化工学报 ›› 2024, Vol. 75 ›› Issue (4): 1333-1354.DOI: 10.11949/0438-1157.20231376
收稿日期:2023-12-26
修回日期:2024-02-18
出版日期:2024-04-25
发布日期:2024-06-07
通讯作者:
徐兆超
作者简介:江文钞(1994—),男,博士研究生,jiangwc2018@ dicp.ac.cn
基金资助:
Wenchao JIANG1,2( ), Zhaochao XU1,2(
), Zhaochao XU1,2( )
)
Received:2023-12-26
Revised:2024-02-18
Online:2024-04-25
Published:2024-06-07
Contact:
Zhaochao XU
摘要:
超分辨显微镜提供超越传统光学显微镜衍射极限的成像能力,彻底改变了细胞生物学领域研究。在这一背景下,具有光稳定性、易于修饰和荧光开关可调等独特性能的有机小分子染料获得了新的发展机遇。聚焦于不同细胞器超分辨成像荧光染料,总结了目前可用的超分辨荧光探针的设计和靶向策略。首先简要介绍了三种主要的超分辨成像技术,包括结构光照明显微镜技术、受激发射损耗显微技术和单分子定位成像技术,以及它们对荧光染料性能的不同要求,并介绍了近五年来用于线粒体、溶酶体、细胞膜、脂滴和细胞核的超分辨成像的荧光染料。最后讨论了该领域当前所面临的挑战。
中图分类号:
江文钞, 徐兆超. 细胞器超分辨成像荧光染料[J]. 化工学报, 2024, 75(4): 1333-1354.
Wenchao JIANG, Zhaochao XU. Fluorescent dyes for super-resolution imaging of organelles[J]. CIESC Journal, 2024, 75(4): 1333-1354.
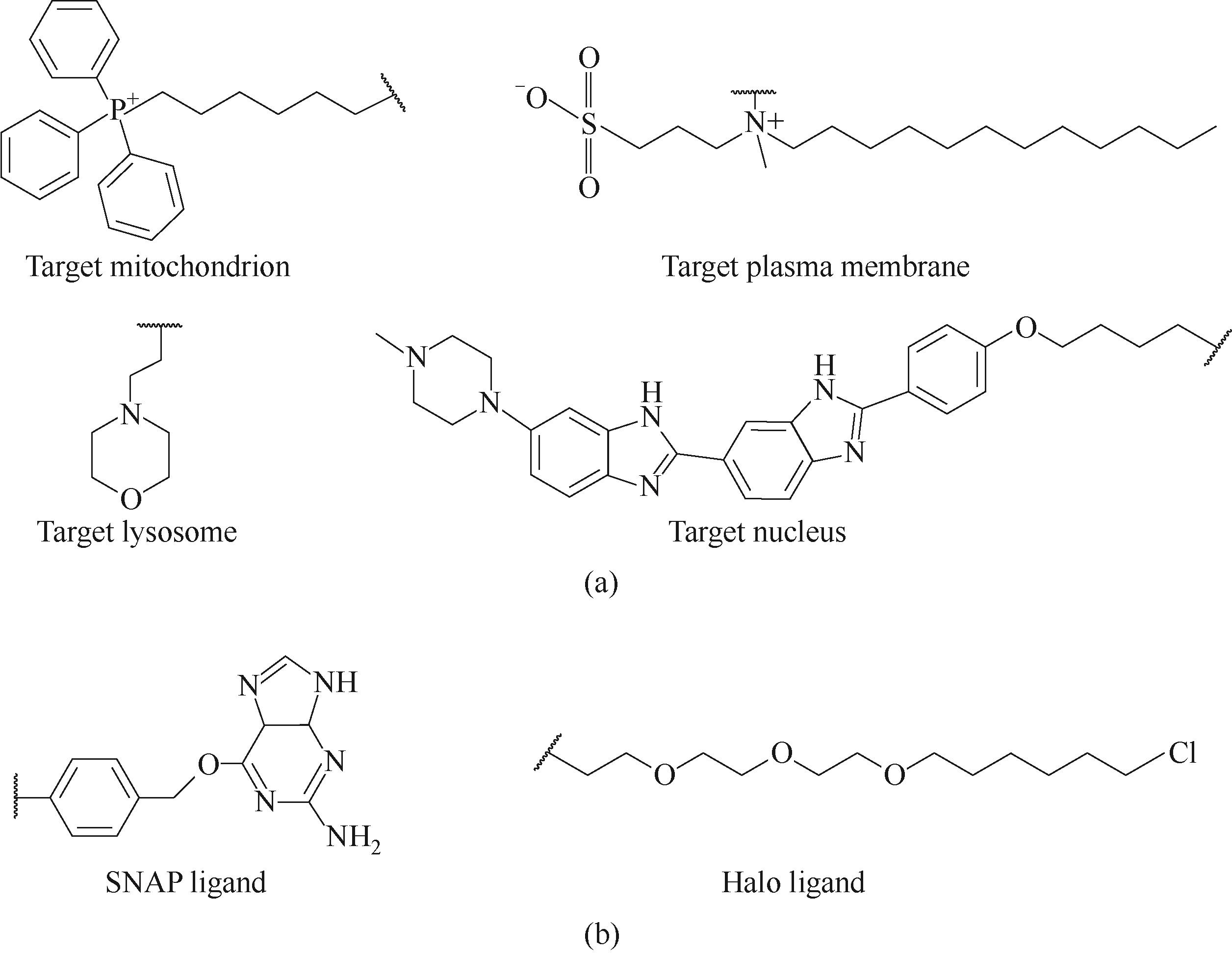
图2 常用细胞器的靶向基团(a)和常用蛋白标签(b)
Fig.2 Ligand groups targeting mitochondria, plasma membrane, lysosomes, and nucleus (a); two universal protein tag ligands (b)

图3 基于正电荷靶向的线粒体超分辨荧光染料结构及其超分辨动态成像
Fig.3 Mitochondrial super-resolution fluorescent dye based on positive charge targeting and its dynamic super-resolution imaging
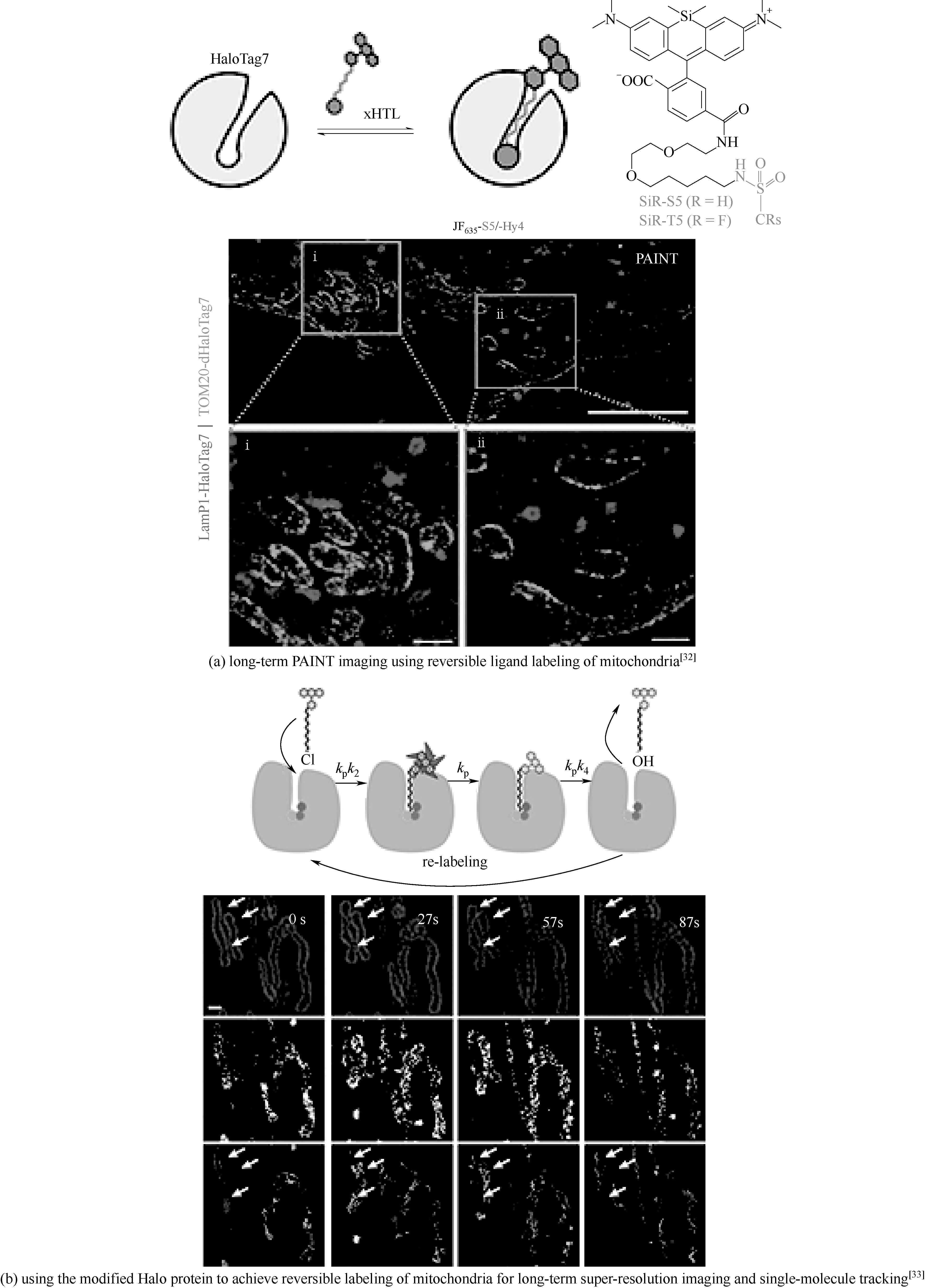
图4 利用改造的蛋白配体或者蛋白标签实现线粒体可逆标记进行长时间超分辨成像
Fig.4 Using modified Halo-ligands or Halotags to achieve reversible labeling of mitochondria for long-term super-resolution imaging
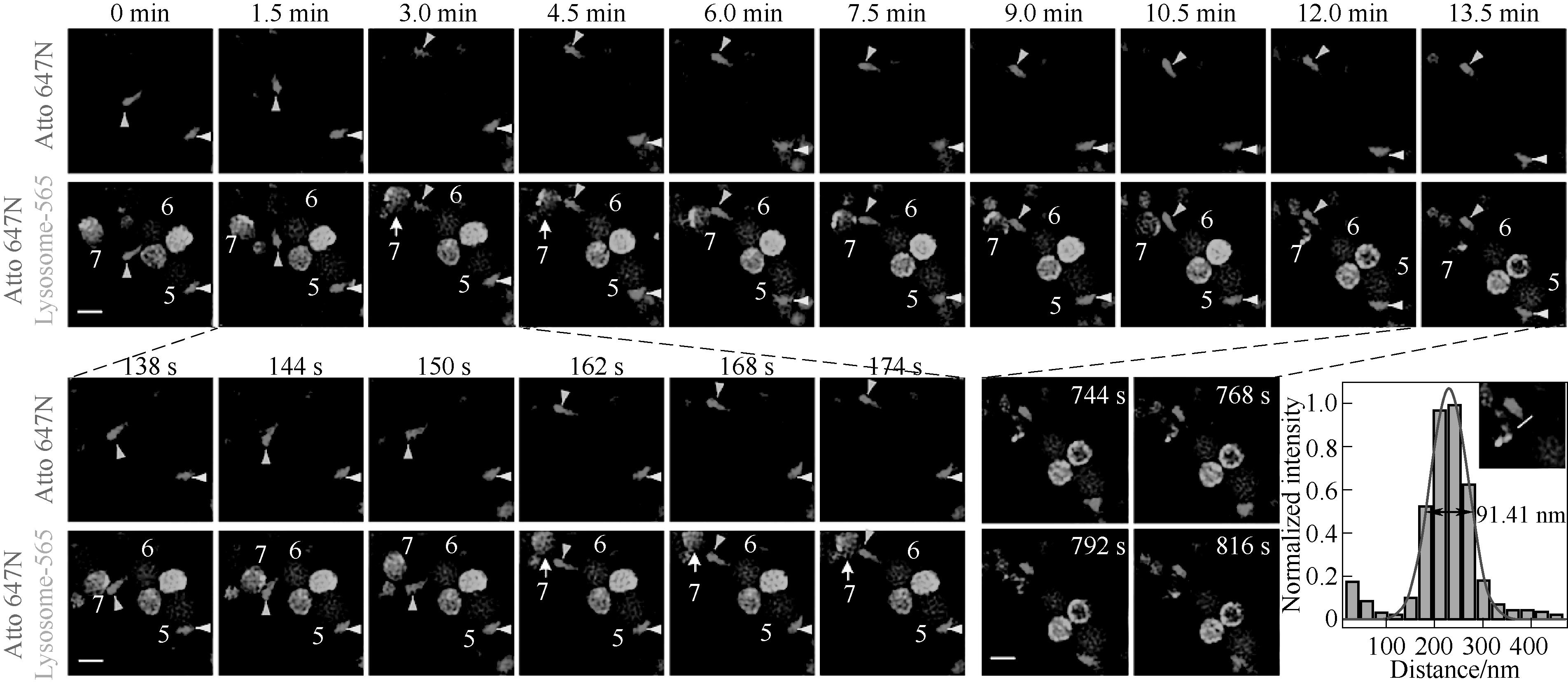
图5 基于细胞穿透肽靶向的溶酶体染料和商业线粒体染料的双色超分辨动态成像
Fig.5 Two-color super-resolution dynamic imaging of lysosomal and mitochondrial dyes by cell-penetrating peptide-targeted lysosomal dyes and commercial mitochondrial dyes[40]
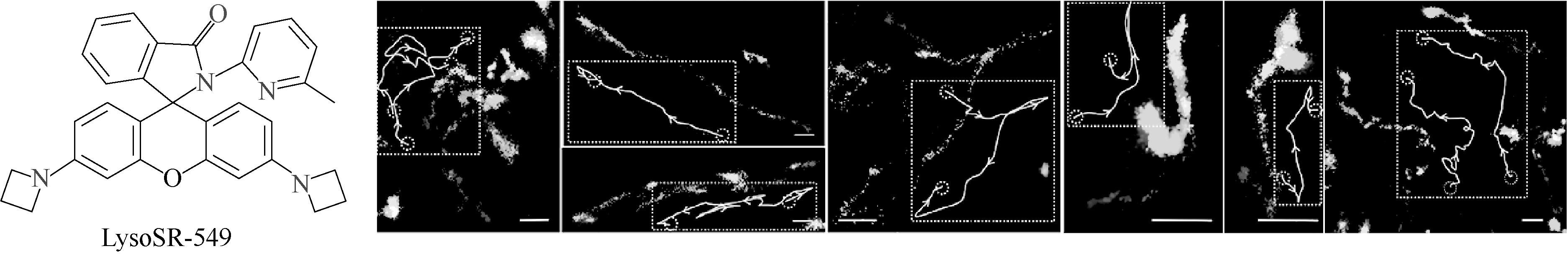
图6 LysoSR-549的结构及其用于溶酶体运动的动态追踪超分辨成像
Fig.6 The structure of LysoSR-549 and its use for dynamic tracking super-resolution imaging of lysosomal movement[41]
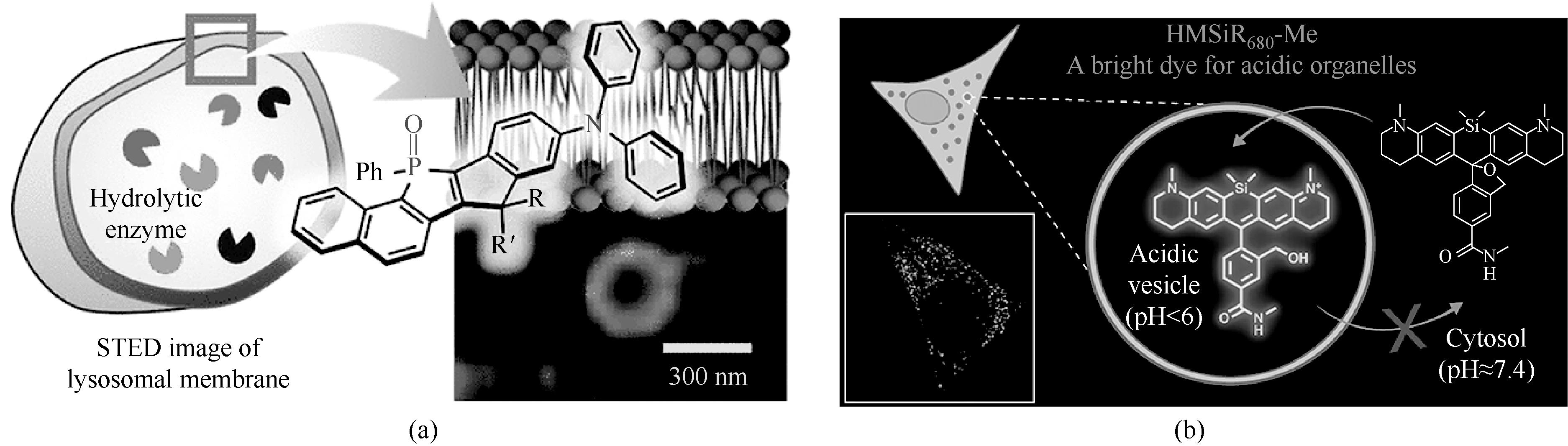
图7 超稳定的溶酶体超分辨染料[42](a)和远红外的溶酶体超分辨染料[43](b)
Fig.7 Ultra-stable lysosomal super-resolution dyes[42] (a) and far-infrared lysosomal super-resolution dyes[43] (b)

图9 细胞膜超分辨荧光染料结构及其3D超分辨动态成像和光谱分辨的超分辨成像
Fig.9 Cell membrane super-resolution fluorescent dyes for 3D super-resolution dynamic imaging and spectral-resolved super-resolution imaging
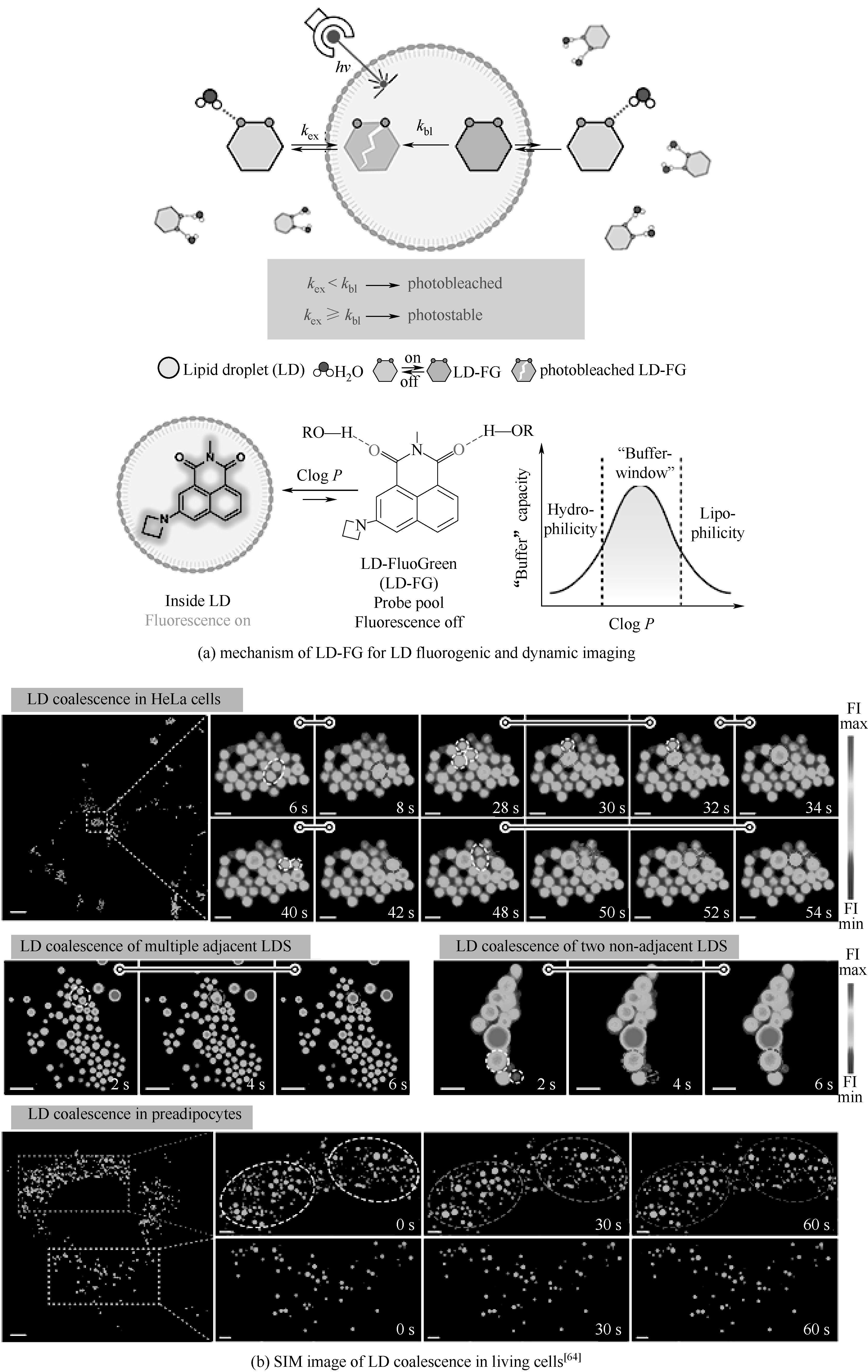
图11 氢键敏感荧光探针通过缓冲策略对脂滴动态的超分辨稳定成像
