化工学报 ›› 2022, Vol. 73 ›› Issue (8): 3699-3707.DOI: 10.11949/0438-1157.20220428
王刚( ), 夏志豪, 李希艳, 张虹, 韩振南, 宋兴飞(
), 夏志豪, 李希艳, 张虹, 韩振南, 宋兴飞( ), 许光文(
), 许光文( )
)
收稿日期:2022-03-25
修回日期:2022-06-30
出版日期:2022-08-05
发布日期:2022-09-06
通讯作者:
宋兴飞,许光文
作者简介:王刚(1996—),男,硕士研究生,gw082032@163.com
基金资助:
Gang WANG( ), Zhihao XIA, Xiyan LI, Hong ZHANG, Zhennan HAN, Xingfei SONG(
), Zhihao XIA, Xiyan LI, Hong ZHANG, Zhennan HAN, Xingfei SONG( ), Guangwen XU(
), Guangwen XU( )
)
Received:2022-03-25
Revised:2022-06-30
Online:2022-08-05
Published:2022-09-06
Contact:
Xingfei SONG, Guangwen XU
摘要:
利用实验室小型流化床装置,通过改变CO2、H2O浓度得到不同品位菱镁矿的轻烧产物,通过分解率、吸碘值、XRD、BET、SEM对轻烧产物的理化性质进行表征,研究了轻烧气氛中CO2、H2O含量对产物活性的影响。结果表明,在原料分解完全时,产物的活性由微观结构决定;增大CO2浓度,轻烧产物的吸碘值逐渐降低,晶粒尺寸由小逐渐变大,表面结构由多孔变为少孔,说明CO2的存在降低了产物活性;增大H2O浓度,轻烧产物的吸碘值会逐渐降低,比表面积由大变小,表面结构由疏松多孔变得致密光滑,H2O通过促进氧化镁的烧结来抑制产物的活性;相同工况下,高品位的菱镁矿轻烧产物活性高于低品位。
中图分类号:
王刚, 夏志豪, 李希艳, 张虹, 韩振南, 宋兴飞, 许光文. 不同气氛下流化床菱镁矿轻烧产物特性研究[J]. 化工学报, 2022, 73(8): 3699-3707.
Gang WANG, Zhihao XIA, Xiyan LI, Hong ZHANG, Zhennan HAN, Xingfei SONG, Guangwen XU. Effect of atmosphere on active performance of light-burned magnesium oxides from calcined magnesite in fluidized bed[J]. CIESC Journal, 2022, 73(8): 3699-3707.
| Compositions | Mass fraction/% | |
|---|---|---|
| High grade | Low grade | |
| MgO | 43.65 | 38.31 |
| SiO2 | 2.92 | 7.89 |
| CaO | 1.50 | 1.62 |
| Al2O3 | 0.29 | 0.94 |
| Fe2O3 | 0.28 | 0.51 |
| others | 0.22 | 0.35 |
| LOI | 51.14 | 50.38 |
表1 两种品位的菱镁矿原料化学组成
Table 1 Chemical composition of two grade magnesite raw materials
| Compositions | Mass fraction/% | |
|---|---|---|
| High grade | Low grade | |
| MgO | 43.65 | 38.31 |
