化工学报 ›› 2022, Vol. 73 ›› Issue (4): 1606-1614.DOI: 10.11949/0438-1157.20211853
李春晖1( ),何辉1,2,何明键1,张萌1,高杨1,矫彩山1(
),何辉1,2,何明键1,张萌1,高杨1,矫彩山1( )
)
收稿日期:2021-12-30
修回日期:2022-01-21
出版日期:2022-04-05
发布日期:2022-04-25
通讯作者:
矫彩山
作者简介:李春晖(1991—),男,博士研究生,基金资助:
Chunhui LI1( ),Hui HE1,2,Mingjian HE1,Meng ZHANG1,Yang GAO1,Caishan JIAO1(
),Hui HE1,2,Mingjian HE1,Meng ZHANG1,Yang GAO1,Caishan JIAO1( )
)
Received:2021-12-30
Revised:2022-01-21
Online:2022-04-05
Published:2022-04-25
Contact:
Caishan JIAO
摘要:
通过恒界面池法研究离子液体1-丁基-3-甲基咪唑双三氟甲磺酰亚胺盐(C4mimNTf2)萃取硝酸溶液中四价铈的动力学,并结合分子动力学模拟分析C4mimNTf2在萃取相界面附近的分布及结构特征。萃取动力学实验通过考察搅拌速率、相界面面积、温度以及两相不同组分浓度对Ce(Ⅳ) 萃取速率的影响,表明萃取过程为水相扩散-界面反应控制模式,且磷酸三丁酯(TBP)和C4mimNTf2间存在动力学的反协同萃取效应。分子动力学模拟分析不同组分在两相中的分布规律,结果验证了C4mimNT2在相界面处存在明显吸附现象。研究表明界面过程对离子液体萃取体系的动力学行为有重要影响,可以为离子液体萃取体系的萃取过程和机理的研究提供一定参考。
中图分类号:
李春晖, 何辉, 何明键, 张萌, 高杨, 矫彩山. 离子液体萃取硝酸中Ce(Ⅳ)的动力学研究[J]. 化工学报, 2022, 73(4): 1606-1614.
Chunhui LI, Hui HE, Mingjian HE, Meng ZHANG, Yang GAO, Caishan JIAO. Extraction kinetics of Ce(Ⅳ) from nitric acid solutions using ionic liquid[J]. CIESC Journal, 2022, 73(4): 1606-1614.
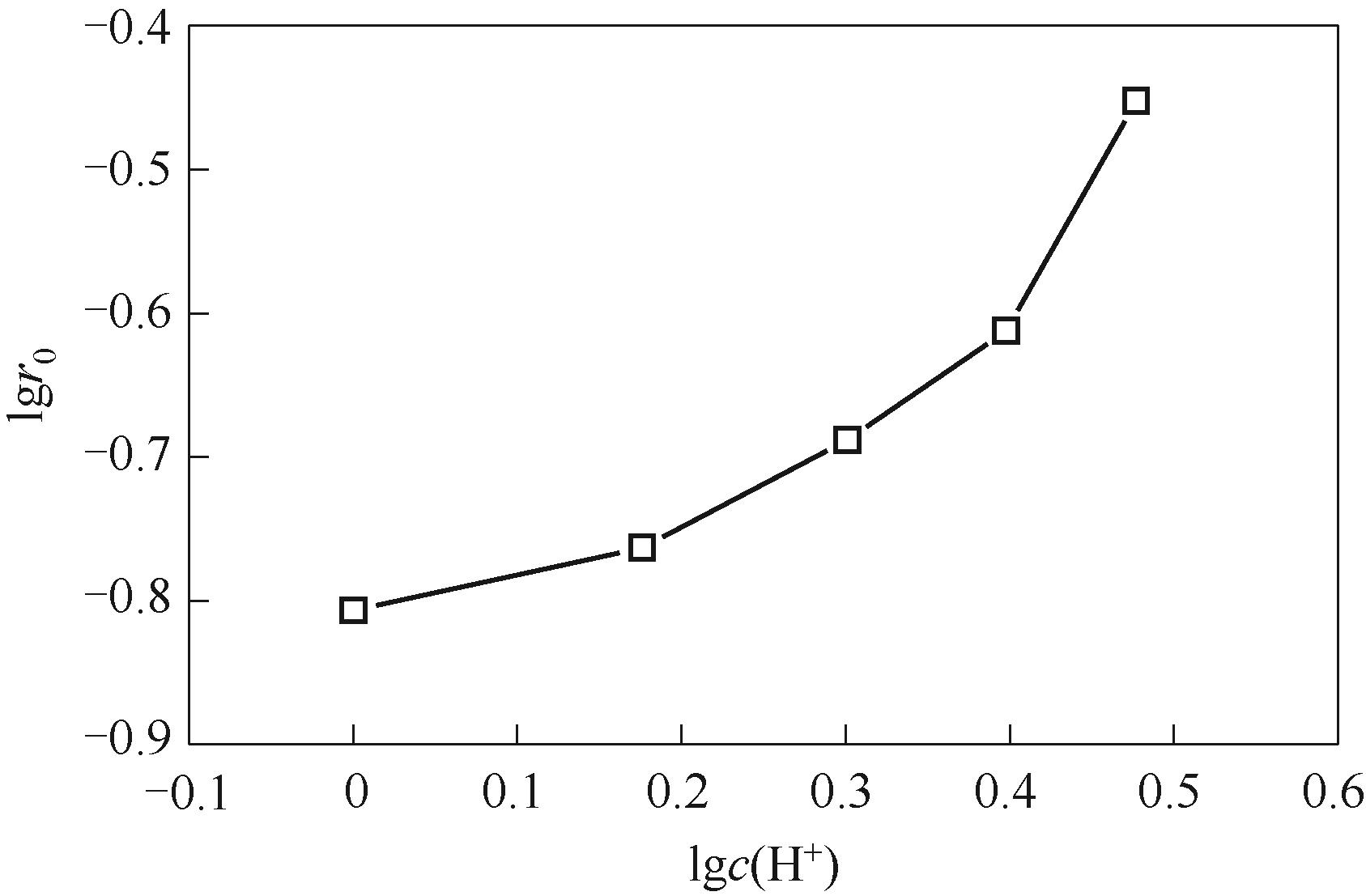
图5 水相氢离子浓度对萃取速率的影响(水相硝酸根浓度c(NO3-) = 3.0 mol·L-1)
Fig.5 Dependence of extraction rate on aqueous hydrogen ion concentration with 3.0 mol·L-1 aqueous nitrate ion
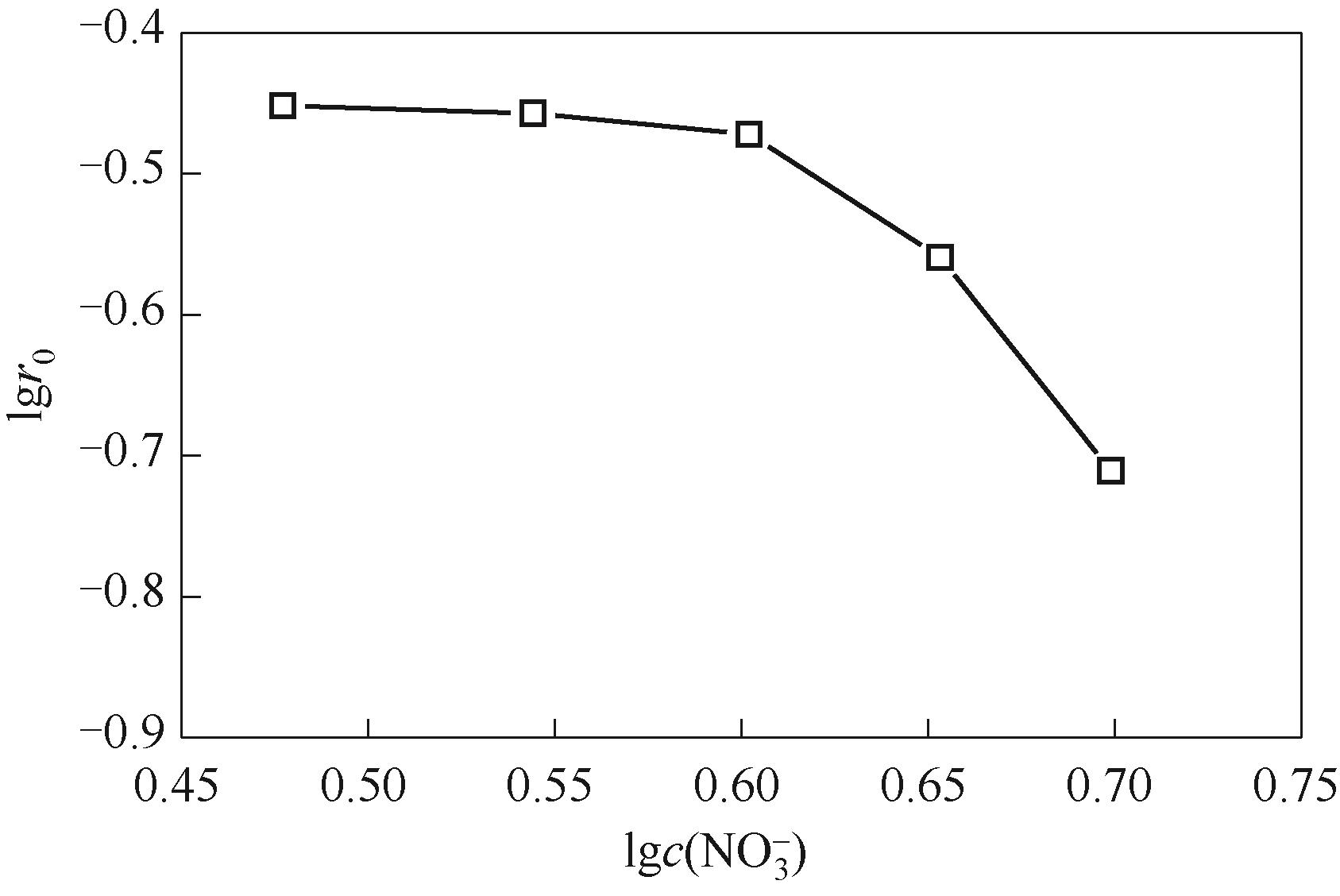
图6 水相硝酸根浓度对萃取速率的影响(水相氢离子浓度c(H+) = 3.0 mol·L-1)
Fig.6 Dependence of extraction rate on aqueous nitrate ion concentration with 3.0 mol·L-1 aqueous hydrogen ion

图10 TBP摩尔分数对萃取速率的影响(TBP和C4mimNTf2总浓度为0.5 mol·L-1)
Fig.10 Dependence of extraction rate on the molar fraction of TBP with total concentration of TBP and C4mimNTf2 at 0.5 mol·L-1
| 1 | 刘海望, 杨涛, 陈庆德, 等. 离子液体体系的萃取行为及其在乏燃料后处理中的应用前景[J]. 核化学与放射化学, 2015, 37(5): 286-309. |
| Liu H W, Yang T, Chen Q D, et al. Extraction behaviors of ionic liquid systems and application perspectives in reprocessing of spent nuclear fuel[J]. Journal of Nuclear and Radiochemistry, 2015, 37(5): 286-309. | |
| 2 | 袁立永, 石伟群, 蓝建慧, 等. 离子液体在核燃料后处理中的应用[J]. 科学通报, 2012, 57(8): 581-593. |