Fig.11 Stable super-resolution imaging of lipid droplet (LD) dynamics via a buffering strategy using hydrogen-bond-sensitive fluorescent probes
| 70 | Bucevičius J, Gilat T, Lukinavičius G. Far-red switching DNA probes for live cell nanoscopy[J]. Chemical Communications, 2020, 56(94): 14797-14800. |
| 71 | Xu J Q, Sun X J, Kim K, et al. Ultrastructural visualization of chromatin in cancer pathogenesis using a simple small-molecule fluorescent probe[J]. Science Advances, 2022, 8(9): eabm8293. |
| 72 | Liu J J, Gu Q M, Du W, et al. Nucleolar RNA in action: ultrastructure revealed during protein translation through a terpyridyl manganese ( Ⅱ ) complex[J]. Biosensors and Bioelectronics, 2022, 203: 114058. |
| 73 | Qiao Q L, Liu W J, Chi W J, et al. Modulation of dynamic aggregation in fluorogenic SNAP-tag probes for long-term super-resolution imaging[J]. Aggregate, 2023, 4(2): e258. |
| 1 | Jaswal S, Kumar J. Review on fluorescent donor-acceptor conjugated system as molecular probes[J]. Materials Today: Proceedings, 2020, 26: 566-580. |
| 2 | Zeng S, Liu X S, Kafuti Y S, et al. Fluorescent dyes based on rhodamine derivatives for bioimaging and therapeutics: recent progress, challenges, and prospects[J]. Chemical Society Reviews, 2023, 52(16): 5607-5651. |
| 3 | Alander J T, Kaartinen I, Laakso A, et al. A review of indocyanine green fluorescent imaging in surgery[J]. International Journal of Biomedical Imaging, 2012, 2012: 940585. |
| 4 | Fu Q F, Shen S Y, Sun P W, et al. Bioorthogonal chemistry for prodrug activation in vivo [J]. Chemical Society Reviews, 2023, 52(22): 7737-7772. |
| 5 | Scinto S L, Bilodeau D A, Hincapie R, et al. Bioorthogonal chemistry[J]. Nature Reviews Methods Primers, 2021, 1: 30. |
| 6 | Lardon N, Wang L, Tschanz A, et al. Systematic tuning of rhodamine spirocyclization for super-resolution microscopy[J]. Journal of the American Chemical Society, 2021, 143(36): 14592-14600. |
| 7 | Sunbul M, Lackner J, Martin A, et al. Super-resolution RNA imaging using a rhodamine-binding aptamer with fast exchange kinetics[J]. Nature Biotechnology, 2021, 39: 686-690. |
| 8 | Liu X G, Qiao Q L, Tian W M, et al. Aziridinyl fluorophores demonstrate bright fluorescence and superior photostability by effectively inhibiting twisted intramolecular charge transfer[J]. Journal of the American Chemical Society, 2016, 138(22): 6960-6963. |