| SiO2 | 2.92 | 7.89 |
| CaO | 1.50 | 1.62 |
| Al2O3 | 0.29 | 0.94 |
| Fe2O3 | 0.28 | 0.51 |
| others | 0.22 | 0.35 |
| LOI | 51.14 | 50.38 |
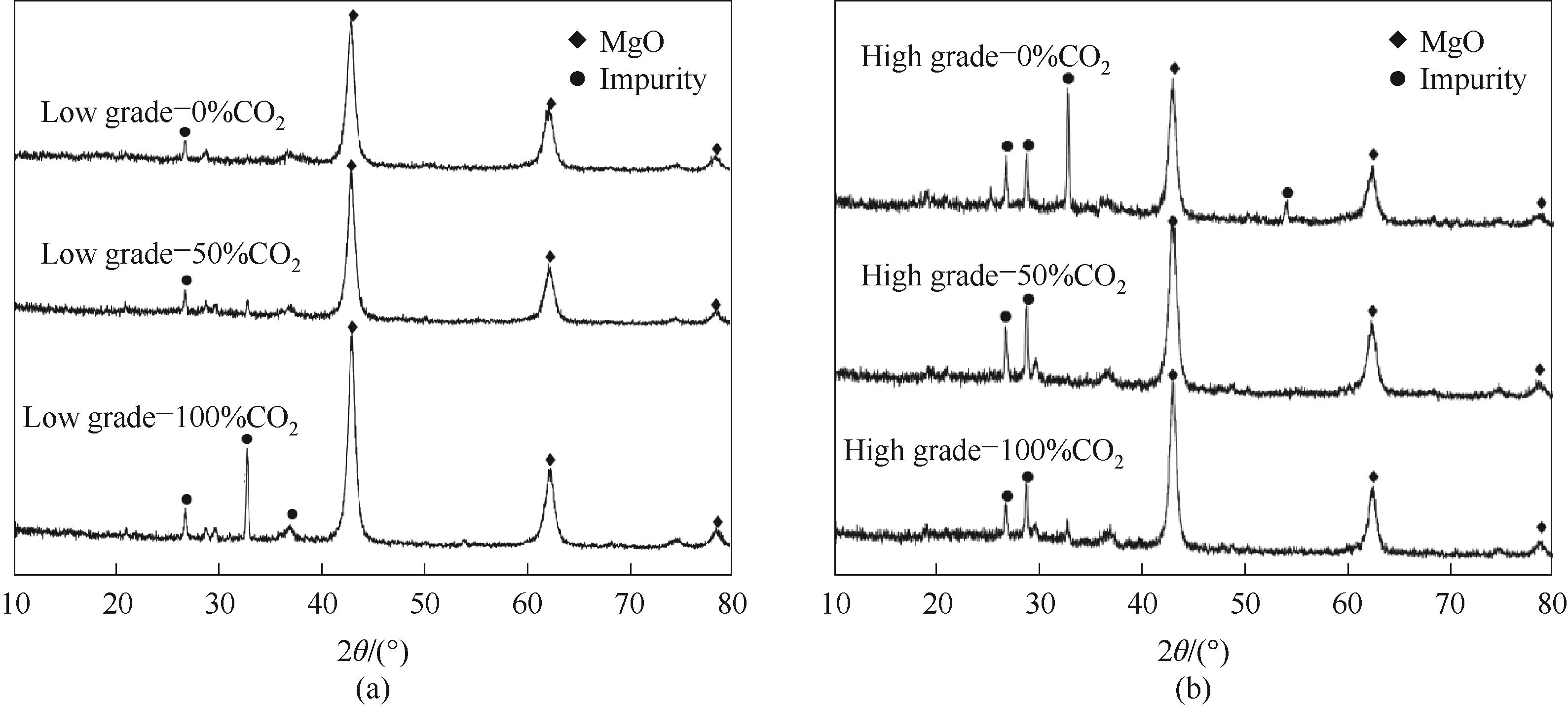
图5 不同CO2气氛下高品位(a)、低品位(b)菱镁矿轻烧产物XRD分析
Fig.5 XRD analysis of light-burned products of high grade (a) and low grade (b) magnesite in different CO2 atmosphere
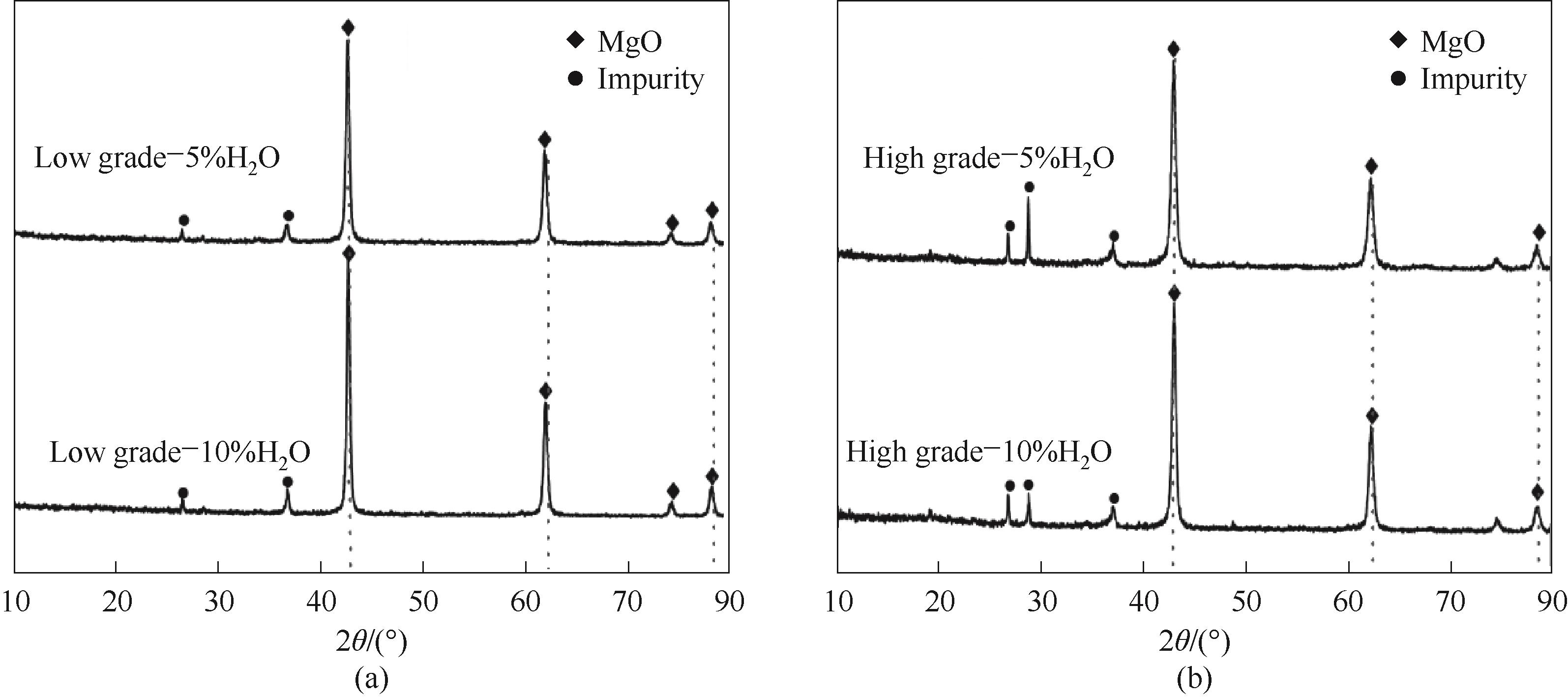
图10 不同H2O气氛下高品位(a)、低品位(b) 菱镁矿轻烧产物XRD分析
Fig.10 XRD analysis of light-burned products of high grade (a) and low grade (b) magnesite in different H2O atmosphere
| 1 | Pehlivan E, Ozkan A M, Dinç S, et al. Adsorption of Cu2+ and Pb2+ ion on dolomite powder[J]. Journal of Hazardous Materials, 2009, 167(1/2/3): 1044-1049. |
| 2 | Abdel-Gawwad H A, Abd El-Aleem S, Zayed A. Stabilization of hazardous lead glass sludge using reactive magnesia via the fabrication of lightweight building bricks[J]. Journal of Hazardous Materials, 2021, 403: 124017. |
| 3 | Canterford J H. Magnesia—an important industrial mineral: a review of processing options and uses[J]. Mineral Processing and Extractive Metallurgy Review, 2007, 2(1/2): 57-104. |
| 4 | 苏莉, 李环, 于景坤. 氧化镁活性与其微观结构的关系[J]. 材料与冶金学报, 2006, 5(4): 308-311. |
| Su L, Li H, Yu J K. Study on the relationship between the activity of the magnesium oxide and its microstructure[J]. Journal of Materials and Metallurgy, 2006, 5(4): 308-311. | |
| 5 | 孙聪, 闫博威, 蔡长庸, 等. 菱镁矿输送床轻烧过程反应与产物微观结构特性[J]. 化工学报, 2020, 71(12): 5735-5744. |
| Sun C, Yan B W, Cai C Y, et al. Characteristics of reaction and product microstructure during light calcination of magnesite in transport bed[J]. CIESC Journal, 2020, 71(12): 5735-5744. | |