| Yuan L Y, Shi W Q, Lan J H, et al. Application of ionic liquids in nuclear fuel reprocessing[J]. Chinese Science Bulletin, 2012, 57(8): 581-593. | |
| 3 | 黄焜, 李晓佩, 徐怡庄, 等. 萃取分离体系分子间弱相互作用的研究进展[J]. 化工学报, 2016, 67(1): 152-164. |
| Huang K, Li X P, Xu Y Z, et al. Research progress on intermolecular weak interaction in extraction and separation system[J]. CIESC Journal, 2016, 67(1): 152-164. | |
| 4 | Sivaramakrishna M, Raut D R, Nayak S, et al. Unusual selective extraction of Pu4+ by some novel diamide ligands in a room temperature ionic liquid[J]. Separation and Purification Technology, 2017, 181: 69-75. |
| 5 | Zuo Y, Liu Y, Chen J, et al. The separation of cerium(Ⅳ) from nitric acid solutions containing thorium(Ⅳ) and lanthanides(Ⅲ) using pure [C8mim]PF6 as extracting phase[J]. Industrial & Engineering Chemistry Research, 2008, 47(7): 2349-2355. |
| 6 | Li C H, He H, He M J, et al. An experimental study on the extraction mechanisms of Ce(Ⅳ) from HNO3 solutions using C4mimNTf2 as extractant[J]. Journal of Radioanalytical and Nuclear Chemistry, 2021: 1-9. |
| 7 | Chotkowski M, Połomski D. Extraction of pertechnetates from HNO3 solutions into ionic liquids[J]. Journal of Radioanalytical and Nuclear Chemistry, 2017, 314(1): 87-92. |
| 8 | Rout A, Venkatesan K A, Srinivasan T G, et al. Tuning the extractive properties of purex solvent using room temperature ionic liquid[J]. Separation Science and Technology, 2013, 48(17): 2576-2581. |
| 9 | Rout A, Venkatesan K A, Srinivasan T G, et al. Ionic liquid extractants in molecular diluents: extraction behavior of europium (Ⅲ) in quarternary ammonium-based ionic liquids[J]. Separation and Purification Technology, 2012, 95: 26-31. |
| 10 | Turanov A N, Karandashev V K, Khvostikov V A. Synergistic extraction of lanthanides (Ⅲ) with mixtures of TODGA and hydrophobic ionic liquid into molecular diluent[J]. Solvent Extraction and Ion Exchange, 2017, 35(7): 461-479. |
| 11 | Turanov A N, Karandashev V K, Boltoeva M, et al. Synergistic extraction of uranium(Ⅵ) with TODGA and hydrophobic ionic liquid mixtures into molecular diluent[J]. Separation and Purification Technology, 2016, 164: 97-106. |