| 9 | Wang C, Chi W J, Qiao Q L, et al. Twisted intramolecular charge transfer (TICT) and twists beyond TICT: from mechanisms to rational designs of bright and sensitive fluorophores[J]. Chemical Society Reviews, 2021, 50(22): 12656-12678. |
| 10 | Wang C, Qiao Q L, Chi W J, et al. Quantitative design of bright fluorophores and AIEgens by the accurate prediction of twisted intramolecular charge transfer (TICT)[J]. Angewandte Chemie International Edition, 2020, 59(25): 10160-10172. |
| 11 | Yadav A, Rao C, Nandi C K. Fluorescent probes for super-resolution microscopy of lysosomes[J]. ACS Omega, 2020, 5(42): 26967-26977. |
| 12 | Jakobs S, Stephan T, Ilgen P, et al. Light microscopy of mitochondria at the nanoscale[J]. Annual Review of Biophysics, 2020, 49: 289-308. |
| 13 | Samanta S, He Y, Sharma A, et al. Fluorescent probes for nanoscopic imaging of mitochondria[J]. Chem, 2019, 5(7): 1697-1726. |
| 14 | Zhao Y Y, Shi W, Li X H, et al. Recent advances in fluorescent probes for lipid droplets[J]. Chemical Communications, 2022, 58(10): 1495-1509. |
| 15 | Stone M B, Shelby S A, Veatch S L. Super-resolution microscopy: shedding light on the cellular plasma membrane[J]. Chemical Reviews, 2017, 117(11): 7457-7477. |
| 16 | Gao P, Pan W, Li N, et al. Fluorescent probes for organelle-targeted bioactive species imaging[J]. Chemical Science, 2019, 10(24): 6035-6071. |
| 17 | Dadina N, Tyson J, Zheng S, et al. Imaging organelle membranes in live cells at the nanoscale with lipid-based fluorescent probes[J]. Current Opinion in Chemical Biology, 2021, 65: 154-162. |
| 18 | Han R C, Li Z H, Fan Y Y, et al. Recent advances in super-resolution fluorescence imaging and its applications in biology[J]. Journal of Genetics and Genomics, 2013, 40(12): 583-595. |
| 19 | Duan X X, Zhang M, Zhang Y H. Organic fluorescent probes for live-cell super-resolution imaging[J]. Frontiers of Optoelectronics, 2023, 16(1): 34. |
| 20 | Nollmann M, Georgieva M. Superresolution microscopy for bioimaging at the nanoscale: from concepts to applications in the nucleus[J]. Research and Reports in Biology, 2015, 2015(6): 157. |
