化工学报 ›› 2022, Vol. 73 ›› Issue (8): 3433-3447.DOI: 10.11949/0438-1157.20220757
葛旺鑫1( ), 朱以华1, 江宏亮2(
), 朱以华1, 江宏亮2( ), 李春忠1,2(
), 李春忠1,2( )
)
收稿日期:2022-05-30
修回日期:2022-07-22
出版日期:2022-08-05
发布日期:2022-09-06
通讯作者:
江宏亮,李春忠
作者简介:葛旺鑫(1998—),男,博士研究生,y20200081@mail.ecust.edu.cn
基金资助:
Wangxin GE1( ), Yihua ZHU1, Hongliang JIANG2(
), Yihua ZHU1, Hongliang JIANG2( ), Chunzhong LI1,2(
), Chunzhong LI1,2( )
)
Received:2022-05-30
Revised:2022-07-22
Online:2022-08-05
Published:2022-09-06
Contact:
Hongliang JIANG, Chunzhong LI
摘要:
基于可再生能源电力将二氧化碳电化学还原(CO2RR)为高价值化学品是实现二氧化碳资源化利用的有效途径。催化剂和电解质组分对界面微环境的调控共同决定了CO2RR的催化性能。尽管在高性能催化剂的设计及制备方面已取得了实质性进展,但电解质组分对界面局部催化环境的影响,以及对CO2RR反应过程的优化机理还未得到充分认识。综述了电解质组分对CO2RR界面微环境调控的研究进展,重点围绕电解质中阳离子、阴离子、溶剂、配体以及添加剂等开展讨论,包括电解质组分对界面化学环境的影响,如界面电场、局部pH、偶极-场作用和界面水结构等,揭示电解质调控的反应机理,以及在改善催化性能中的重要作用。本文从电解质调控角度出发,为设计高催化性能电解体系提供新的研究思路,推动CO2RR领域发展。
中图分类号:
葛旺鑫, 朱以华, 江宏亮, 李春忠. 二氧化碳电还原的电解质研究进展[J]. 化工学报, 2022, 73(8): 3433-3447.
Wangxin GE, Yihua ZHU, Hongliang JIANG, Chunzhong LI. Research progress on electrolytes for carbon dioxide electroreduction[J]. CIESC Journal, 2022, 73(8): 3433-3447.
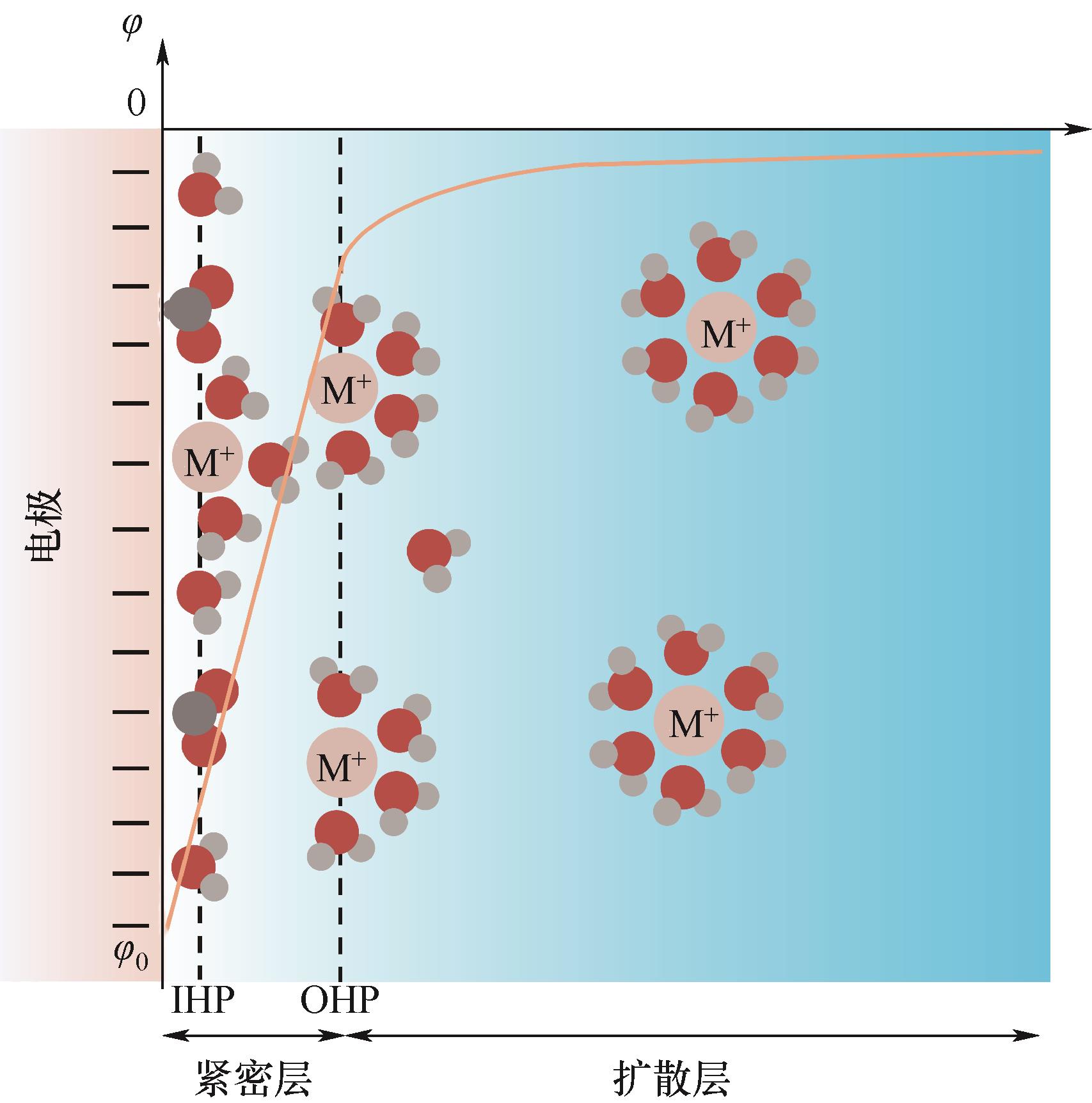
图1 基于GCS模型的EDL结构示意图[13](由紧密层(IHP和OHP层)和扩散层组成,黄色曲线表示电势与距离电极表面距离的关系。粉色、红色、灰色和黑灰色小球分别代表碱金属阳离子、氧原子、氢原子和碳原子)
Fig.1 Schematic illustration of the EDL structure based on Gouy-Chapman-Stern model[13]
| Cation | pKa | ||
|---|---|---|---|
| Bulk | Cu (Surf) | Ag (Surf) | |
| Li+ | 13.6 | 11.64 | 13.16 |
| Na+ | 14.2 | 10.26 | 11.44 |
| K+ | 14.5 | 7.95 | 8.49 |
| Rb+ | 14.6 | 6.97 | 7.23 |
| Cs+ | 14.7 | 4.31 | 4.32 |
表1 碱金属阳离子在本体溶液以及Cu电极和Ag电极表面的水解pKa值[18]
Table 1 Hydrolysis pKa values of alkali metal cations in bulk solution and on the surface of Cu and Ag electrodes[18]
| Cation | pKa | ||
|---|---|---|---|
| Bulk | Cu (Surf) | Ag (Surf) | |
| Li+ | 13.6 | 11.64 | 13.16 |
| Na+ | 14.2 | 10.26 | 11.44 |
| K+ | 14.5 | 7.95 | 8.49 |
| Rb+ | 14.6 | 6.97 | 7.23 |
| Cs+ | 14.7 | 4.31 | 4.32 |

图8 非质子型溶剂中碳酸盐和CO的歧化反应机理(上)以及*CO2-二聚形成草酸盐机理(下)[86]
Fig.8 Suggested reaction mechanism for the disproportionation to carbonate and carbon monoxide (above) and the dimerization to oxalate (below) in aprotic media[86]
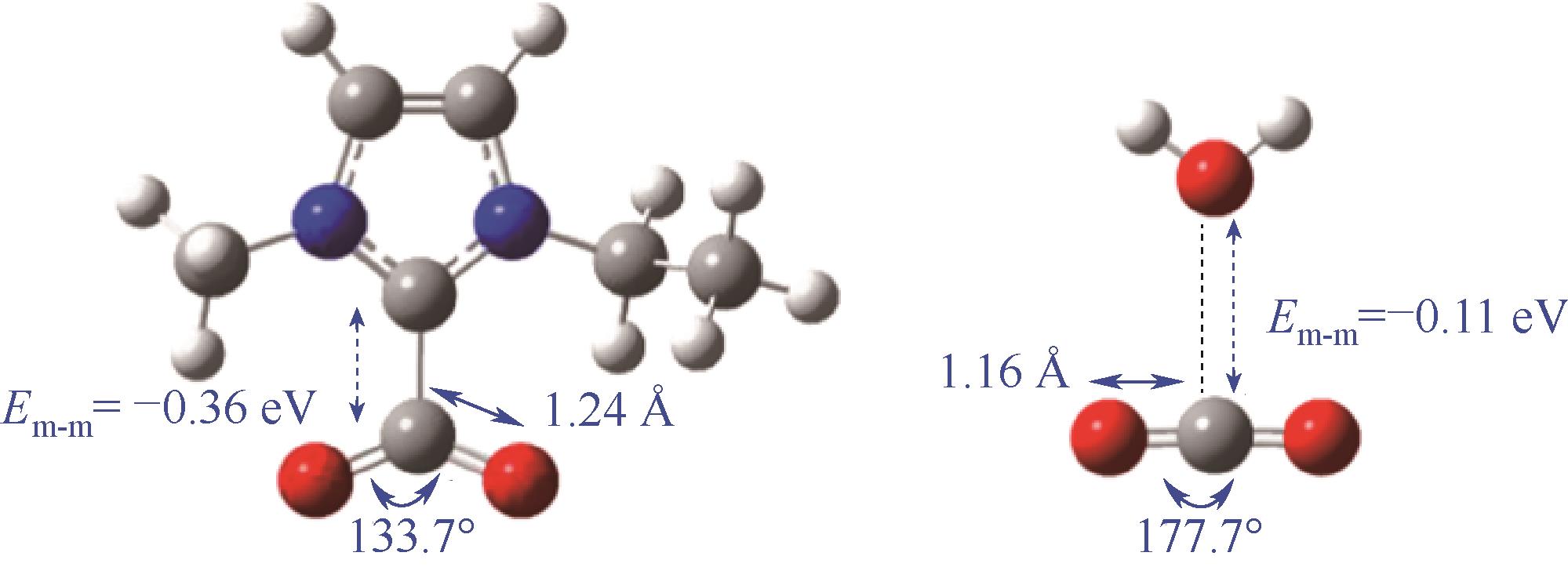
图11 离子液体的作用机制:相比于[H2O-CO2]构型,Emim-与CO2通过形成[Emim–CO2]络合物构型活化CO2[102]
Fig.11 Role of ionic liquid in CO2RR: Emim- and CO2 by forming [Emim-CO2] complex configuration compared to [H2O-CO2][102]
| 1 | Earth System Research Laboratory. Monthly Average Mauna Loa CO2 [OL]. [2022-05-05] . |
| 2 | Tan X Y, Yu C, Ren Y W, et al. Recent advances in innovative strategies for the CO2 electroreduction reaction[J]. Energy & Environmental Science, 2021, 14(2): 765-780. |
| 3 | Wang G X, Chen J X, Ding Y C, et al. Electrocatalysis for CO2 conversion: from fundamentals to value-added products[J]. Chemical Society Reviews, 2021, 50(8): 4993-5061. |
| 4 | Aomchad V, Cristòfol À, Della Monica F, et al. Recent progress in the catalytic transformation of carbon dioxide into biosourced organic carbonates[J]. Green Chemistry, 2021, 23(3): 1077-1113. |
