化工学报 ›› 2022, Vol. 73 ›› Issue (8): 3586-3596.DOI: 10.11949/0438-1157.20220614
收稿日期:2022-05-05
修回日期:2022-07-19
出版日期:2022-08-05
发布日期:2022-09-06
通讯作者:
辛忠
作者简介:戴文华(1994—),女,博士研究生,18317136869@163.com
基金资助:Received:2022-05-05
Revised:2022-07-19
Online:2022-08-05
Published:2022-09-06
Contact:
Zhong XIN
摘要:
为了提高Cu/ZrO2催化剂在二氧化碳加氢制甲醇中的催化活性,制备了一系列不同Si/Zr的Si-ZrO2载体并负载5%(质量分数)Cu得到了Cu/Si-ZrO2催化剂。对所制备的催化剂进行了X射线衍射(XRD)、N2物理吸脱附(BET)、X射线光电子能谱(XPS)、氢气程序升温还原(H2-TPR)、二氧化碳程序升温脱附(CO2-TPD)及高分辨透射电子显微镜 (HRTEM) 的表征。结果表明,Si的掺杂使得Cu/ZrO2体系获得了稳定的晶相,大的比表面积和更多的碱性位点,尤其是中强碱性位点,同时产生了更多的氧空位,促进了CO2的吸附和转化,因此得到了更高活性的催化剂。当Si与Zr的摩尔比为0.2时,在质量空速为6000 ml·g-1·h-1,温度为220℃、压力为3.0 MPa,V(H2)∶V(CO2)=3∶1(体积比)条件下,催化剂的CO2转化率为4.6%,CH3OH选择性为85%。
中图分类号:
戴文华, 辛忠. Si掺杂对Cu/ZrO2催化CO2加氢制甲醇性能的影响[J]. 化工学报, 2022, 73(8): 3586-3596.
Wenhua DAI, Zhong XIN. Effect of Si-doped Cu/ZrO2 on the performance of catalysts for CO2 hydrogenation to methanol[J]. CIESC Journal, 2022, 73(8): 3586-3596.
| 催化剂 | 比表面积①/(m2·g-1) | 孔容②/(cm3·g-1) | 平均孔径/ nm | 铜比表面积③/(m2·g-1) |
|---|---|---|---|---|
| Cu/ZrO2 | 43 | 0.17 | 15.5 | 2.02 |
| Cu/0.1Si-ZrO2 | 162 | 0.43 | 10.7 | 6.31 |
| Cu/0.2Si-ZrO2 | 188 | 0.37 | 7.8 | 8.89 |
| Cu/0.3Si-ZrO2 | 252 | 0.30 | 4.8 | 6.19 |
| Cu/0.4Si-ZrO2 | 272 | 0.41 | 7.2 | 6.12 |
表1 催化剂的理化性质
Table 1 Physical and chemical properties of catalysts
| 催化剂 | 比表面积①/(m2·g-1) | 孔容②/(cm3·g-1) | 平均孔径/ nm | 铜比表面积③/(m2·g-1) |
|---|---|---|---|---|
| Cu/ZrO2 | 43 | 0.17 | 15.5 | 2.02 |
| Cu/0.1Si-ZrO2 | 162 | 0.43 | 10.7 | 6.31 |
| Cu/0.2Si-ZrO2 | 188 | 0.37 | 7.8 | 8.89 |
| Cu/0.3Si-ZrO2 | 252 | 0.30 | 4.8 | 6.19 |
| Cu/0.4Si-ZrO2 | 272 | 0.41 | 7.2 | 6.12 |
| 催化剂 | Cu物种/% | |
|---|---|---|
| CuO | Cu-Si-Zr氧化物 | |
| Cu/ZrO2 | 71.7 | 28.3 |
| Cu/0.1Si-ZrO2 | 59.4 | 40.6 |
| Cu/0.2Si-ZrO2 | 35.1 | 64.9 |
表2 Cu/xSi-ZrO2催化剂中Cu的化学价态
Table 2 Cu valence of Cu/xSi-ZrO2 catalysts
| 催化剂 | Cu物种/% | |
|---|---|---|
| CuO | Cu-Si-Zr氧化物 | |
| Cu/ZrO2 | 71.7 | 28.3 |
| Cu/0.1Si-ZrO2 | 59.4 | 40.6 |
| Cu/0.2Si-ZrO2 | 35.1 | 64.9 |
| 催化剂 | O物种/% | |
|---|---|---|
| 晶格氧 | 缺陷氧 | |
| Cu/ZrO2 | 77.7 | 22.3 |
| Cu/0.1Si-ZrO2 | 70.9 | 29.1 |
| Cu/0.2Si-ZrO2 | 59.3 | 40.7 |
| Cu/0.3Si-ZrO2 | 60.6 | 39.4 |
| Cu/0.4Si-ZrO2 | 64.8 | 35.2 |
表3 Cu/xSi-ZrO2催化剂中O的化学价态
Table 3 O valence of Cu/xSi-ZrO2 catalysts
| 催化剂 | O物种/% | |
|---|---|---|
| 晶格氧 | 缺陷氧 | |
| Cu/ZrO2 | 77.7 | 22.3 |
| Cu/0.1Si-ZrO2 | 70.9 | 29.1 |
