化工学报 ›› 2022, Vol. 73 ›› Issue (4): 1597-1605.DOI: 10.11949/0438-1157.20211630
收稿日期:2021-11-15
修回日期:2022-01-06
出版日期:2022-04-05
发布日期:2022-04-25
通讯作者:
曹海山
作者简介:刘碧强(1995—),男,博士后,基金资助:Received:2021-11-15
Revised:2022-01-06
Online:2022-04-05
Published:2022-04-25
Contact:
Haishan CAO
摘要:
多孔吸附材料广泛应用于分离提纯、气体储存和工业催化等领域,吸附等温线的测定对研究吸附性质具有重要意义。针对传统容积法易受管路温度均匀性影响的问题,介绍了一种基于流量校准的吸附测量方法。分析了两种方法的误差传递,并对比了结构参数、物性参数和仪表精度对测量结果的影响。分析结果显示,增大校准球体积和样品室容积可提升传统容积法测量精度,提升样品量、比过剩吸附量、密度和仪表精度,可提升两种方法的测量精度。相比传统容积法,基于流量校准的吸附测量方法误差因子数量更少,可实现更低的测量误差。研究成果对提升容积法吸附测量精度具有指导意义。
中图分类号:
刘碧强, 曹海山. 基于流量校准的吸附测量方法及误差分析[J]. 化工学报, 2022, 73(4): 1597-1605.
Biqiang LIU, Haishan CAO. Adsorption measurement method based on flow calibration and its error analysis[J]. CIESC Journal, 2022, 73(4): 1597-1605.
| 平衡状态 | 工质 | 状态 | 气体分配系统 | 校准腔 | 样品室(包括过渡段) | 气体吸附量 |
|---|---|---|---|---|---|---|
| 1 | 氦气 | 气体分配系统充气 | ||||
| 2 | 氦气 | 连通校准腔 | ||||
| 3 | 氦气 | 校准腔内加入校准球 | ||||
| 4 | 氦气 | 连通加入样品后的样品室 | ||||
| 5 | 氦气 | 调节样品室温度至测量温度 | ||||
| 6 | 吸附气体 | 气体分配系统充气 | ||||
| 7 | 吸附气体 | 连通加入样品的样品室,调节样品室温度至测量温度 |
表1 吸附测量系统的各平衡状态
Table 1 Various equilibrium states of the adsorption measurement system
| 平衡状态 | 工质 | 状态 | 气体分配系统 | 校准腔 | 样品室(包括过渡段) | 气体吸附量 |
|---|---|---|---|---|---|---|
| 1 | 氦气 | 气体分配系统充气 | ||||
| 2 | 氦气 | 连通校准腔 | ||||
| 3 | 氦气 | 校准腔内加入校准球 | ||||
| 4 | 氦气 | 连通加入样品后的样品室 | ||||
| 5 | 氦气 | 调节样品室温度至测量温度 | ||||
| 6 | 吸附气体 | 气体分配系统充气 | ||||
| 7 | 吸附气体 | 连通加入样品的样品室,调节样品室温度至测量温度 |
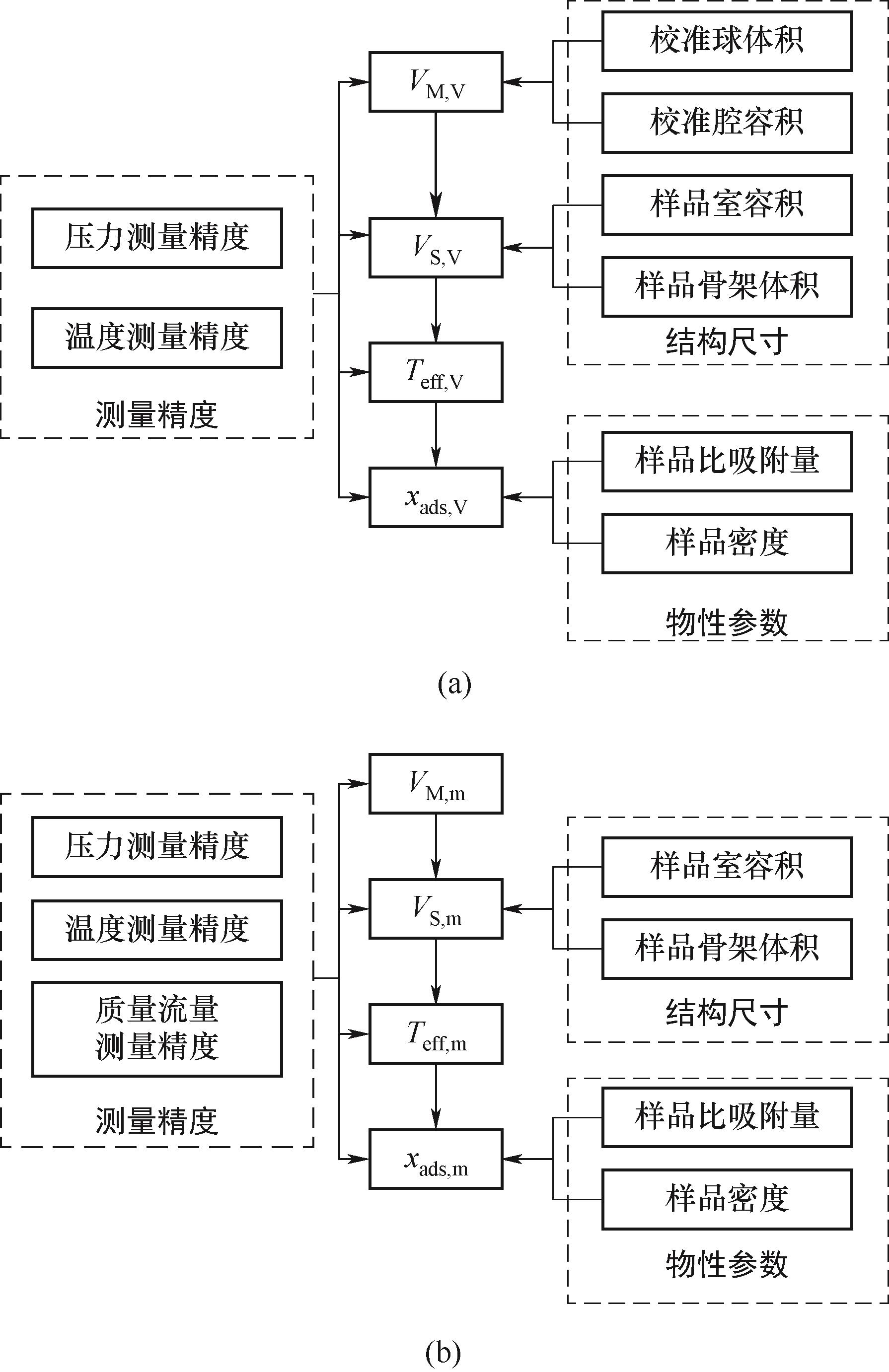
图3 各因素影响(a) 容积法;(b) 基于流量校准的吸附测量
Fig.3 Influence of various factors in volumetric adsorption measurement (a) and adsorption measurement based on flow calibration (b)
| 容积法 | 基于流量校准的容积法 | |||
|---|---|---|---|---|
| 8 | 6 | |||
| 5 | 4 | |||
| 8 | 4 | |||
| 10 | 5 | |||
表2 两种方法测量不确定度表达式参数
Table 2 Parameters of measurement uncertainty expression in both methods
| 容积法 | 基于流量校准的容积法 | |||
|---|---|---|---|---|
| 8 | 6 | |||
| 5 | 4 | |||
| 8 | 4 | |||
| 10 | 5 | |||
| 参数 | 数值 |
|---|---|
| 恒温箱温度/K | |
| 校准腔容积/m3 | |
| 气体分配管路系统容积/m3 | |
| 压力测量误差限 | ±0.05%×量程 |
| 质量流量测量误差限 | ±0.2%×读数 |
表3 基本参数设定