| 21 | Chung K K H, Zhang Z, Kidd P, et al. Fluorogenic DNA-PAINT for faster, low-background super-resolution imaging[J]. Nature Methods, 2022, 19: 554-559. |
| 22 | Zhai R X, Fang B, Lai Y Q, et al. Small-molecule fluorogenic probes for mitochondrial nanoscale imaging[J]. Chemical Society Reviews, 2023, 52(3): 942-972. |
| 23 | Fang H B, Chen Y C, Geng S S, et al. Super-resolution imaging of mitochondrial HClO during cell ferroptosis using a near-infrared fluorescent probe[J]. Analytical Chemistry, 2022, 94(51): 17904-17912. |
| 24 | Li W, Pan W H, Huang M N, et al. Disulfide-reduction-triggered spontaneous photoblinking Cy5 probe for nanoscopic imaging of mitochondrial dynamics in live cells[J]. Analytical Chemistry, 2021, 93(4): 2596-2602. |
| 25 | Wang H W, Fang B, Peng B, et al. Recent advances in chemical biology of mitochondria targeting[J]. Frontiers in Chemistry, 2021, 9: 683220. |
| 26 | Yang Z G, Xiong J, Zhang W, et al. A reversibly intramolecular cyclization Cy5 optical probe for stochastic optical reconstruction microscopy in live cell mitochondria[J]. Acta Chimica Sinica, 2020, 78(2): 130. |
| 27 | Zhang J, Samanta S, Wang J L, et al. Study on a novel probe for stimulated emission depletion super-resolution imaging of mitochondria[J]. Acta Physica Sinica, 2020, 69(16): 168702. |
| 28 | Wang C G, Taki M, Sato Y, et al. A photostable fluorescent marker for the superresolution live imaging of the dynamic structure of the mitochondrial cristae[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(32): 15817-15822. |
| 29 | Zheng S, Dadina N, Mozumdar D, et al. Long-term super-resolution inner mitochondrial membrane imaging with a lipid probe[J]. Nature Chemical Biology, 2024, 20: 83-92. |
| 30 | Ren W, Ge X C, Li M Q, et al. Visualization of mitochondrial cristae and mtDNA evolvement and interactions with super-resolution microscopy[J]. bioRxiv, 2022. DOI: 10.1101/2022.12.26.521907 . |
| 31 | Yang X S, Yang Z G, Wu Z Y, et al. Mitochondrial dynamics quantitatively revealed by STED nanoscopy with an enhanced squaraine variant probe[J]. Nature Communications, 2020, 11: 3699. |
| 32 | Kompa J, Bruins J, Glogger M, et al. Exchangeable HaloTag ligands for super-resolution fluorescence microscopy[J]. Journal of the American Chemical Society, 2023, 145(5): 3075-3083. |