| 6 | Eubank W R. Calcination studies of magnesium oxides[J]. Journal of the American Ceramic Society, 1951, 34(8): 225-229. |
| 7 | Mo L W, Deng M, Tang M S. Effects of calcination condition on expansion property of MgO-type expansive agent used in cement-based materials[J]. Cement and Concrete Research, 2010, 40(3): 437-446. |
| 8 | 刘欣伟, 冯雅丽, 李浩然, 等. 菱镁矿制备轻烧氧化镁及其水化动力学研究[J]. 中南大学学报(自然科学版), 2011, 42(12): 3912-3917. |
| Liu X W, Feng Y L, Li H R, et al. Preparation of light-burned magnesia from magnesite and its hydration kinetics[J]. Journal of Central South University (Science and Technology), 2011, 42(12): 3912-3917. | |
| 9 | van der Merwe E M, Strydom C, Botha A. Hydration of medium reactive industrial magnesium oxide with magnesium acetate[J]. Journal of Thermal Analysis and Calorimetry, 2004, 77(1): 49-56. |
| 10 | Erşahan H, Ekmekyapar A, Sevim F. Flash calcination of a magnesite ore in a free-fall reactor and leaching of magnesia[J]. International Journal of Mineral Processing, 1994, 42(1/2): 121-136. |
| 11 | 张强, 何宏平, 陶奇. 菱镁矿煅烧参数优化及其产物水化动力学解析[J]. 中国有色金属学报, 2016, 26(3): 699-706. |
| Zhang Q, He H P, Tao Q. Parameters optimization of magnesite calcination and hydration dynamics of optimized sample[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(3): 699-706. | |
| 12 | Hu N Y, Scaroni A W. Calcination of pulverized limestone particles under furnace injection conditions[J]. Fuel, 1996, 75(2): 177-186. |
| 13 | 仲兆平, Telfer Marnie, 章名耀, 等. Caroline石灰石热分解实验研究[J]. 燃烧科学与技术, 2001, 7(2): 110-114. |
| Zhong Z P, Telfer M, Zhang M Y, et al. Experimental study on pyrolysis of Caroline limestone[J]. Journal of Combustion Science and Technology, 2001, 7(2): 110-114. | |
