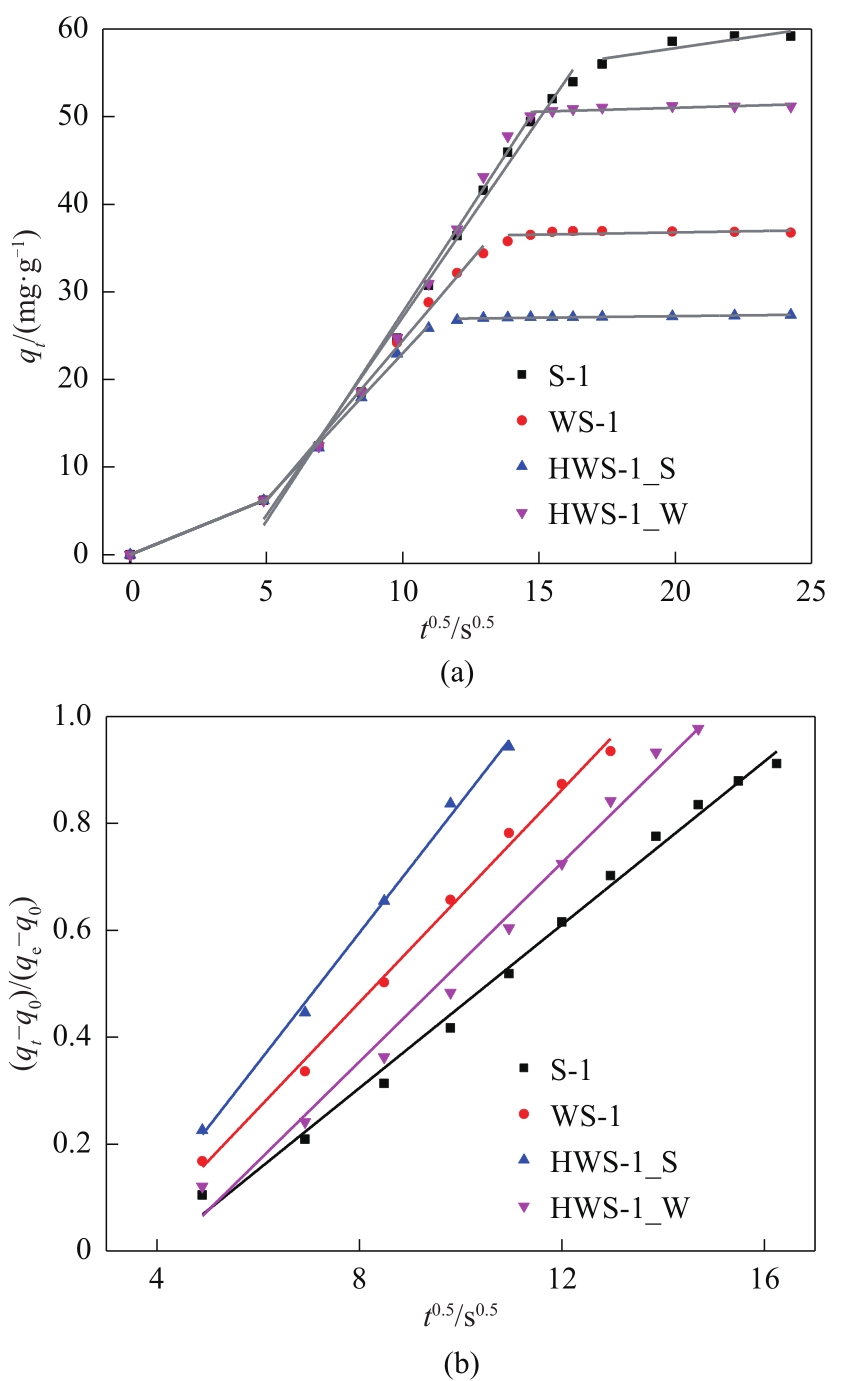
图8 微孔和中空分子筛吸附丙酮过程的Weber-Morris模型拟合(a)和表观扩散系数线性拟合(b)
Fig.8 W-M model curves (a) and fitting of apparent diffusion coefficients (b) of parent and hollow zeolites adsorbents
| 样品 | 穿透时间 | 饱和吸附量 | (Qwet/Qdry)/% | ||
|---|---|---|---|---|---|
| RH=0 | RH=10% | RH=0 | RH=10% | ||
| S-1 | 120 | 96 | 59.2 | 49.7 | 83.9 |
| HWS-1_S | 48 | 36 | 27.4 | 21.5 | 78.5 |
| WS-1 | 80 | 60 | 36.8 | 32.1 | 87.2 |
| HWS-1_W*② | 135 | 100 | 42.3 | 36.4 | 86.0 |
| HWS-1_W | 160 | 120 | 51.2 | 40.4 | 78.9 |
| HWS-1_W_R③ | 156 | 110 | 50.1 | 38.8 | 77.4 |
表4 干气和10%湿度条件下W掺杂中空分子筛丙酮吸附性能
Table 4 Acetone adsorption breakthrough times and saturation adsorption capacities of parent and corresponding hollow zeolite adsorbents at RH = 0 and RH = 10%
| 样品 | 穿透时间 | 饱和吸附量 | (Qwet/Qdry)/% | ||
|---|---|---|---|---|---|
| RH=0 | RH=10% | RH=0 | RH=10% | ||
| S-1 | 120 | 96 | 59.2 | 49.7 | 83.9 |
| HWS-1_S | 48 | 36 | 27.4 | 21.5 | 78.5 |
| WS-1 | 80 | 60 | 36.8 | 32.1 | 87.2 |
| HWS-1_W*② | 135 | 100 | 42.3 | 36.4 | 86.0 |
| HWS-1_W | 160 | 120 | 51.2 | 40.4 | 78.9 |
| HWS-1_W_R③ | 156 | 110 | 50.1 | 38.8 | 77.4 |
| 1 | He C, Cheng J, Zhang X, et al. Recent advances in the catalytic oxidation of volatile organic compounds: a review based on pollutant sorts and sources[J]. Chemical Reviews, 2019, 119(7): 4471-4568. |
| 2 | Maudhuit A, Raillard C, Héquet V, et al. Adsorption phenomena in photocatalytic reactions: the case of toluene, acetone and heptane[J]. Chemical Engineering Journal, 2011, 170(2/3): 464-470. |
| 3 | 孙静, 董一霖, 李法齐, 等. Co3O4改性USY分子筛吸附和催化氧化甲苯特性研究[J]. 化工学报, 2021, 72(6): 3306-3315. |
| Sun J, Dong Y L, Li F Q, et al. Study on adsorption and catalytic oxidation characteristics of toluene on Co3O4 modified USY molecular sieve[J]. CIESC Journal, 2021, 72(6): 3306-3315. | |
| 4 | 高君安, 王伟, 张傑, 等. 用于高湿度废气中甲苯吸附净化的疏水型ZSM-5分子筛的合成及其吸附性能研究[J]. 化工学报, 2020, 71(1): 337-343. |
| Gao J A, Wang W, Zhang J, et al. Study on synthesis and adsorption performance of hydrophobic ZSM-5 zeolites for removal of toluene in high-humidity exhaust gas[J]. CIESC Journal, 2020, 71(1): 337-343. | |
| 5 | Sui H, Liu J J, He L, et al. Adsorption and desorption of binary mixture of acetone and ethyl acetate on silica gel[J]. Chemical Engineering Science, 2019, 197: 185-194. |
| 6 | Baek S W, Kim J R, Ihm S K. Design of dual functional adsorbent/catalyst system for the control of VOC’s by using metal-loaded hydrophobic Y-zeolites[J]. Catalysis Today, 2004, 93/94/95: 575-581. |
| 7 | Ouzzine M, Romero-Anaya A J, Lillo-Ródenas M A, et al. Spherical activated carbons for the adsorption of a real multicomponent VOC mixture[J]. Carbon, 2019, 148: 214-223. |
| 8 | Yang C T, Miao G, Pi Y H, et al. Abatement of various types of VOCs by adsorption/catalytic oxidation: a review[J]. Chemical Engineering Journal, 2019, 370: 1128-1153. |