| 33 | Holtmannspötter M, Wienbeuker E, Dellmann T, et al. Reversible live-cell labeling with retro-engineered HaloTags enables long-term high- and super-resolution imaging[J]. Angewandte Chemie International Edition, 2023, 62(18): e202219050. |
| 34 | Fan M T, An H Y, Wang C F, et al. STED imaging the dynamics of lysosomes by dually fluorogenic Si-rhodamine[J]. Chemistry, 2021, 27(37): 9620-9626. |
| 35 | Liu L Y, Fang H B, Chen Q X, et al. Multiple-color platinum complex with super-large stokes shift for super-resolution imaging of autolysosome escape[J]. Angewandte Chemie International Edition, 2020, 59(43): 19229-19236. |
| 36 | Lv Z, Man Z W, Cui H T, et al. Red AIE luminogens with tunable organelle specific anchoring for live cell dynamic super resolution imaging[J]. Advanced Functional Materials, 2021, 31(10): 2009329. |
| 37 | Wang H, Fang G Q, Chen H M, et al. Lysosome-targeted biosensor for the super-resolution imaging of lysosome-mitochondrion interaction[J]. Frontiers in Pharmacology, 2022, 13: 865173. |
| 38 | Ye Z W, Zheng Y, Peng X J, et al. Surpassing the background barrier for multidimensional single-molecule localization super-resolution imaging: a case of lysosome-exclusively turn-on probe[J]. Analytical Chemistry, 2022, 94(22): 7990-7995. |
| 39 | Pan D, Hu Z, Qiu F W, et al. A general strategy for developing cell-permeable photo-modulatable organic fluorescent probes for live-cell super-resolution imaging[J]. Nature Communications, 2014, 5: 5573. |
| 40 | Han Y B, Li M H, Qiu F W, et al. Cell-permeable organic fluorescent probes for live-cell long-term super-resolution imaging reveal lysosome-mitochondrion interactions[J]. Nature Communications, 2017, 8: 1307. |
| 41 | Qiao Q L, Liu W J, Chen J, et al. An acid-regulated self-blinking fluorescent probe for resolving whole-cell lysosomes with long-term nanoscopy[J]. Angewandte Chemie International Edition, 2022, 61(21): e202202961. |
| 42 | Wang C G, Taki M, Kajiwara K, et al. Phosphole-oxide-based fluorescent probe for super-resolution stimulated emission depletion live imaging of the lysosome membrane[J]. ACS Materials Letters, 2020, 2(7): 705-711. |
| 43 | Lesiak L, Dadina N, Zheng S, et al. A bright, photostable, and far-red dye that enables multicolor, time-lapse, and super-resolution imaging of acidic organelles[J]. ACS Central Science, 2023, 10(1): 19-27. |