| 5 | Martínez N P, Isaacs M, Nanda K K. Paired electrolysis for simultaneous generation of synthetic fuels and chemicals[J]. New Journal of Chemistry, 2020, 44(15): 5617-5637. |
| 6 | Zhong Y, Wang S, Li M, et al. Rational design of copper-based electrocatalysts and electrochemical systems for CO2 reduction: from active sites engineering to mass transfer dynamics[J]. Materials Today Physics, 2021, 18: 100354. |
| 7 | Wu Z Z, Gao F Y, Gao M R. Regulating the oxidation state of nanomaterials for electrocatalytic CO2 reduction[J]. Energy & Environmental Science, 2021, 14(3): 1121-1139. |
| 8 | Liu J L, Guo C X, Vasileff A, et al. Nanostructured 2D materials: prospective catalysts for electrochemical CO2 reduction[J]. Small Methods, 2017, 1(1/2): 1600006. |
| 9 | Wang Y F, Han P, Lv X M, et al. Defect and interface engineering for aqueous electrocatalytic CO2 reduction[J]. Joule, 2018, 2(12): 2551-2582. |
| 10 | Hahn C, Jaramillo T F. Using Microenvironments to control reactivity in CO2 electrocatalysis[J]. Joule, 2020, 4(2): 292-294. |
| 11 | Wagner A, Sahm C D, Reisner E. Towards molecular understanding of local chemical environment effects in electro- and photocatalytic CO2 reduction[J]. Nature Catalysis, 2020, 3(10): 775-786. |
| 12 | Edwardes Moore E, Cobb S J, Coito A M, et al. Understanding the local chemical environment of bioelectrocatalysis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(4): e2114097119. |
| 13 | Stern O. Zur theorie der elektrolytischen doppelschicht[J]. Zeitschrift Für Elektrochemie und Angewandte Physikalische Chemie, 1924, 30(21/22): 508–516. |
| 14 | Pan B B, Wang Y H, Li Y G. Understanding and leveraging the effect of cations in the electrical double layer for electrochemical CO2 reduction[J]. Chem Catalysis, 2022, 2(6): 1267-1276. |
| 15 | Waegele M M, Gunathunge C M, Li J Y, et al. How cations affect the electric double layer and the rates and selectivity of electrocatalytic processes[J]. The Journal of Chemical Physics, 2019, 151(16): 160902. |
| 16 | Ringe S, Clark E L, Resasco J, et al. Understanding cation effects in electrochemical CO2 reduction[J]. Energy & Environmental Science, 2019, 12(10): 3001-3014. |
| 17 | Gu J, Liu S, Ni W Y, et al. Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium[J]. Nature Catalysis, 2022, 5(4): 268-276. |
| 18 | Singh M R, Kwon Y, Lum Y, et al. Hydrolysis of electrolyte cations enhances the electrochemical reduction of CO2 over Ag and Cu[J]. Journal of the American Chemical Society, 2016, 138(39): 13006-13012. |
| 19 | Resasco J, Chen L D, Clark E, et al. Promoter effects of alkali metal cations on the electrochemical reduction of carbon dioxide[J]. Journal of the American Chemical Society, 2017, 139(32): 11277-11287. |