| 12 | 李洲. HS-LIX65N从氯化物介质中萃铜的萃取动力学研究[J]. 化工学报, 1988, 39(3): 299-308. |
| Li Z. Studies on the Kinetics of extracting copper with HS-LIX65N from chloride mediums[J]. Journal of Chemical Industry and Engineering(China), 1988, 39(3): 299-308. | |
| 13 | 蒋盼, 张盼良, 曾乐林, 等. 反应萃取分离2-苯基丁酸对映体的动力学[J]. 化工学报, 2017, 68(1): 163-169. |
| Jiang P, Zhang P L, Zeng L L, et al. Kinetics of reaction extraction separation of 2-phenylbutyric acid enantiomers[J]. CIESC Journal, 2017, 68(1): 163-169. | |
| 14 | Wang Y L, Wang Y B, Zhou H Y, et al. Extraction kinetics of mixed rare earth elements with bifunctional ionic liquid using a constant interfacial area cell[J]. RSC Advances, 2017, 7(63): 39556-39563. |
| 15 | Yang H L, Chen J, Zhang D L, et al. Kinetics of cerium(Ⅳ) and fluoride extraction from sulfuric solutions using bifunctional ionic liquid extractant (Bif-ILE) [A336][P204][J]. Transactions of Nonferrous Metals Society of China, 2014, 24(6): 1937-1945. |
| 16 | Yang H L, Chen J, Wang W, et al. Extraction kinetics of lanthanum in chloride medium by bifunctional ionic liquid [A336][CA-12] using a constant interfacial cell with laminar flow[J]. Chinese Journal of Chemical Engineering, 2014, 22(10): 1174-1177. |
| 17 | Sypula M, Ouadi A, Gaillard C, et al. Kinetics of metal extraction in ionic liquids: Eu3+/HNO3// TODGA/[C1C4im][Tf2N] as a case study[J]. RSC Advances, 2013, 3(27): 10736-10744. |
| 18 | Sieffert N, Wipff G. The [BMI][Tf 2N] ionic liquid/water binary system: a molecular dynamics study of phase separation and of the liquid–liquid interface[J]. The Journal of Physical Chemistry B, 2006, 110(26): 13076-13085. |
| 19 | Chevrot G, Schurhammer R, Wipff G. Molecular dynamics simulations of the aqueous interface with the [BMI][PF 6] ionic liquid: comparison of different solvent models[J]. Physical Chemistry Chemical Physics, 2006, 8(36): 4166-4174. |
| 20 | 邰德荣, 仝继红, 李守成. 30%TBP(煤油)/UO2(NO3)2-HNO3体系中铈的某些化学性质[J]. 核科学与工程, 1996, 16(2): 154-159. |