| 44 | Takakura H, Zhang Y D, Erdmann R S, et al. Long time-lapse nanoscopy with spontaneously blinking membrane probes[J]. Nature Biotechnology, 2017, 35: 773-780. |
| 45 | Thompson A D, Omar M H, Rivera-Molina F, et al. Long-term live-cell STED nanoscopy of primary and cultured cells with the plasma membrane HIDE probe DiI-SiR[J]. Angewandte Chemie International Edition, 2017, 56(35): 10408-10412. |
| 46 | Lorizate M, Terrones O, Nieto-Garai J A, et al. Super-resolution microscopy using a bioorthogonal-based cholesterol probe provides unprecedented capabilities for imaging nanoscale lipid heterogeneity in living cells[J]. Small Methods, 2021, 5(9): e2100430. |
| 47 | Aparin I O, Yan R, Pelletier R, et al. Fluorogenic dimers as bright switchable probes for enhanced super-resolution imaging of cell membranes[J]. Journal of the American Chemical Society, 2022, 144(39): 18043-18053. |
| 48 | Danylchuk D I, Moon S, Xu K, et al. Switchable solvatochromic probes for live-cell super-resolution imaging of plasma membrane organization[J]. Angewandte Chemie International Edition, 2019, 58(42): 14920-14924. |
| 49 | Moon S, Yan R, Kenny S J, et al. Spectrally resolved, functional super-resolution microscopy reveals nanoscale compositional heterogeneity in live-cell membranes[J]. Journal of the American Chemical Society, 2017, 139(32): 10944-10947. |
| 50 | Yan R, Chen K, Xu K. Probing nanoscale diffusional heterogeneities in cellular membranes through multidimensional single-molecule and super-resolution microscopy[J]. Journal of the American Chemical Society, 2020, 142(44): 18866-18873. |
| 51 | Cao M Y, Zhu T, Zhao M Y, et al. Structure rigidification promoted ultrabright solvatochromic fluorescent probes for super-resolution imaging of cytosolic and nuclear lipid droplets[J]. Analytical Chemistry, 2022, 94(30): 10676-10684. |
| 52 | Liu G N, Zheng H L, Zhou R, et al. Ultrabright organic fluorescent probe for quantifying the dynamics of cytosolic/nuclear lipid droplets[J]. Biosensors and Bioelectronics, 2023, 241: 115707. |
| 53 | Connor D O, Byrne A, Berselli G B, et al. Mega-stokes pyrene ceramide conjugates for STED imaging of lipid droplets in live cells[J]. The Analyst, 2019, 144(5): 1608-1621. |