| 20 | Li J, Li X, Gunathunge C M, et al. Hydrogen bonding steers the product selectivity of electrocatalytic CO reduction[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(19): 9220-9229. |
| 21 | Resasco J, Lum Y, Clark E, et al. Effects of anion identity and concentration on electrochemical reduction of CO2 [J]. ChemElectroChem, 2018, 5(7): 1064-1072. |
| 22 | de Salles Pupo M M, Kortlever R. Electrolyte effects on the electrochemical reduction of CO2 [J]. ChemPhysChem, 2019, 20(22): 2926-2935. |
| 23 | Zhu Q S, Murphy C J, Baker L R. Opportunities for electrocatalytic CO2 reduction enabled by surface ligands[J]. Journal of the American Chemical Society, 2022, 144(7): 2829-2840. |
| 24 | Li L, Liu Y P, Le J B, et al. Unraveling molecular structures and ion effects of electric double layers at metal water interfaces [J]. Cell Reports Physical Science, 2022, 3(2): 100759. |
| 25 | Shan W Y, Liu R, Zhao H C, et al. In situ surface-enhanced Raman spectroscopic evidence on the origin of selectivity in CO2 electrocatalytic reduction[J]. ACS Nano, 2020, 14(9): 11363-11372. |
| 26 | Jin L, Seifitokaldani A. In situ spectroscopic methods for electrocatalytic CO2 reduction[J]. Catalysts, 2020, 10(5): 481. |
| 27 | Serva A, Salanne M, Havenith M, et al. Size dependence of hydrophobic hydration at electrified gold/water interfaces[J]. Proceedings of the National Academy of Sciences of the United States of America, 2021, 118(15): e2023867118. |
| 28 | Baldelli S. Surface structure at the ionic liquid-electrified metal interface[J]. Accounts of Chemical Research, 2008, 41(3): 421-431. |
| 29 | Le J B, Fan Q Y, Li J Q, et al. Molecular origin of negative component of Helmholtz capacitance at electrified Pt(111)/water interface[J]. Science Advances, 2020, 6(41): eabb1219. |
| 30 | Baldelli S. Probing electric fields at the ionic liquid-electrode interface using sum frequency generation spectroscopy and electrochemistry[J]. The Journal of Physical Chemistry B, 2005, 109(27): 13049-13051. |
| 31 | Jouny M, Luc W, Jiao F. High-rate electroreduction of carbon monoxide to multi-carbon products[J]. Nature Catalysis, 2018, 1(10): 748-755. |
| 32 | Pérez-Gallent E, Marcandalli G, Figueiredo M C, et al. Structure- and potential-dependent cation effects on CO reduction at copper single-crystal electrodes[J]. Journal of the American Chemical Society, 2017, 139(45): 16412-16419. |
| 33 | Gunathunge C M, Ovalle V J, Waegele M M. Probing promoting effects of alkali cations on the reduction of CO at the aqueous electrolyte/copper interface[J]. Physical Chemistry Chemical Physics: PCCP, 2017, 19(44): 30166-30172. |
| 34 | Hussain G, Pérez-Martínez L, Le J B, et al. How cations determine the interfacial potential profile: relevance for the CO2 reduction reaction[J]. Electrochimica Acta, 2019, 327: 135055. |
| 35 | Monteiro M C O, Dattila F, Hagedoorn B, et al. Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution[J]. Nature Catalysis, 2021, 4(8): 654-662. |