| Tai D R, Tong J H, Li S C. Studies on some chemical properties of cerium in the system of 30% TBP(OK)/UO2(NO3)2-HNO3 [J]. Chinese Journal of Nuclear Science and Engineering, 1996, 16(2): 154-159. | |
| 21 | 王辉, 贾永芬, 魏艳, 等. TBP-煤油从硝酸介质中萃取Tc(Ⅶ)的动力学研究[J]. 核化学与放射化学, 2010, 32(4): 193-198. |
| Wang H, Jia Y F, Wei Y, et al. Extraction kinetics of technetium(Ⅶ) between TBP-kerosine and nitric acid medium[J]. Journal of Nuclear and Radiochemistry, 2010, 32(4): 193-198. | |
| 22 | 吴瑞林, 朱利亚. 某些稀土元素共存下银合金中铈的光度测定[J]. 冶金分析, 1990, 10(5): 17-19. |
| Wu R L, Zhu L Y. Photometric determination of cerium coexistent with some other rare earth elements in silver alloys[J]. Metallurgical Analysis, 1990, 10(5): 17-19. | |
| 23 | Singh M B, Patil S R, Lohi A A, et al. Insight into nitric acid extraction and aggregation of N,N,N′,N′-tetraoctyl diglycolamide (TODGA) in organic solutions by molecular dynamics simulation[J]. Separation Science and Technology, 2018, 53(9): 1361-1371. |
| 24 | Gaillard C, Mazan V, Georg S, et al. Acid extraction to a hydrophobic ionic liquid: the role of added tributylphosphate investigated by experiments and simulations[J]. Physical Chemistry Chemical Physics, 2012, 14(15): 5187-5199. |
| 25 | Jorgensen W L, Chandrasekhar J, Madura J D, et al. Comparison of simple potential functions for simulating liquid water[J]. The Journal of Chemical Physics, 1983, 79(2): 926-935. |
| 26 | Noroozi J, Smith W. Force-field-based computational study of the thermodynamics of a large set of aqueous alkanolamine solvents for post-combustion CO2 capture[J]. Journal of Chemical Information and Modeling, 2021, 61(9): 4497-4513. |
| 27 | Wang J M, Wolf R M, Caldwell J W, et al. Development and testing of a general amber force field[J]. Journal of Computational Chemistry, 2004, 25(9): 1157-1174. |
| 28 | Neese F. The ORCA program system[J]. WIREs Computational Molecular Science, 2012, 2(1): 73-78. |
| 29 | Stephens P J, Devlin F J, Chabalowski C F, et al. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields[J]. The Journal of Physical Chemistry, 1994, 98(45): 11623-11627. |