| Cu/0.2Si-ZrO2 | 59.3 | 40.7 |
| Cu/0.3Si-ZrO2 | 60.6 | 39.4 |
| Cu/0.4Si-ZrO2 | 64.8 | 35.2 |
| 催化剂 | 总碱性位点/(µmol·g-1) | 弱碱性 位点/ (µmol·g-1) | 中强碱性 位点/ (µmol·g-1) | 中强碱密度/(µmol·m-2) |
|---|---|---|---|---|
| Cu/ZrO2 | 35.6 | 18.4 | 17.2 | 0.40 |
| Cu/0.1Si-ZrO2 | 229.5 | 190.6 | 38.9 | 0.24 |
| Cu/0.2Si-ZrO2 | 230.5 | 19.1 | 211.4 | 1.12 |
| Cu/0.3Si-ZrO2 | 207.4 | 14.0 | 193.4 | 0.77 |
| Cu/0.4Si-ZrO2 | 187.4 | 2.9 | 184.5 | 0.68 |
表4 催化剂表面碱性位点密度
Table 4 The basic sites density of catalysts
| 催化剂 | 总碱性位点/(µmol·g-1) | 弱碱性 位点/ (µmol·g-1) | 中强碱性 位点/ (µmol·g-1) | 中强碱密度/(µmol·m-2) |
|---|---|---|---|---|
| Cu/ZrO2 | 35.6 | 18.4 | 17.2 | 0.40 |
| Cu/0.1Si-ZrO2 | 229.5 | 190.6 | 38.9 | 0.24 |
| Cu/0.2Si-ZrO2 | 230.5 | 19.1 | 211.4 | 1.12 |
| Cu/0.3Si-ZrO2 | 207.4 | 14.0 | 193.4 | 0.77 |
| Cu/0.4Si-ZrO2 | 187.4 | 2.9 | 184.5 | 0.68 |
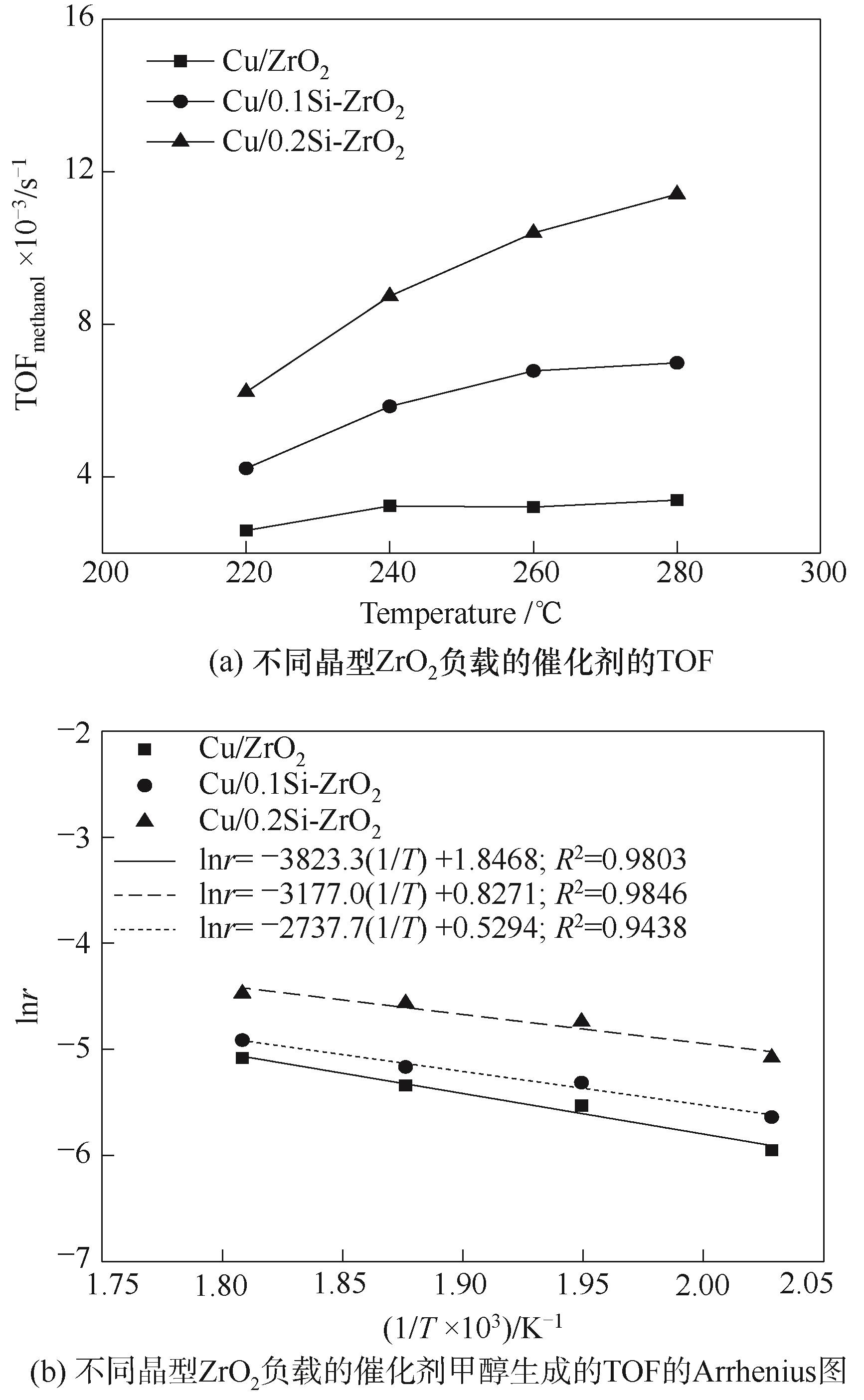
图10 Cu/ZrO2, Cu/0.1Si-ZrO2和Cu/0.2Si-ZrO2催化剂甲醇生成的TOF和Arrhenius图
Fig.10 TOF and Arrhenius plots of TOF for the formation of methanol of Cu/ZrO2, Cu/0.1Si-ZrO2 and Cu/0.2Si-ZrO2 catalysts
| 1 | Zhong J W, Yang X F, Wu Z L, et al. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol[J]. Chemical Society Reviews, 2020, 49(5): 1385-1413. |
| 2 | Dang S S, Yang H Y, Gao P, et al. A review of research progress on heterogeneous catalysts for methanol synthesis from carbon dioxide hydrogenation[J]. Catalysis Today, 2019, 330: 61-75. |
| 3 | Ren M H, Zhang Y M, Wang X, et al. Catalytic hydrogenation of CO2 to methanol: a review[J]. Catalysts, 2022, 12(4): 403. |
| 4 | Bao J, Yang G H, Yoneyama Y, et al. Significant advances in C1 catalysis: highly efficient catalysts and catalytic reactions[J]. ACS Catalysis, 2019, 9(4): 3026-3053. |
| 5 | Fang K G, Li D B, Lin M G, et al. A short review of heterogeneous catalytic process for mixed alcohols synthesis via syngas[J]. Catalysis Today, 2009, 147(2): 133-138. |
| 6 | Li K Z, Chen J G. CO2 hydrogenation to methanol over ZrO2-containing catalysts: insights into ZrO2 induced synergy[J]. ACS Catalysis, 2019, 9(9): 7840-7861. |
| 7 | Scotti N, Bossola F, Zaccheria F, et al. Copper–zirconia catalysts: powerful multifunctional catalytic tools to approach sustainable processes[J]. Catalysts, 2020, 10(2): 168. |
| 8 | Sharma P, Sebastian J, Ghosh S, et al. Recent advances in hydrogenation of CO2 into hydrocarbons via methanol intermediate over heterogeneous catalysts[J]. Catalysis Science & Technology, 2021, 11(5): 1665-1697. |