| 36 | Kim H, Park H S, Hwang Y J, et al. Surface-morphology-dependent electrolyte effects on gold-catalyzed electrochemical CO2 reduction[J]. The Journal of Physical Chemistry C, 2017, 121(41): 22637-22643. |
| 37 | Briega-Martos V, Sarabia F J, Climent V, et al. Cation effects on interfacial water structure and hydrogen peroxide reduction on Pt(111)[J]. ACS Measurement Science Au, 2021, 1(2): 48-55. |
| 38 | Wang Y H, Zheng S S, Yang W M, et al. In situ Raman spectroscopy reveals the structure and dissociation of interfacial water[J]. Nature, 2021, 600(7887): 81-85. |
| 39 | Schizodimou A, Kyriacou G. Acceleration of the reduction of carbon dioxide in the presence of multivalent cations[J]. Electrochimica Acta, 2012, 78: 171-176. |
| 40 | Monteiro M C O, Dattila F, López N, et al. The role of cation acidity on the competition between hydrogen evolution and CO2 reduction on gold electrodes[J]. Journal of the American Chemical Society, 2022, 144(4): 1589-1602. |
| 41 | Moradzaman M, Mul G. Optimizing CO coverage on rough copper electrodes: effect of the partial pressure of CO and electrolyte anions (pH) on selectivity toward ethylene[J]. The Journal of Physical Chemistry C, 2021, 125(12): 6546-6554. |
| 42 | Dunwell M, Lu Q, Heyes J M, et al. The central role of bicarbonate in the electrochemical reduction of carbon dioxide on gold[J]. Journal of the American Chemical Society, 2017, 139(10): 3774-3783. |
| 43 | Dunwell M, Yang X, Setzler B P, et al. Examination of near-electrode concentration gradients and kinetic impacts on the electrochemical reduction of CO2 using surface-enhanced infrared spectroscopy[J]. ACS Catalysis, 2018, 8(5): 3999-4008. |
| 44 | Wuttig A, Yoon Y, Ryu J, et al. Bicarbonate is not a general acid in Au-catalyzed CO2 electroreduction[J]. Journal of the American Chemical Society, 2017, 139(47): 17109-17113. |
| 45 | Gutiérrez-Sánchez O, Daems N, Offermans W, et al. The inhibition of the proton donor ability of bicarbonate promotes the electrochemical conversion of CO2 in bicarbonate solutions[J]. Journal of CO2 Utilization, 2021, 48: 101521. |
| 46 | Jackson M N, Jung O, Lamotte H C, et al. Donor-dependent promotion of interfacial proton-coupled electron transfer in aqueous electrocatalysis[J]. ACS Catalysis, 2019, 9(4): 3737-3743. |
| 47 | Verma S, Lu X, Ma S C, et al. The effect of electrolyte composition on the electroreduction of CO2 to CO on Ag based gas diffusion electrodes[J]. Physical Chemistry Chemical Physics: PCCP, 2016, 18(10): 7075-7084. |
| 48 | Hsieh Y C, Senanayake S D, Zhang Y, et al. Effect of chloride anions on the synthesis and enhanced catalytic activity of silver nanocoral electrodes for CO2 electroreduction[J]. ACS Catalysis, 2015, 5(9): 5349-5356. |
| 49 | Huang Y, Ong C W, Yeo B S. Effects of electrolyte anions on the reduction of carbon dioxide to ethylene and ethanol on copper (100) and (111) surfaces[J]. ChemSusChem, 2018, 11(18): 3299-3306. |