| 30 | Grimme S, Ehrlich S, Goerigk L. Effect of the damping function in dispersion corrected density functional theory[J]. Journal of Computational Chemistry, 2011, 32(7): 1456-1465. |
| 31 | Schäfer A, Huber C, Ahlrichs R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr[J]. The Journal of Chemical Physics, 1994, 100(8): 5829-5835. |
| 32 | Lu T, Chen F W. Multiwfn: a multifunctional wavefunction analyzer[J]. Journal of Computational Chemistry, 2012, 33(5): 580-592. |
| 33 | Zhang J, Lu T. Efficient evaluation of electrostatic potential with computerized optimized code[J]. Physical Chemistry Chemical Physics, 2021, 23(36): 20323-20328. |
| 34 | Martínez L, Andrade R, Birgin E G, et al. PACKMOL: a package for building initial configurations for molecular dynamics simulations[J]. Journal of Computational Chemistry, 2009, 30(13): 2157-2164. |
| 35 | Van Der Spoel D, Lindahl E, Hess B, et al. GROMACS: fast, flexible, and free[J]. Journal of Computational Chemistry, 2005, 26(16): 1701-1718. |
| 36 | Essmann U, Perera L, Berkowitz M L, et al. A smooth particle mesh Ewald method[J]. The Journal of Chemical Physics, 1995, 103(19): 8577-8593. |
| 37 | Bussi G, Donadio D, Parrinello M. Canonical sampling through velocity rescaling[J]. The Journal of Chemical Physics, 2007, 126(1): 014101. |
| 38 | Berendsen H J C, Postma J P M, van Gunsteren W F, et al. Molecular dynamics with coupling to an external bath[J]. The Journal of Chemical Physics, 1984, 81(8): 3684-3690. |
| 39 | Parrinello M, Rahman A. Polymorphic transitions in single crystals: a new molecular dynamics method[J]. Journal of Applied Physics, 1981, 52(12): 7182-7190. |
| 40 | Xiao C X, Huang K, Liu H Z. A novel constant interfacial area cell for determining the extraction kinetics of Er(Ⅲ) from chloride medium [J]. Chinese Journal of Chemical Engineering, 2018, 26(6): 1435-1441. |
| 41 | 於静芬, 刘涤非, 吴雄武, 等. 界面化学反应控制的萃取动力学研究——新协萃体系D2EHPA-MEHPA萃取Al3+动力学及反应机理[J]. 铀矿冶, 1989, 8(2): 18-28. |