| 54 | Wu M Y, Leung J K, Kam C, et al. A near-infrared AIE probe for super-resolution imaging and nuclear lipid droplet dynamic study[J]. Materials Chemistry Frontiers, 2021, 5(7): 3043-3049. |
| 55 | Xu H K, Zhang H H, Liu G, et al. Coumarin-based fluorescent probes for super-resolution and dynamic tracking of lipid droplets[J]. Analytical Chemistry, 2019, 91(1): 977-982. |
| 56 | Zhang C Y, Shao H R, Zhang J, et al. Long-term live-cell lipid droplet-targeted biosensor development for nanoscopic tracking of lipid droplet-mitochondria contact sites[J]. Theranostics, 2021, 11(16): 7767-7778. |
| 57 | Zheng X J, Zhu W C, Ni F, et al. Simultaneous dual-colour tracking lipid droplets and lysosomes dynamics using a fluorescent probe[J]. Chemical Science, 2018, 10(8): 2342-2348. |
| 58 | Zheng X J, Zhu W C, Ni F, et al. A specific bioprobe for super-resolution fluorescence imaging of lipid droplets[J]. Sensors and Actuators B: Chemical, 2018, 255: 3148-3154. |
| 59 | 周日, 王晨光, 卢革宇. 用于细胞脂滴超分辨荧光成像的有机荧光探针研究进展[J]. 中国光学, 2022, 15(6): 1228-1242. |
| Zhou R, Wang C G, Lu G Y. Advances in organic fluorescent probes for super-resolution imaging of cellular lipid droplets[J]. Chinese Optics, 2022, 15(6): 1228-1242. | |
| 60 | Taki M, Kajiwara K, Yamaguchi E, et al. Fused thiophene-S, S-dioxide-based super-photostable fluorescent marker for lipid droplets[J]. ACS Materials Letters, 2021, 3(1): 42-49. |
| 61 | Zhou R, Wang C G, Liang X S, et al. Stimulated emission depletion (STED) super-resolution imaging with an advanced organic fluorescent probe: visualizing the cellular lipid droplets at the unprecedented nanoscale resolution[J]. ACS Materials Letters, 2021, 3(5): 516-524. |
| 62 | Liu G N, Peng G S, Dai J N, et al. STED nanoscopy imaging of cellular lipid droplets employing a superior organic fluorescent probe[J]. Analytical Chemistry, 2021, 93(44): 14784-14791. |
| 63 | Zhou R, Liu G N, Li D, et al. An advanced organic molecular probe for multimodal fluorescence imaging of cellular lipid droplets[J]. Sensors and Actuators B: Chemical, 2023, 387: 133772. |
| 64 | Chen J, Wang C, Liu W J, et al. Stable super-resolution imaging of lipid droplet dynamics through a buffer strategy with a hydrogen-bond sensitive fluorogenic probe[J]. Angewandte Chemie International Edition, 2021, 60(47): 25104-25113. |