| 50 | Gao D F, Sinev I, Scholten F, et al. Selective CO2 electroreduction to ethylene and multicarbon alcohols via electrolyte-driven nanostructuring[J]. Angewandte Chemie International Edition, 2019, 58(47): 17047-17053. |
| 51 | Garg S, Li M R, Wu Y M, et al. Understanding the effects of anion interactions with Ag electrodes on electrochemical CO2 reduction in choline halide electrolytes[J]. ChemSusChem, 2021, 14(12): 2601-2611. |
| 52 | Gao D F, Mccrum I T, Deo S, et al. Activity and selectivity control in CO2 electroreduction to multicarbon products over CuO x catalysts via electrolyte design[J]. ACS Catalysis, 2018, 8(11): 10012-10020. |
| 53 | Ma W C, Xie S J, Liu T T, et al. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C—C coupling over fluorine-modified copper[J]. Nature Catalysis, 2020, 3(6): 478-487. |
| 54 | Deng B W, Huang M, Zhao X L, et al. Interfacial electrolyte effects on electrocatalytic CO2 reduction[J]. ACS Catalysis, 2021, 12(1): 331-362. |
| 55 | Verma S, Hamasaki Y, Kim C, et al. Insights into the low overpotential electroreduction of CO2 to CO on a supported gold catalyst in an alkaline flow electrolyzer[J]. ACS Energy Letters, 2018, 3(1): 193-198. |
| 56 | Zosel J, Oelßner W, Decker M, et al. The measurement of dissolved and gaseous carbon dioxide concentration[J]. Measurement Science and Technology, 2011, 22(7): 072001. |
| 57 | Vayenas C G, White R E, Gamboa-Aldeco M E. Modern Aspects of Electrochemistry[M]. New York: Springer New York, 2008. |
| 58 | Gao D F, Wang J, Wu H H, et al. pH effect on electrocatalytic reduction of CO2 over Pd and Pt nanoparticles[J]. Electrochemistry Communications, 2015, 55: 1-5. |
| 59 | Hori Y, Murata A, Takahashi R. Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution[J]. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, 1989, 85(8): 2309-2326. |
| 60 | Hori Y, Takahashi R, Yoshinami Y, et al. Electrochemical reduction of CO at a copper electrode[J]. The Journal of Physical Chemistry B, 1997, 101(36): 7075-7081. |
| 61 | Gu J, Héroguel F, Luterbacher J, et al. Densely packed, ultra small SnO nanoparticles for enhanced activity and selectivity in electrochemical CO2 reduction[J]. Angewandte Chemie International Edition, 2018, 57(11): 2943-2947. |
| 62 | Chen C Z, Zhang B, Zhong J H, et al. Selective electrochemical CO2 reduction over highly porous gold films[J]. Journal of Materials Chemistry A, 2017, 5(41): 21955-21964. |
| 63 | Souza M L, Lima F H B. Dibenzyldithiocarbamate-functionalized small gold nanoparticles as selective catalysts for the electrochemical reduction of CO2 to CO[J]. ACS Catalysis, 2021, 11(19): 12208-12219. |
| 64 | Zhong H X, Qiu Y L, Li X F, et al. Ordered cone-structured tin directly grown on carbon paper as efficient electrocatalyst for CO2 electrochemical reduction to formate[J]. Journal of Energy Chemistry, 2021, 55: 236-243. |