| Yu J F, Liu D F, Wu X W, et al. Investigation on kinetics and mechanism of aluminum extraction by a new synergistic system D2EHPA-MEHPA[J]. Uranium Mining and Metallurgy, 1989, 8(2): 18-28. | |
| 42 | Levin V I, Korpusov G V, Man'ko N M, et al. Extraction of tetravalent cerium by organic solvents[J]. Soviet Atomic Energy, 1964, 15(2): 828-835. |
| 43 | 秦启宗, 周祖铭, 杨永乐, 等. 溶剂萃取动力学的研究(Ⅰ): 磷酸三丁酯萃取硝酸铈(Ⅳ)的速率与机理[J]. 复旦学报(自然科学版), 1981, 20(2): 148-154. |
| Qin Q Z, Zhou Z M, Yang Y L, et al. Kinetic studies on the solvent extraction (Ⅰ):extraction rate and mechanism of cerium (Ⅳ) nitrate in the TBP-HNO3 system[J]. Journal of Fudan University (Natural Science), 1981, 20(2): 148-154. | |
| 44 | 刘梅堂, 浦敏锋, 马鸿文. 阴离子Gemini表面活性剂在油/水界面行为的分子动力学模拟[J]. 高等学校化学学报, 2012, 33(6): 1319-1325. |
| Liu M T, Pu M F, Ma H W. Molecular dynamics simulation on adsorbing behavior of anionic gemini surfactants at decane/water interface[J]. Chemical Journal of Chinese Universities, 2012, 33(6): 1319-1325. | |
| 45 | Matsumoto R, Thompson M W, Cummings P T. Ion pairing controls physical properties of ionic liquid-solvent mixtures[J]. The Journal of Physical Chemistry B, 2019, 123(46): 9944-9955. |
| [1] | 周晓庆, 李春煜, 杨光, 蔡爱峰, 吴静怡. 液滴撞击不同曲率过冷波纹面结冰动力学行为及机理研究[J]. 化工学报, 2023, 74(S1): 141-153. |
| [2] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [3] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [4] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [5] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [6] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [7] | 肖明堃, 杨光, 黄永华, 吴静怡. 浸没孔液氧气泡动力学数值研究[J]. 化工学报, 2023, 74(S1): 87-95. |
| [8] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [9] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [10] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [11] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [12] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [13] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [14] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [15] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号