Table 3 Basic parameter set
| 参数 | 数值 |
|---|---|
| 恒温箱温度/K | |
| 校准腔容积/m3 | |
| 气体分配管路系统容积/m3 | |
| 压力测量误差限 | ±0.05%×量程 |
| 质量流量测量误差限 | ±0.2%×读数 |

图11 比过剩吸附量相对不确定度和比过剩吸附量(a)、样品密度(b)的关系
Fig.11 Relationship between relative uncertainty of excess adsorption amount and excess adsorption amount (a), sample density (b)

图12 比过剩吸附量相对不确定度与压力测量精度(a)、温度测量精度(b)的关系
Fig.12 Relationship between relative uncertainty of excess adsorption amount and pressure measurement accuracy (a), temperature measurement accuracy (b)
| 1 | Zhou D D, Chen P, Wang C . et al. Intermediate-sized molecular sieving of styrene from larger and smaller analogues[J]. Nature Materials, 2019, 18: 994-998. |
| 2 | Finsy V, Ma L, Alaerts L, et al. Separation of CO2/CH4 mixtures with the MIL-53(Al) metal–organic framework[J]. Microporous Mesoporous Materials, 2009, 120: 221-227. |
| 3 | Li H, Li L B, Lin R B, et al. Porous metal-organic frameworks for gas storage and separation: status and challenges[J]. EnergyChem, 2019, 1(1): 10006. |
| 4 | Dinca M, Long J R, Hydrogen storage in microporous metal-organic frameworks with exposed metal sites[J]. Angewandte Chemie International Edition, 2008, 47: 6766-6779. |
| 5 | Rowsell J L C, Millward A R, Park K S, et al. Hydrogen sorption in functionalized metal-organic frameworks[J]. Journal of the American Chemical Society, 2004, 126: 5666–5667. |
| 6 | Vo T S, Hossain M M, Jeong H M, et al. Heavy metal removal applications using adsorptive membranes[J]. Nano Convergence, 2020, 7: 36. |
| 7 | Piumetti Marco. A brief history of the science of catalysis (Ⅰ): From the early concepts to single-site heterogeneous catalysts[J]. Chimica Oggi, 2014, 32(6): 22-27. |
| 8 | 赵振国. 吸附作用应用原理[M]. 北京: 化学工业出版社, 2005: 11. |
| Zhao Z G. Principle of Application of Adsorption[M]. Beijing: Chemical Industry Press, 2005: 11. | |
| 9 | Tzabar N, Nitrogen Grossman G., methane, and ethane sorption on activated carbon[J]. Cryogenics, 2011, 51: 499-508. |
| 10 | Tzabar N, ter Brake H J M. Adsorption isotherms and Sips models of nitrogen, methane, ethane, and propane on commercial activated carbons and polyvinylidene chloride[J]. Adsorption, 2016, 22: 901-914. |