| 65 | Mu S J, Li L, Zhao R J, et al. Molecular-scale insights into electrochemical reduction of CO2 on hydrophobically modified Cu surfaces[J]. ACS Applied Materials & Interfaces, 2021, 13(40): 47619-47628. |
| 66 | Buckley A K, Cheng T, Oh M H, et al. Approaching 100% selectivity at low potential on Ag for electrochemical CO2 reduction to CO using a surface additive[J]. ACS Catalysis, 2021, 11(15): 9034-9042. |
| 67 | Zhang Z Q, Banerjee S, Thoi V S, et al. Reorganization of interfacial water by an amphiphilic cationic surfactant promotes CO2 reduction[J]. The Journal of Physical Chemistry Letters, 2020, 11(14): 5457-5463. |
| 68 | Tao Z X, Wu Z S, Wu Y S, et al. Activating copper for electrocatalytic CO2 reduction to formate via molecular interactions[J]. ACS Catalysis, 2020, 10(16): 9271-9275. |
| 69 | Ge W X, Chen Y X, Fan Y, et al. Dynamically formed surfactant assembly at the electrified electrode-electrolyte interface boosting CO2 electroreduction[J]. Journal of the American Chemical Society, 2022, 144(14): 6613-6622. |
| 70 | Sarkar S, Maitra A, Banerjee S, et al. Electric fields at metal-surfactant interfaces: a combined vibrational spectroscopy and capacitance study[J]. The Journal of Physical Chemistry B, 2020, 124(7): 1311-1321. |
| 71 | Banerjee S, Zhang Z Q, Hall A S, et al. Surfactant perturbation of cation interactions at the electrode-electrolyte interface in carbon dioxide reduction[J]. ACS Catalysis, 2020, 10(17): 9907-9914. |
| 72 | Banerjee S, Han X, Thoi V S. Modulating the electrode-electrolyte interface with cationic surfactants in carbon dioxide reduction[J]. ACS Catalysis, 2019, 9(6): 5631-5637. |
| 73 | Zhong Y, Xu Y, Ma J, et al. An artificial electrode/electrolyte interface for CO2 electroreduction by cation surfactant self-assembly[J]. Angewandte Chemie (International Ed. in English), 2020, 59(43): 19095-19101. |
| 74 | Xing Z, Hu L, Ripatti D S, et al. Enhancing carbon dioxide gas-diffusion electrolysis by creating a hydrophobic catalyst microenvironment[J]. Nature Communications, 2021, 12: 136. |
| 75 | Xing Z, Hu X, Feng X F. Tuning the microenvironment in gas-diffusion electrodes enables high-rate CO2 electrolysis to formate[J]. ACS Energy Letters, 2021, 6(5): 1694-1702. |
| 76 | Liang H Q, Zhao S Q, Hu X M, et al. Hydrophobic copper interfaces boost electroreduction of carbon dioxide to ethylene in water[J]. ACS Catalysis, 2021, 11(2): 958-966. |
| 77 | Wei X, Yin Z L, Lyu K J, et al. Highly selective reduction of CO2 to C2+ hydrocarbons at copper/polyaniline interfaces[J]. ACS Catalysis, 2020, 10(7): 4103-4111. |
| 78 | Kim C, Eom T, Jee M S, et al. Insight into electrochemical CO2 reduction on surface-molecule-mediated Ag nanoparticles[J]. ACS Catalysis, 2017, 7(1): 779-785. |
| 79 | Chen X Y, Chen J F, Alghoraibi N M, et al. Electrochemical CO2-to-ethylene conversion on polyamine-incorporated Cu electrodes[J]. Nature Catalysis, 2021, 4(1): 20-27. |