| 9 | Wang W W, Qu Z P, Song L X, et al. CO2 hydrogenation to methanol over Cu/CeO2 and Cu/ZrO2 catalysts: tuning methanol selectivity via metal-support interaction[J]. Journal of Energy Chemistry, 2020, 40: 22-30. |
| 10 | Wang Z Q, Xu Z N, Zhang M J, et al. Insight into composition evolution in the synthesis of high-performance Cu/SiO2 catalysts for CO2 hydrogenation[J]. RSC Advances, 2016, 6(30): 25185-25190. |
| 11 | Wang Z Q, Xu Z N, Peng S Y, et al. High-performance and long-lived Cu/SiO2 nanocatalyst for CO2 hydrogenation[J]. ACS Catalysis, 2015, 5(7): 4255-4259. |
| 12 | Xiao J, Mao D S, Guo X M, et al. Effect of TiO2, ZrO2, and TiO2-ZrO2 on the performance of CuO-ZnO catalyst for CO2 hydrogenation to methanol[J]. Applied Surface Science, 2015, 338: 146-153. |
| 13 | Witoon T, Chalorngtham J, Dumrongbunditkul P, et al. CO2 hydrogenation to methanol over Cu/ZrO2 catalysts: effects of zirconia phases[J]. Chemical Engineering Journal, 2016, 293: 327-336. |
| 14 | Marcos F C F, Cavalcanti F M, Petrolini D D, et al. Effect of operating parameters on H2/CO2 conversion to methanol over Cu-Zn oxide supported on ZrO2 polymorph catalysts: characterization and kinetics[J]. Chemical Engineering Journal, 2022, 427: 130947. |
| 15 | Numpilai T, Kidkhunthod P, Cheng C K, et al. CO2 hydrogenation to methanol at high reaction temperatures over In2O3/ZrO2 catalysts: influence of calcination temperatures of ZrO2 support[J]. Catalysis Today, 2021, 375: 298-306. |
| 16 | Marcos F C F, Assaf J M, Giudici R, et al. Surface interaction of CO2/H2 mixture on mesoporous ZrO2: effect of crystalline polymorph phases[J]. Applied Surface Science, 2019, 496: 143671. |
| 17 | Tada S, Katagiri A, Kiyota K, et al. Cu species incorporated into amorphous ZrO2 with high activity and selectivity in CO2-to-methanol hydrogenation[J]. The Journal of Physical Chemistry C, 2018, 122(10): 5430-5442. |
| 18 | Yamamura T, Tada S, Kikuchi R, et al. Effect of Sm doping on CO2-to-methanol hydrogenation of Cu/amorphous-ZrO2 catalysts[J]. The Journal of Physical Chemistry C, 2021, 125(29): 15899-15909. |
| 19 | Oshima K, Nakajima S, Tada S, et al. Dimethyl ether synthesis from CO2-H2 mixture over Cu/amorphous-ZrO2 mixed with FER-type zeolite[J]. Journal of the Japan Petroleum Institute, 2020, 63(6): 388-393. |
