化工学报 ›› 2024, Vol. 75 ›› Issue (11): 3857-3869.DOI: 10.11949/0438-1157.20240639
收稿日期:2024-06-07
修回日期:2024-07-07
出版日期:2024-11-25
发布日期:2024-12-26
通讯作者:
金万勤
作者简介:赵静(1989—),女,博士,教授, zhaojingmem@njtech.edu.cn
基金资助:
Jing ZHAO1( ), Gongping LIU1, Wanqin JIN1(
), Gongping LIU1, Wanqin JIN1( ), Nanping XU2
), Nanping XU2
Received:2024-06-07
Revised:2024-07-07
Online:2024-11-25
Published:2024-12-26
Contact:
Wanqin JIN
摘要:
限域传质分离膜面向分子/离子的亚纳米尺度高精度分离,是目前国内外膜领域研究的热点和难点。厘清膜内的限域传质效应,形成共性传质机制及调控方法,是突破trade-off效应,实现超常膜分离性能的关键途径。从限域传质通道构筑、限域传质机理及限域传质分离膜应用三个方面入手,对限域传质分离膜近年来的研究进展进行举例介绍与讨论,并对限域传质分离膜的未来发展方向进行了思考与展望,以期为高性能膜材料的设计与应用提供参考。
中图分类号:
赵静, 刘公平, 金万勤, 徐南平. 限域传质分离膜的精密构筑与应用[J]. 化工学报, 2024, 75(11): 3857-3869.
Jing ZHAO, Gongping LIU, Wanqin JIN, Nanping XU. Precision construction and application of separation membranes based on confined mass transfer mechanism[J]. CIESC Journal, 2024, 75(11): 3857-3869.