| 80 | Lin C, Xu Z F, Kong D X, et al. Lysine-functionalized SnO2 for efficient CO2 electroreduction into formate[J]. ChemNanoMat, 2022, 8(5): e202200020. |
| 81 | Aeshala L M, Uppaluri R, Verma A. Electrochemical conversion of CO2 to fuels: tuning of the reaction zone using suitable functional groups in a solid polymer electrolyte[J]. Physical Chemistry Chemical Physics: PCCP, 2014, 16(33): 17588-17594. |
| 82 | de Arquer F P G, Dinh C T, Ozden A, et al. CO2 electrolysis to multicarbon products at activities greater than 1 A·cm-2 [J]. Science, 2020, 367(6478): 661-666. |
| 83 | Gupta K, Bersani M, Darr J A. Highly efficient electro-reduction of CO2 to formic acid by nano-copper[J]. Journal of Materials Chemistry A, 2016, 4(36): 13786-13794. |
| 84 | Yan Z F, Hitt J L, Zeng Z C, et al. Improving the efficiency of CO2 electrolysis by using a bipolar membrane with a weak-acid cation exchange layer[J]. Nature Chemistry, 2021, 13(1): 33-40. |
| 85 | Kim C, Bui J C, Luo X Y, et al. Tailored catalyst microenvironments for CO2 electroreduction to multicarbon products on copper using bilayer ionomer coatings[J]. Nature Energy, 2021, 6(11): 1026-1034. |
| 86 | Kaiser U, Heitz E. Zum Mechanismus der elektrochemischen Dimerisierung von CO2 zu Oxalsäure[J]. Berichte der Bunsengesellschaft für physikalische Chemie, 1973, 77(10/11): 818-823. |
| 87 | Gennaro A, Isse A A, Vianello E. Solubility and electrochemical determination of CO2 in some dipolar aprotic solvents[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1990, 289(1): 203-215. |
| 88 | Aljabour A, Coskun H, Apaydin D H, et al. Nanofibrous cobalt oxide for electrocatalysis of CO2 reduction to carbon monoxide and formate in an acetonitrile-water electrolyte solution[J]. Applied Catalysis B: Environmental, 2018, 229: 163-170. |
| 89 | Kaneco S, Katsumata H, Suzuki T, et al. Electrochemical reduction of CO2 to methane at the Cu electrode in methanol with sodium supporting salts and its comparison with other alkaline salts[J]. Energy & Fuels, 2006, 20(1): 409-414. |
| 90 | Kaneco S, Iiba K, Katsumata H, et al. Effect of sodium cation on the electrochemical reduction of CO2 at a copper electrode in methanol[J]. Journal of Solid State Electrochemistry, 2007, 11(4): 490-495. |
| 91 | Berto T C, Zhang L H, Hamers R J, et al. Electrolyte dependence of CO2 electroreduction: tetraalkylammonium ions are not electrocatalysts[J]. ACS Catalysis, 2015, 5(2): 703-707. |
| 92 | Chu A T, Surendranath Y. Aprotic solvent exposes an altered mechanism for copper-catalyzed ethylene electrosynthesis[J]. Journal of the American Chemical Society, 2022, 144(12): 5359-5365. |
| 93 | Cui Y D, He B, Liu X M, et al. Ionic liquids-promoted electrocatalytic reduction of carbon dioxide[J]. Industrial & Engineering Chemistry Research, 2020, 59(46): 20235-20252. |
| 94 | Cadena C, Anthony J L, Shah J K, et al. Why is CO2 so soluble in imidazolium-based ionic liquids?[J]. Journal of the American Chemical Society, 2004, 126(16): 5300-5308. |