| 9 | Zhang X Y, Gao B, Creamer A E, et al. Adsorption of VOCs onto engineered carbon materials: a review[J]. Journal of Hazardous Materials, 2017, 338: 102-123. |
| 10 | Zhang L, Peng Y X, Zhang J, et al. Adsorptive and catalytic properties in the removal of volatile organic compounds over zeolite-based materials[J]. Chinese Journal of Catalysis, 2016, 37(6): 800-809. |
| 11 | Shah I K, Pre P, Alappat B J. Effect of thermal regeneration of spent activated carbon on volatile organic compound adsorption performances[J]. Journal of the Taiwan Institute of Chemical Engineers, 2014, 45(4): 1733-1738. |
| 12 | Veerapandian S K P, de Geyter N, Giraudon J M, et al. The use of zeolites for VOCs abatement by combining non-thermal plasma, adsorption, and/or catalysis: a review[J]. Catalysts, 2019, 9(1): 98. |
| 13 | Song W, Justice R E, Jones C A, et al. Size-dependent properties of nanocrystalline silicalite synthesized with systematically varied crystal sizes[J]. Langmuir, 2004, 20(11): 4696-4702. |
| 14 | Li X Q, Zhang L, Yang Z Q, et al. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: a review[J]. Separation and Purification Technology, 2020, 235: 116213. |
| 15 | Lou F J, Zhang G H, Ren L M, et al. Impacts of nano-scale pore structure and organic amine assembly in porous silica on the kinetics of CO2 adsorptive separation[J]. Nano Research, 2021, 14(9): 3294-3302. |
| 16 | Chen L H, Sun M H, Wang Z, et al. Hierarchically structured zeolites: from design to application[J]. Chemical Reviews, 2020, 120(20): 11194-11294. |
| 17 | Li Y, Li L, Yu J H. Applications of zeolites in sustainable chemistry[J]. Chem, 2017, 3(6): 928-949. |
| 18 | Feng A H, Yu Y, Mi L, et al. Structural, textural and toluene adsorption properties of NH4HF2 and alkali modified USY zeolite[J]. Microporous and Mesoporous Materials, 2019, 290: 109646. |
| 19 | Feng A H, Mi L, Yu Y, et al. Development of intracrystalline mesoporosity in NH4HF2-etched NaY zeolites by surfactant-templating and its effect on toluene adsorption[J]. Chemical Engineering Journal, 2020, 390: 124529. |
| 20 | Cosseron A F, Daou T J, Tzanis L, et al. Adsorption of volatile organic compounds in pure silica CHA, BEA, MFI and STT-type zeolites[J]. Microporous and Mesoporous Materials, 2013, 173: 147-154. |
| 21 | Zhu L L, Shen D K, Luo K H. A critical review on VOCs adsorption by different porous materials: species, mechanisms and modification methods[J]. Journal of Hazardous Materials, 2020, 389: 122102. |
| 22 | Dai C Y, Zhang A F, Li L L, et al. Synthesis of hollow nanocubes and macroporous monoliths of silicalite-1 by alkaline treatment[J]. Chemistry of Materials, 2013, 25(21): 4197-4205. |