| 20 | Zhang X P, Zhang Q D, Tsubaki N, et al. Carbon dioxide reforming of methane over Ni nanoparticles incorporated into mesoporous amorphous ZrO2 matrix[J]. Fuel, 2015, 147: 243-252. |
| 21 | Nagase H, Naito R, Tada S, et al. Ru nanoparticles supported on amorphous ZrO2 for CO2 methanation[J]. Catalysis Science & Technology, 2020, 10(14): 4522-4531. |
| 22 | Tada S, Kayamori S, Honma T, et al. Design of interfacial sites between Cu and amorphous ZrO2 dedicated to CO2-to-methanol hydrogenation[J]. ACS Catalysis, 2018, 8(9): 7809-7819. |
| 23 | Tada S, Oshima K, Noda Y, et al. Effects of Cu precursor types on the catalytic activity of Cu/ZrO2 toward methanol synthesis via CO2 hydrogenation[J]. Industrial & Engineering Chemistry Research, 2019, 58(42): 19434-19445. |
| 24 | Larmier K, Liao W C, Tada S, et al. CO2-to-methanol hydrogenation on zirconia-supported copper nanoparticles: reaction intermediates and the role of the metal-support interface[J]. Angewandte Chemie, 2017, 129(9): 2358-2363. |
| 25 | Jia M Y, Gao W G, Wang H, et al. Effect of silica promoter on performance of CuO-ZnO-ZrO2 catalyst for methanol synthesis from CO2 hydrogenation[J]. Applied Mechanics and Materials, 2014, 556/557/558/559/560/561/562: 117-122. |
| 26 | Phongamwong T, Chantaprasertporn U, Witoon T, et al. CO2 hydrogenation to methanol over CuO-ZnO-ZrO2-SiO2 catalysts: effects of SiO2 contents[J]. Chemical Engineering Journal, 2017, 316: 692-703. |
| 27 | Uchiyama S, Isobe T, Matsushita S, et al. Preparation of porous spherical ZrO2-SiO2 composite particles using templating and its solid acidity by H2SO4 treatment[J]. Journal of Materials Science, 2012, 47(1): 341-349. |
| 28 | Lam E, Larmier K, Wolf P, et al. Isolated Zr surface sites on silica promote hydrogenation of CO2 to CH3OH in supported Cu catalysts[J]. Journal of the American Chemical Society, 2018, 140(33): 10530-10535. |
| 29 | Lam E, Larmier K, Tada S, et al. Zr(Ⅳ) surface sites determine CH3OH formation rate on Cu/ZrO2/SiO2-CO2 hydrogenation catalysts[J]. Chinese Journal of Catalysis, 2019, 40(11): 1741-1748. |
| 30 | Pyen S, Hong E, Shin M, et al. Acidity of co-precipitated SiO2-ZrO2 mixed oxides in the acid-catalyzed dehydrations of iso-propanol and formic acid[J]. Molecular Catalysis, 2018, 448: 71-77. |