| 95 | Tan X X, Sun X F, Han B X. Ionic liquid-based electrolytes for CO2 electroreduction and CO2 electroorganic transformation[J]. National Science Review, 2021, 9(4): nwab022. |
| 96 | Lim H-K, Kim H. The mechanism of room-temperature ionic-liquid-based electrochemical CO2 reduction: a review[J]. Molecules (Basel, Switzerland), 2017, 22(4): E536. |
| 97 | Alvarez-Guerra M, Albo J, Alvarez-Guerra E, et al. Ionic liquids in the electrochemical valorisation of CO2 [J]. Energy & Environmental Science, 2015, 8(9): 2574-2599. |
| 98 | Rosen B A, Salehi-Khojin A, Thorson M R, et al. Ionic liquid-mediated selective conversion of CO2 to CO at low overpotentials[J]. Science, 2011, 334(6056): 643-644. |
| 99 | Barrosse-Antle L E, Compton R G. Reduction of carbon dioxide in 1-butyl-3-methylimidazolium acetate[J]. Chemical Communications (Cambridge, England), 2009, 25: 3744-3746. |
| 100 | Kumar B, Asadi M, Pisasale D, et al. Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction[J]. Nature Communications, 2013, 4: 2819. |
| 101 | Liu S B, Tao H B, Liu Q, et al. Rational design of silver sulfide nanowires for efficient CO2 electroreduction in ionic liquid[J]. ACS Catalysis, 2018, 8(2): 1469-1475. |
| 102 | Yu S, Jain P K. Plasmonic photosynthesis of C1—C3 hydrocarbons from carbon dioxide assisted by an ionic liquid[J]. Nature Communications, 2019, 10: 2022. |
| 103 | Zhu P, Wang H T. High-purity and high-concentration liquid fuels through CO2 electroreduction[J]. Nature Catalysis, 2021, 4(11): 943-951. |
| 104 | Xia C, Zhu P, Jiang Q, et al. Continuous production of pure liquid fuel solutions via electrocatalytic CO2 reduction using solid-electrolyte devices[J]. Nature Energy, 2019, 4(9): 776-785. |
| 105 | Zheng T T, Zhang M L, Wu L H, et al. Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering[J]. Nature Catalysis, 2022, 5(5): 388-396. |
| [1] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [2] | 周晓庆, 李春煜, 杨光, 蔡爱峰, 吴静怡. 液滴撞击不同曲率过冷波纹面结冰动力学行为及机理研究[J]. 化工学报, 2023, 74(S1): 141-153. |
| [3] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [4] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [5] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [6] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [7] | 傅予, 刘兴翀, 王瀚雨, 李海敏, 倪亚飞, 邹文静, 雷月, 彭永姗. F3EACl修饰层对钙钛矿太阳能电池性能提升的研究[J]. 化工学报, 2023, 74(8): 3554-3563. |
| [8] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [9] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [10] | 林典, 江国梅, 徐秀彬, 赵波, 刘冬梅, 吴旭. 硅基类液防原油黏附涂层的研制及其减阻性能研究[J]. 化工学报, 2023, 74(8): 3438-3445. |
| [11] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [12] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [13] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [14] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [15] | 张贲, 王松柏, 魏子亚, 郝婷婷, 马学虎, 温荣福. 超亲水多孔金属结构驱动的毛细液膜冷凝及传热强化[J]. 化工学报, 2023, 74(7): 2824-2835. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号