| 23 | Medeiros-Costa I C, Dib E, Nesterenko N, et al. Silanol defect engineering and healing in zeolites: opportunities to fine-tune their properties and performances[J]. Chemical Society Reviews, 2021, 50(19): 11156-11179. |
| 24 | Grand J, Talapaneni S N, Vicente A, et al. One-pot synthesis of silanol-free nanosized MFI zeolite[J]. Nature Materials, 2017, 16(10): 1010-1015. |
| 25 | Grand J, Talapaneni S N, Aleksandrov H A, et al. Hydrophobic tungsten-containing MFI-type zeolite films for exhaust gas detection[J]. ACS Applied Materials & Interfaces, 2019, 11(13): 12914-12919. |
| 26 | Dubray F, Moldovan S, Kouvatas C, et al. Direct evidence for single molybdenum atoms incorporated in the framework of MFI zeolite nanocrystals[J]. Journal of the American Chemical Society, 2019, 141(22): 8689-8693. |
| 27 | Guo Y, Quan X, Lu N, et al. High photocatalytic capability of self-assembled nanoporous WO3 with preferential orientation of (002) planes[J]. Environmental Science & Technology, 2007, 41(12): 4422-4427. |
| 28 | Zhang H Y, Yang X T, Song X J, et al. Hydrothermal synthesis of tungsten-tin bimetallic MFI type zeolites and their catalytic properties for cyclohexene epoxidation[J]. Microporous and Mesoporous Materials, 2020, 303: 110277. |
| 29 | Wang X, You Q, Wu Y S, et al. Tungsten-substituted Silicalite-1 with an interconnected hollow structure for catalytic epoxidation of cyclohexene[J]. Microporous and Mesoporous Materials, 2021, 317: 111028. |
| 30 | Dai C Y, Zhang S H, Zhang A F, et al. Hollow zeolite encapsulated Ni–Pt bimetals for sintering and coking resistant dry reforming of methane[J]. Journal of Materials Chemistry A, 2015, 3(32): 16461-16468. |
| 31 | Wang Y S, Jia H, Fang X, et al. CO2 and water vapor adsorption properties of framework hybrid W-ZSM-5/silicalite-1 prepared from RHA[J]. RSC Advances, 2020, 10(41): 24642-24652. |
| 32 | Wu H Y, Zhang X L, Yang C Y, et al. Alkali-hydrothermal synthesis and characterization of W-MCM-41 mesoporous materials with various Si/W molar ratios[J]. Applied Surface Science, 2013, 270: 590-595. |
| 33 | Watmanee S, Suriye K, Praserthdam P, et al. Formation of isolated tungstate sites on hierarchical structured SiO2- and HY zeolite-supported WO x catalysts for propene metathesis[J]. Journal of Catalysis, 2019, 376: 150-160. |
| 34 | Hu Q, Li J J, Hao Z P, et al. Dynamic adsorption of volatile organic compounds on organofunctionalized SBA-15 materials[J]. Chemical Engineering Journal, 2009, 149(1/2/3): 281-288. |
| 35 | Li X F, Wang J, Guo Y Y, et al. Adsorption and desorption characteristics of hydrophobic hierarchical zeolites for the removal of volatile organic compounds[J]. Chemical Engineering Journal, 2021, 411: 128558. |
| 36 | Chandak M V, Lin Y S. Hydrophobic zeolites as adsorbents for removal of volatile organic compounds from air[J]. Environmental Technology, 1998, 19(9): 941-948. |