| 31 | Gu J, Xin Z, Tao M, et al. Effect of Si-modified zirconia on the properties of MoO3/Si-ZrO2 catalysts for sulfur-resistant CO methanation[J]. Applied Catalysis A: General, 2019, 575: 230-237. |
| 32 | Wang J J, Zeng J Y, Mo X T, et al. A measurement system for brake connectors in automobiles[J]. Assembly Automation, 2006, 26(3): 195-199. |
| 33 | Wang F, Liu Y W, Gan Y H, et al. Study on the modification of Cu-based catalysts with cupric silicate for methanol synthesis from synthesis gas[J]. Fuel Processing Technology, 2013, 110: 190-196. |
| 34 | Karelovic A, Galdames G, Medina J C, et al. Mechanism and structure sensitivity of methanol synthesis from CO2 over SiO2-supported Cu nanoparticles[J]. Journal of Catalysis, 2019, 369: 415-426. |
| 35 | Li Z Y, Hao H G, Lu J J, et al. Role of the Cu-ZrO2 interface in the hydrogenation of levulinic acid to γ-valerolactone[J]. Journal of Energy Chemistry, 2021, 61: 446-458. |
| 36 | Gervasini A, Manzoli M, Martra G, et al. Dependence of copper species on the nature of the support for dispersed CuO catalysts[J]. The Journal of Physical Chemistry. B, 2006, 110(15): 7851-7861. |
| 37 | Chen H, Cui H S, Lv Y, et al. CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts: effects of ZnO morphology and oxygen vacancy[J]. Fuel, 2022, 314: 123035. |
| 38 | Ye K H, Li K S, Lu Y R, et al. An overview of advanced methods for the characterization of oxygen vacancies in materials[J]. TrAC Trends in Analytical Chemistry, 2019, 116: 102-108. |
| 39 | Zhuang G X, Chen Y W, Zhuang Z Y, et al. Oxygen vacancies in metal oxides: recent progress towards advanced catalyst design[J]. Science China Materials, 2020, 63(11): 2089-2118. |
| 40 | Singh R, Tripathi K, Pant K K. Investigating the role of oxygen vacancies and basic site density in tuning methanol selectivity over Cu/CeO2 catalyst during CO2 hydrogenation[J]. Fuel, 2021, 303: 121289. |
| 41 | Arena F, Italiano G, Barbera K, et al. Solid-state interactions, adsorption sites and functionality of Cu-ZnO/ZrO2 catalysts in the CO2 hydrogenation to CH3OH[J]. Applied Catalysis A: General, 2008, 350(1): 16-23. |
| 42 | Han F N, Liu H P, Cheng W Q, et al. Highly selective conversion of CO2 to methanol on the CuZnO–ZrO2 solid solution with the assistance of plasma[J]. RSC Advances, 2020, 10(56): 33620-33627. |
| 43 | Wang X H, Zhao J X, Li Y, et al. Effects of surface acid-base properties of ZrO2 on the direct synthesis of DMC from CO2 and methanol: a combined DFT and experimental study[J]. Chemical Engineering Science, 2021, 229: 116018. |