| 11 | Brandani S, Mangano E, Net Sarkisov L., excess and absolute adsorption and adsorption of helium[J]. Adsorption, 2016, 22: 261-276. |
| 12 | Liu J H, Cao H S, Shi Y X, et al. Enhanced methane delivery in MIL-101(Cr) by means of subambient cooling[J]. Energy & Fuels, 2021, 35: 6898-6908. |
| 13 | 张辉, 刘应书, 李永玲, 等. 基于容量法的高压静态吸附仪的研制与应用[J]. 低温与特气, 2010, 28(5): 20-23. |
| Zhang H, Liu Y S, Li Y L, et al. Manufacture and application of the volumetric adsorption analyzer at high pressure[J]. Low Temperature and Specialty Gases, 2010, 28(5): 20-23. | |
| 14 | 周尚文, 薛华庆, 郭伟, 等. 基于重量法的页岩气超临界吸附特征实验研究[J]. 煤炭学报, 2016, 41(11): 2806-2812. |
| Zhou S W, Xue H Q, Guo W, et al.Supercritical isothermal adsorption characteristics of shale gas based on gravimetric method[J].Journal of China Coal Society, 2016, 41(11): 2806-2812. | |
| 15 | 俞凌杰, 范明, 陈红宇, 等. 富有机质页岩高温高压重量法等温吸附实验[J]. 石油学报, 2015, 36(5): 557-563. |
| Yu L J, Fan M, Chen H Y, et al. Isothermal adsorption experiment of organic-rich shale under high temperature and pressure using gravimetric method[J]. Acta Petrolei Sinica, 2015, 36(5): 557-563. | |
| 16 | 汤进华, 梁晓怿, 龙东辉, 等. 活性炭孔结构和表面官能团对吸附甲醛性能影响[J]. 炭素技术, 2007(3): 21-25. |
| Tang J H, Liang X Y, Long D H, et al. Effects of micropore and functional groups of activated carbon on adsorption behavior of formaldehyde[J]. Carbon Techniques, 2007(3): 21-25. | |
| 17 | Gregg S J, Sing K S W. Adsorption, Surface Area and Porosity[M]. London: Academic Press, 1982. |
| 18 | 中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. 气体吸附BET法测定固态物质比表面积: [S]. 北京: 中国标准出版社, 2017. |
| General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China. Determination of the specific surface area of solids by gas adsorption using the BET method: [S]. Beijing: Standards Press of China, 2017. | |
| 19 | 近藤精一, 石川达雄, 安部郁夫. 吸附科学[M]. 2版. 李国希, 译. 北京: 化学工业出版社, 2006: 185. |
| Kondo S, Ishikawa T, Abe I. Adsorption Science[M]. 2nd ed. Li G X, trans. Beijing: Chemical Industry Press, 2006: 185. | |