| 37 | Mi Z R, Li J, Lu T T, et al. Reducing the dosage of the organic structure-directing agent in the crystallization of pure silica zeolite MFI (silicalite-1) for volatile organic compounds (VOCs) adsorption[J]. Inorganic Chemistry Frontiers, 2021, 8(13): 3354-3362. |
| 38 | Guo M, Liu Q, Lu S, et al. Synthesis of silanol-rich MCM-48 with mixed surfactants and their application in acetone adsorption: equilibrium, kinetic, and thermodynamic studies[J]. Langmuir, 2020, 36(39): 11528-11537. |
| 39 | Fang H J, Zheng A M, Chu Y Y, et al. 13C chemical shift of adsorbed acetone for measuring the acid strength of solid acids: a theoretical calculation study[J]. The Journal of Physical Chemistry C, 2010, 114(29): 12711-12718. |
| 40 | Huang S S, Deng W, Zhang L, et al. Adsorptive properties in toluene removal over hierarchical zeolites[J]. Microporous and Mesoporous Materials, 2020, 302: 110204. |
| 41 | Zhou J, Fan W, Wang Y D, et al. The essential mass transfer step in hierarchical/nano zeolite: surface diffusion[J]. National Science Review, 2020, 7(11): 1630-1632. |
| 42 | Zhu Z G, Xu H, Jiang J G, et al. Hydrophobic nanosized all-silica beta zeolite: efficient synthesis and adsorption application[J]. ACS Applied Materials & Interfaces, 2017, 9(32): 27273-27283. |
| 43 | Bal'Zhinimaev B S, Paukshtis E A, Toktarev A V, et al. Effect of water on toluene adsorption over high silica zeolites[J]. Microporous and Mesoporous Materials, 2019, 277: 70-77. |
| 44 | Iyoki K, Kikumasa K, Onishi T, et al. Extremely stable zeolites developed via designed liquid-mediated treatment[J]. Journal of the American Chemical Society, 2020, 142(8): 3931-3938. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [3] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [4] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [5] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [6] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [7] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [8] | 王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588. |
| [9] | 王蕾, 王磊, 白云龙, 何柳柳. SA膜状锂离子筛的制备及其锂吸附性能[J]. 化工学报, 2023, 74(5): 2046-2056. |
| [10] | 陈韶云, 徐东, 陈龙, 张禹, 张远方, 尤庆亮, 胡成龙, 陈建. 单层聚苯胺微球阵列结构的制备及其吸附性能[J]. 化工学报, 2023, 74(5): 2228-2238. |
| [11] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [12] | 李辰鑫, 潘艳秋, 何流, 牛亚宾, 俞路. 基于碳微晶结构的炭膜模型及其气体分离模拟[J]. 化工学报, 2023, 74(5): 2057-2066. |
| [13] | 肖川宝, 李林洋, 刘武锋, 钟年丙, 解泉华, 钟登杰, 常海星. 光催化与离子交换吸附耦合有效去除2,4,6-三氯苯酚[J]. 化工学报, 2023, 74(4): 1587-1597. |
| [14] | 潘煜, 王子航, 王佳韵, 王如竹, 张华. 基于可得然-氯化锂复合吸附剂的除湿换热器热湿性能研究[J]. 化工学报, 2023, 74(3): 1352-1359. |
| [15] | 吴选军, 王超, 曹子健, 蔡卫权. 数据与物理信息混合驱动的固定床吸附穿透深度学习模型[J]. 化工学报, 2023, 74(3): 1145-1160. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号