| 44 | Sohn J R, Jang H J. Characterization of ZrO2-SiO2 unmodified or modified with H2SO4 and acid catalysis[J]. Journal of Molecular Catalysis, 1991, 64(3): 349-360. |
| 45 | Gao P, Li F, Zhao N, et al. Influence of modifier (Mn, La, Ce, Zr and Y) on the performance of Cu/Zn/Al catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol[J]. Applied Catalysis A: General, 2013, 468: 442-452. |
| 46 | Chagas L H, Zonetti P C, Matheus C R V, et al. The role of the oxygen vacancies in the synthesis of 1,3-butadiene from ethanol[J]. ChemCatChem, 2019, 11(22): 5625-5632. |
| 47 | Stangeland K, Navarro H H, Huynh H L, et al. Tuning the interfacial sites between copper and metal oxides (Zn, Zr, In) for CO2 hydrogenation to methanol[J]. Chemical Engineering Science, 2021, 238: 116603. |
| 48 | 蔡中杰, 田盼, 黄忠亮, 等. 基于生物模板制备二氧化碳加氢反应的Cu/ZnO催化剂[J]. 化工学报, 2021, 72(7): 3668-3679. |
| Cai Z J, Tian P, Huang Z L, et al. Preparation of Cu/ZnO nanocatalysts based on bio-templates for CO2 hydrogenation[J]. CIESC Journal, 2021, 72(7): 3668-3679. | |
| 49 | 刘洋洋, 孙超, Malhi H S, 等. 载体对铁基催化剂结构及CO2加氢制烯烃反应性能的影响特性[J]. 化工学报, 2020, 71(10): 4652-4662. |
| Liu Y Y, Sun C, Singh M, et al. Effects of identities of supports on Fe-based catalyst and their consequences on activities of CO2 hydrogenation to olefins[J]. CIESC Journal, 2020, 71(10): 4652-4662. |
| [1] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [2] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [3] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [4] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [5] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [6] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [7] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [8] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [9] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [10] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [11] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [12] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [13] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [14] | 康超, 乔金鹏, 杨胜超, 彭超, 付元鹏, 刘斌, 刘建荣, Aleksandrova Tatiana, 段晨龙. 煤矸石中有价关键金属活化提取研究进展[J]. 化工学报, 2023, 74(7): 2783-2799. |
| [15] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号