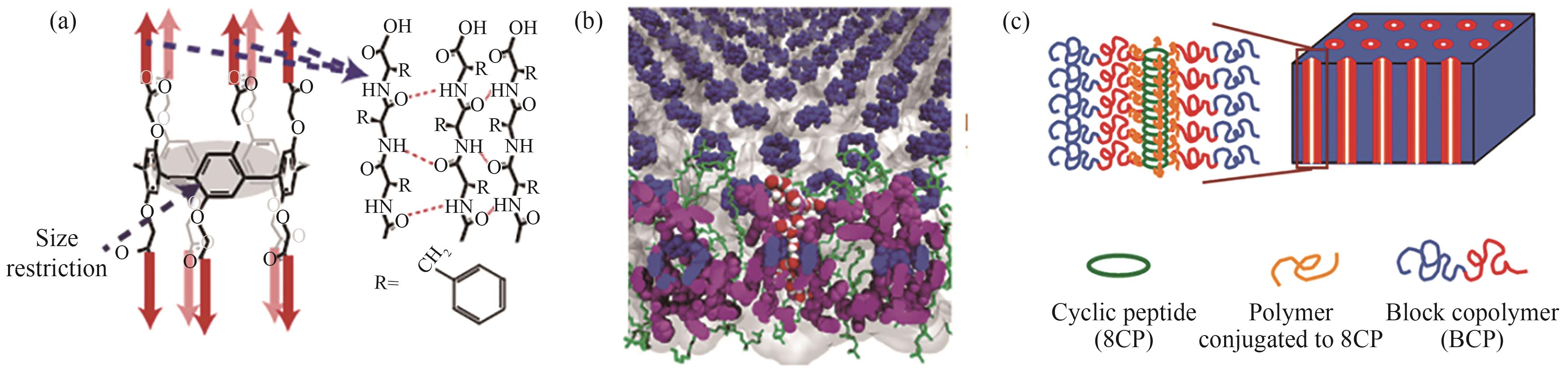
图1 (a)柱芳烃组装形成纳米管示意图[8];(b)柱芳烃纳米管镶嵌于双分子层结构示意图[8];(c)环肽分子与嵌段共聚物共组装形成纳米管结构示意图[17]
Fig.1 Schematic diagrams of formation of nanotube through assembly of pillar[5]arene[8] (a); embedding of nanotubes in bilayers[8] (b), assembly of cyclic peptide with the assistance of block copolymer[17](c)
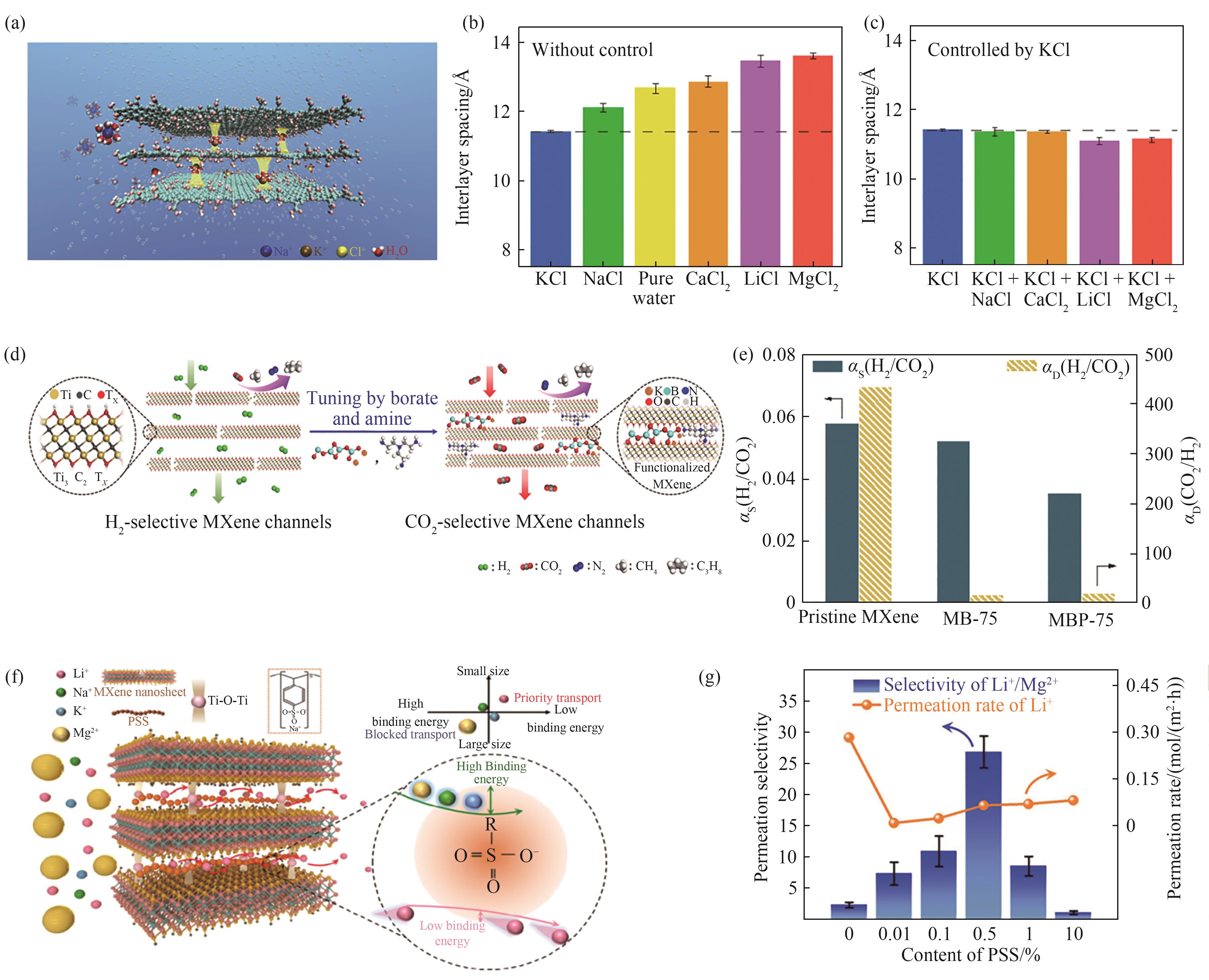
图2 (a)K+固定GO膜层间距实现离子截留示意图[29];(b)GO膜在纯水及不同盐溶液中的层间距[29];(c)经KCl浸泡后GO膜在不同盐溶液中的层间距[29];(d)MXene膜内通道从“扩散主导型”变为“溶解主导型”示意图[20];(e)MXene膜改性前后溶解选择性与扩散选择性变化(MB:MXene+硼酸;MBP:Mxene+硼酸+聚乙烯亚胺)[20];(f)MXene/PSS复合膜内Li+快速传递亚纳米通道示意图[21];(g)MXene/PSS复合膜离子渗透速率及选择性随PSS含量变化[21]