| 65 | Bucevičius J, Lukinavičius G, Gerasimaitė R. The use of hoechst dyes for DNA staining and beyond[J]. Chemosensors, 2018, 6(2): 18. |
| 66 | Nakamura A, Takigawa K, Kurishita Y, et al. Hoechst tagging: a modular strategy to design synthetic fluorescent probes for live-cell nucleus imaging[J]. Chemical Communications, 2014, 50(46): 6149-6152. |
| 67 | Lukinavičius G, Blaukopf C, Pershagen E, et al. SiR-Hoechst is a far-red DNA stain for live-cell nanoscopy[J]. Nature Communications, 2015, 6: 8497. |
| 68 | Zhang X D, Ye Z W, Zhang X F, et al. A targetable fluorescent probe for dSTORM super-resolution imaging of live cell nucleus DNA[J]. Chemical Communications, 2019, 55(13): 1951-1954. |
| 69 | Bucevičius J, Keller-Findeisen J, Gilat T, et al. Rhodamine-Hoechst positional isomers for highly efficient staining of heterochromatin[J]. Chemical Science, 2019, 10(7): 1962-1970. |
| [1] | 吴立盛, 刘杰, 王添添, 罗正鸿, 周寅宁. 开环易位烯烃聚合物的动态交联改性研究进展[J]. 化工学报, 2024, 75(4): 1118-1136. |
| [2] | 肖扬可, 常印龙, 李平, 王文俊, 李伯耿, 刘平伟. 动态化学交联聚烯烃类弹性体研究进展[J]. 化工学报, 2024, 75(4): 1394-1413. |
| [3] | 董益斌, 熊敬超, 王敬宇, 汪守康, 王亚飞, 黄群星. 融合激光雷达料位测算的锅炉燃烧优化模型预测控制[J]. 化工学报, 2024, 75(3): 924-935. |
| [4] | 王沛, 段睿明, 张广儒, 金万勤. 光热驱动的膜分离生物甲烷制氢过程建模与仿真分析[J]. 化工学报, 2024, 75(3): 967-973. |
| [5] | 谈莹莹, 刘晓庆, 王林, 黄鲤生, 李修真, 王占伟. R1150/R600a自复叠制冷循环开机动态特性实验研究[J]. 化工学报, 2023, 74(S1): 213-222. |
| [6] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [7] | 宋嘉豪, 王文. 斯特林发动机与高温热管耦合运行特性研究[J]. 化工学报, 2023, 74(S1): 287-294. |
| [8] | 张思雨, 殷勇高, 贾鹏琦, 叶威. 双U型地埋管群跨季节蓄热特性研究[J]. 化工学报, 2023, 74(S1): 295-301. |
| [9] | 仪显亨, 周骛, 蔡小舒, 蔡天意. 光纤后向动态光散射测量纳米颗粒的浓度适用范围研究[J]. 化工学报, 2023, 74(8): 3320-3328. |
| [10] | 黄可欣, 李彤, 李桉琦, 林梅. 加装旋转叶轮T型通道流场的模态分解[J]. 化工学报, 2023, 74(7): 2848-2857. |
| [11] | 邵伟明, 韩文学, 宋伟, 杨勇, 陈灿, 赵东亚. 基于分布式贝叶斯隐马尔可夫回归的动态软测量建模方法[J]. 化工学报, 2023, 74(6): 2495-2502. |
| [12] | 袁子涵, 王淑彦, 邵宝力, 谢磊, 陈曦, 马一玫. 基于幂律液固曳力模型流化床内湿颗粒流动特性的研究[J]. 化工学报, 2023, 74(5): 2000-2012. |
| [13] | 闫沛伟, 张曼铮, 肖猛, 苗政. 地热能有机朗肯循环系统控制策略研究[J]. 化工学报, 2023, 74(12): 4810-4819. |
| [14] | 邓均锐, 李泽宇, 陈嘉衍. 面向动力电池热安全的准被动式热移出系统[J]. 化工学报, 2023, 74(11): 4679-4687. |
| [15] | 李祥宇, 隋璘, 马君霞, 熊伟丽. 基于时序迁移与双流加权的ONLSTM软测量建模[J]. 化工学报, 2023, 74(11): 4622-4633. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号