| 14 | 李振山, 房凡, 蔡宁生. 高浓度CO2下CaCO3循环煅烧试验与模拟[J]. 热能动力工程, 2007, 22(6): 642-646, 689. |
| Li Z S, Fang F, Cai N S. CaCO3 circulating calcination tests and its simulation at a high concentration of CO2 [J]. Journal of Engineering for Thermal Energy and Power, 2007, 22(6): 642-646, 689. | |
| 15 | Wang Y, Lin S Y, Suzuki Y. Limestone calcination with CO2 capture ( Ⅱ ) : Decomposition in CO2/steam and CO2/N2 atmospheres[J]. Energy & Fuels, 2008, 22(4): 2326-2331. |
| 16 | Abanades J C, Alvarez D. Conversion limits in the reaction of CO2 with lime[J]. Energy & Fuels, 2003, 17(2): 308-315. |
| 17 | Sun P, Grace J R, Lim C J, et al. Investigation of attempts to improve cyclic CO2 capture by sorbent hydration and modification[J]. Industrial & Engineering Chemistry Research, 2008, 47(6): 2024-2032. |
| 18 | Wang Y, Lin S Y, Suzuki Y. Experimental study on CO2 capture conditions of a fluidized bed limestone decomposition reactor[J]. Fuel Processing Technology, 2010, 91(8): 958-963. |
| 19 | 关岩, 毕万利, 张玲. 低品位菱镁矿煅烧制备活性氧化镁试验[J]. 冶金能源, 2015, 34(4): 33-36. |
| Guan Y, Bi W L, Zhang L. Experiment of the preparation of activated MgO by calcining low grade magnesite[J]. Energy for Metallurgical Industry, 2015, 34(4): 33-36. | |
| 20 | 孙文华, 崔崇, 张宏华, 等. MgO的晶粒大小和晶格畸变与水化活性的关系[J]. 武汉工业大学学报, 1991, 13(4): 21-24. |
| Sun W H, Cui C, Zhang H H, et al. Relationship between crystalline size and lattice distortion of MgO and its activity[J]. China Civil Engineering Journal, 1991, 13(4): 21-24. | |
| 21 | 李维翰, 尚红霞, 李盛栋. 轻烧氧化镁粉活性的研究[J]. 武汉钢铁学院学报, 1992, 15(1): 30-37. |