Fig.2 (a) A schematic of how K+ ions fix the interlayer spacing of GO membrane such that other cations are rejected[29]; (b) Interlayer spacings for GO membranes immersed in pure water or in various salt solutions[29]; (c) Interlayer spacings of GO membranes that were soaked in KCl solution, followed by being immersed in various salt solutions[29]; (d) Schematic of the transformation of MXene channels from “diffusion-controlled” to “solution-controlled”[20]; (e) Change of sorption selectivity and diffusion selectivity after chemical tuning of MXene membrane (MB: MXene+borate; MBP: MXene+borate+PEI)[20]; (f) Schematic of the fast transport subnanochannels for Li+ in MXene/PSS composite membranes[21]; (g) The PSS content dependent ion permeation rates and permselectivity of MXene/PSS composite membrane[21]
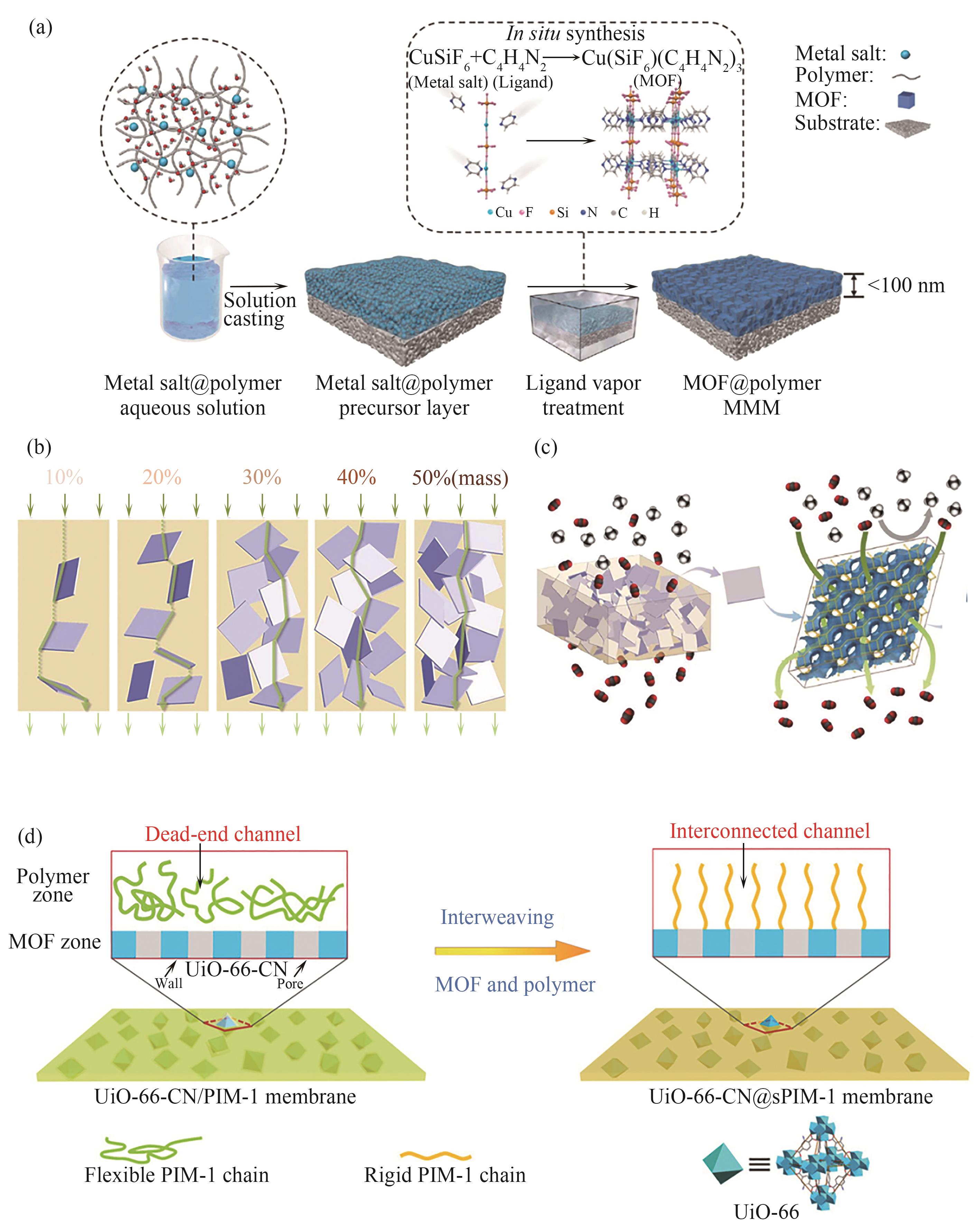