| 20 | 赵会民, 林丹, 杨刚, 等. 有机胺修饰具有较大孔径介孔材料的二氧化碳吸附性能[J]. 物理化学学报, 2012, 28(4): 985-992. |
| Zhao H M, Lin D, Yang G, et al. Adsorption capacity of carbon dioxide on amine modified mesoporous materials with larger pore sizes[J]. Acta Phys. -Chim. Sin., 2012, 28(4): 985-992. | |
| 21 | 汤涛, 张淑华. 用IR-电子天平重量吸附法快速测定催化剂表面酸度[J]. 分析试验室, 2004(6): 57-59. |
| Tang T, Zhang S H. Determination of surface acidity on catalysts by IR-electronic balance with mass adsorption[J]. Chinese Journal of Analysis Laboratory, 2004(6): 57-59. | |
| 22 | 周尚文, 王红岩, 薛华庆, 等. 页岩过剩吸附量与绝对吸附量的差异及页岩气储量计算新方法[J]. 天然气工业, 2016, 36(11): 12-20. |
| Zhou S W, Wang H Y, Xue H Q, et al. Difference between excess and absolute adsorption capacity of shale and a new shale gas reserve calculation method[J]. Natural Gas Industry, 2016, 36(11): 12-20. | |
| 23 | Belmabkhout Y, Frere M, Weireld G D. High-pressure adsorption measurements: a comparative study of the volumetric and gravimetric methods[J]. Measurement Science and Technology, 2004, 15: 848-858. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [3] | 仪显亨, 周骛, 蔡小舒, 蔡天意. 光纤后向动态光散射测量纳米颗粒的浓度适用范围研究[J]. 化工学报, 2023, 74(8): 3320-3328. |
| [4] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [5] | 闫琳琦, 王振雷. 基于STA-BiLSTM-LightGBM组合模型的多步预测软测量建模[J]. 化工学报, 2023, 74(8): 3407-3418. |
| [6] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [7] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [8] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [9] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [10] | 邵伟明, 韩文学, 宋伟, 杨勇, 陈灿, 赵东亚. 基于分布式贝叶斯隐马尔可夫回归的动态软测量建模方法[J]. 化工学报, 2023, 74(6): 2495-2502. |
| [11] | 王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588. |
| [12] | 王蕾, 王磊, 白云龙, 何柳柳. SA膜状锂离子筛的制备及其锂吸附性能[J]. 化工学报, 2023, 74(5): 2046-2056. |
| [13] | 陈韶云, 徐东, 陈龙, 张禹, 张远方, 尤庆亮, 胡成龙, 陈建. 单层聚苯胺微球阵列结构的制备及其吸附性能[J]. 化工学报, 2023, 74(5): 2228-2238. |
| [14] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [15] | 李辰鑫, 潘艳秋, 何流, 牛亚宾, 俞路. 基于碳微晶结构的炭膜模型及其气体分离模拟[J]. 化工学报, 2023, 74(5): 2057-2066. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号