| Li W H, Shang H X, Li S D. Activity of light-burned magnesia powder[J]. Journal of Wuhan Iron and Steel University, 1992, 15(1): 30-37. | |
| 22 | Wong B, Pask J A. Experimental analysis of sintering of MgO compacts[J]. Journal of the American Ceramic Society, 1979, 62(3/4): 141-146. |
| [1] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [2] | 吕龙义, 及文博, 韩沐达, 李伟光, 高文芳, 刘晓阳, 孙丽, 王鹏飞, 任芝军, 张光明. 铁基导电材料强化厌氧去除卤代有机污染物:研究进展及未来展望[J]. 化工学报, 2023, 74(8): 3193-3202. |
| [3] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [4] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [5] | 杨峥豪, 何臻, 常玉龙, 靳紫恒, 江霞. 生物质快速热解下行式流化床反应器研究进展[J]. 化工学报, 2023, 74(6): 2249-2263. |
| [6] | 陈巨辉, 张谦, 舒崚峰, 李丹, 徐鑫, 刘晓刚, 赵晨希, 曹希峰. 基于DEM方法的旋转流化床纳米颗粒流动特性研究[J]. 化工学报, 2023, 74(6): 2374-2381. |
| [7] | 张媛媛, 曲江源, 苏欣欣, 杨静, 张锴. 循环流化床燃煤机组SNCR脱硝过程气液传质和反应特性[J]. 化工学报, 2023, 74(6): 2404-2415. |
| [8] | 朱理想, 罗默也, 张晓东, 龙涛, 余冉. 醌指纹法指示三氯乙烯污染土功能微生物活性应用研究[J]. 化工学报, 2023, 74(6): 2647-2654. |
| [9] | 徐文超, 孙志高, 李翠敏, 李娟, 黄海峰. 静态条件下表面活性剂E-1310对HCFC-141b水合物生成的影响[J]. 化工学报, 2023, 74(5): 2179-2185. |
| [10] | 袁子涵, 王淑彦, 邵宝力, 谢磊, 陈曦, 马一玫. 基于幂律液固曳力模型流化床内湿颗粒流动特性的研究[J]. 化工学报, 2023, 74(5): 2000-2012. |
| [11] | 葛运通, 王玮, 李楷, 肖帆, 于志鹏, 宫敬. 多相分散体系中微油滴与改性二氧化硅表面间作用力的AFM研究[J]. 化工学报, 2023, 74(4): 1651-1659. |
| [12] | 衣思敏, 马亚丽, 刘伟强, 张金帅, 岳岩, 郑强, 贾松岩, 李雪. 微晶菱镁矿蒸氨及水化动力学研究[J]. 化工学报, 2023, 74(4): 1578-1586. |
| [13] | 王锋, 陈钰, 裴鸿艳, 刘东东, 张静, 张立新. 1,2,4-𫫇二唑类衍生物的设计、合成及抗菌活性[J]. 化工学报, 2023, 74(3): 1390-1398. |
| [14] | 王倩, 李神勇, 康帅, 庞薇, 郝龙龙, 秦身钧. 粉煤灰分质高效利用预处理技术的研究进展[J]. 化工学报, 2023, 74(3): 1010-1032. |
| [15] | 张娜, 潘鹤林, 牛波, 张亚运, 龙东辉. 酚醛树脂热裂解反应机理的密度泛函理论研究[J]. 化工学报, 2023, 74(2): 843-860. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号