图3 (a)“固态溶剂法”制备超薄高填充MOFs混合基质膜示意图[53];(b)不同填充量下非取向片状分子筛在高分子基质中的分布示意图[54];(c)具有准连续分子筛相的混合基质膜及片状Na-SSZ-39分子筛三维通道中CO2渗透(绿色箭头)路径示意图[54];(d)通过UiO-66与PIM-1结合构建CO2高速传递路径示意图[43]
Fig.3 Schematic diagram of fabricating ultrathin and high-loading MOFs mixed matrix membranes via a solid-solvent processing strategy[53]; (b) Illustration of the nonaligned zeolite platelet distribution in the polymer matrix with different zeolite loadings[54]; (c) Illustration of the mixed matrix membrane with quasi-continuous zeolite phase and the unhindered CO2 permeation (indicated with green arrows) through the 3D-channel system of platelet-shaped Na-SSZ-39 filler[54]; (d) Schematic diagram of building CO2 transport freeways through interweaving UiO-66 and PIM-1[43]
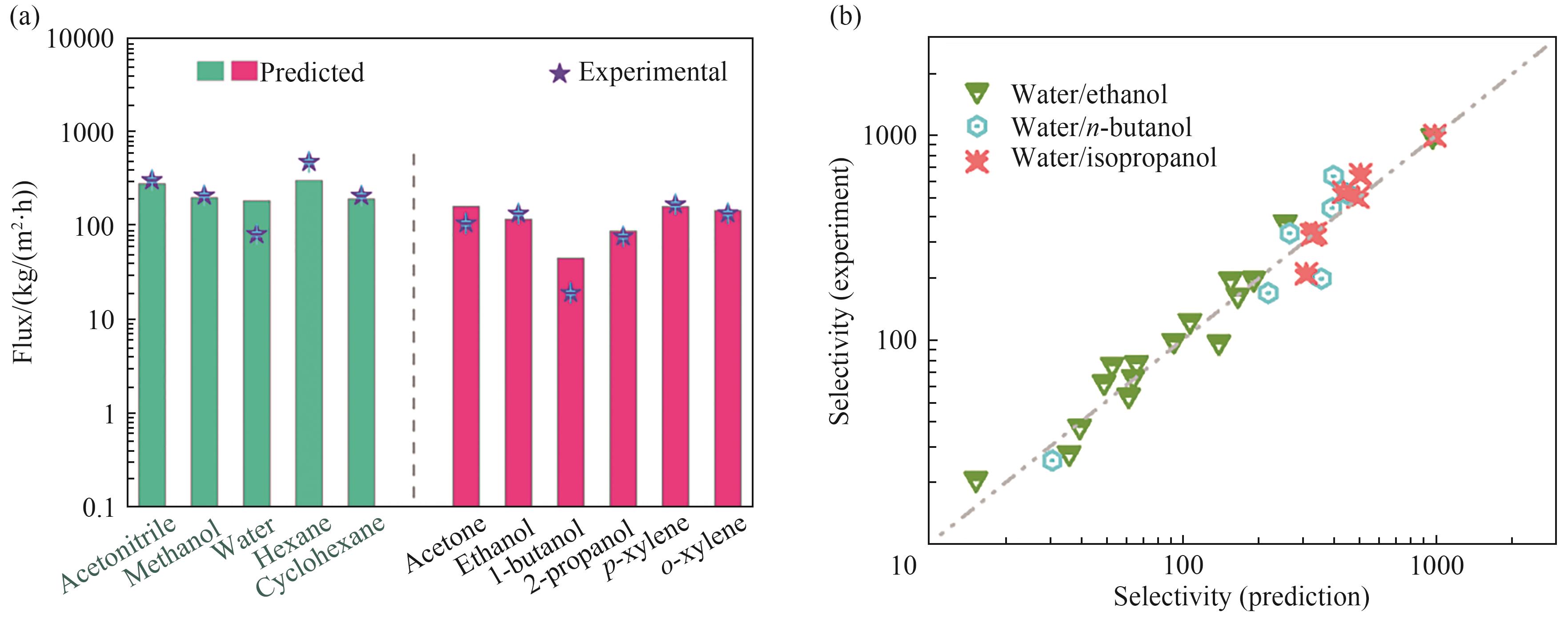
图4 (a)不同溶剂分子在Ti3C2/graphene通道内的通量测定值与计算值[60];(b)石墨烯膜对不同水/醇体系(水/乙醇、水/正丁醇、水/异丙醇)分离选择性的实验值与计算值[61]
Fig.4 (a) The experimental fluxes (purple stars) and predicted fluxes (bars) of different solvents through Ti3C2/graphene channels[60]; (b) The experimental and predicted selectivities of different water/alcohol systems (water/ethanol, water/n-butanol, and water/isopropanol) in graphene-based membranes[61]
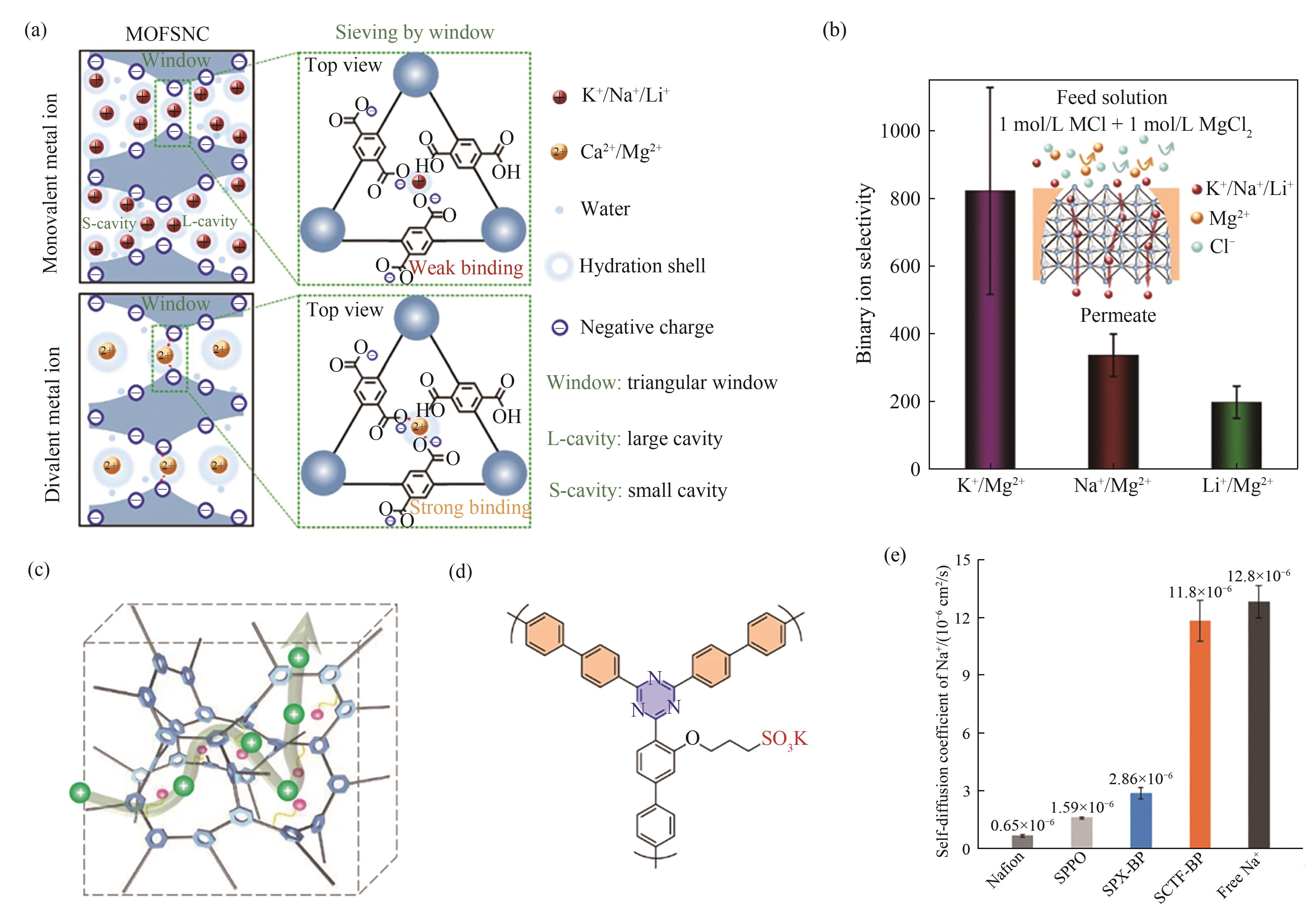
图5 (a)UiO-66-(COOH)2亚纳米通道(MOFSNC)的离子筛分机理及(b)二元离子选择性(K+/Mg2+、Na+/Mg2+、Li+/Mg2+)[63];(c)磺酸共价三嗪框架膜内刚性离子通道示意图[65];(d)联苯共价三嗪框架(SCTF-BP)分子结构[65];(e)不同膜内及水溶液中Na+扩散系数[65]
Fig.5 (a) Ion sieving mechanism and (b) binary K+/Mg2+, Na+/Mg2+ and Li+/Mg2+ selectivities in the UiO-66-(COOH)2 subnanochannels (MOFSNC)[63]; (c) Schematic diagram of the rigid ion channels in sulfonated SCTF (SCTF) membrane[65]; (d) Molecular structure of SCTF- biphenyl (SCTF-BP)[65]; (e) Diffusion coefficients of Na+ in water and different membrane samples[65]

图6 (a)用于膜蒸馏的亲水梯度结构COF膜示意图[66];(b)孔径对水蒸发通量的影响(分子模拟)[66];(c)通过COF纳米片与纳米带结合形成的COF膜内通道结构与作用力示意图[67];(d)温度对COF膜渗透通量的影响[67];(e)Ti3C2/石墨烯复合膜结构示意图[60];(f)温度对不同组成Ti3C2/石墨烯复合膜水通量的影响[60]
Fig.6 (a) Schematic illustration of the COF membrane with hydrophilicity gradient for membrane distillation[66]; (b) Water evaporation flux as a function of pore diameter (molecular dynamics simulations)[66]; (c) Schematic for the channel structures and interactions in the COF membranes formed through binding of COF nanosheets and nanoribbons[67]; (d) Temperature-dependent permeation flux of COF membrane[67]; (e) Schematic illustration of the Ti3C2-graphene membrane structure[60]; (f) Water flux of the Ti3C2-graphene hetero-channel membranes under different feed temperatures[60]
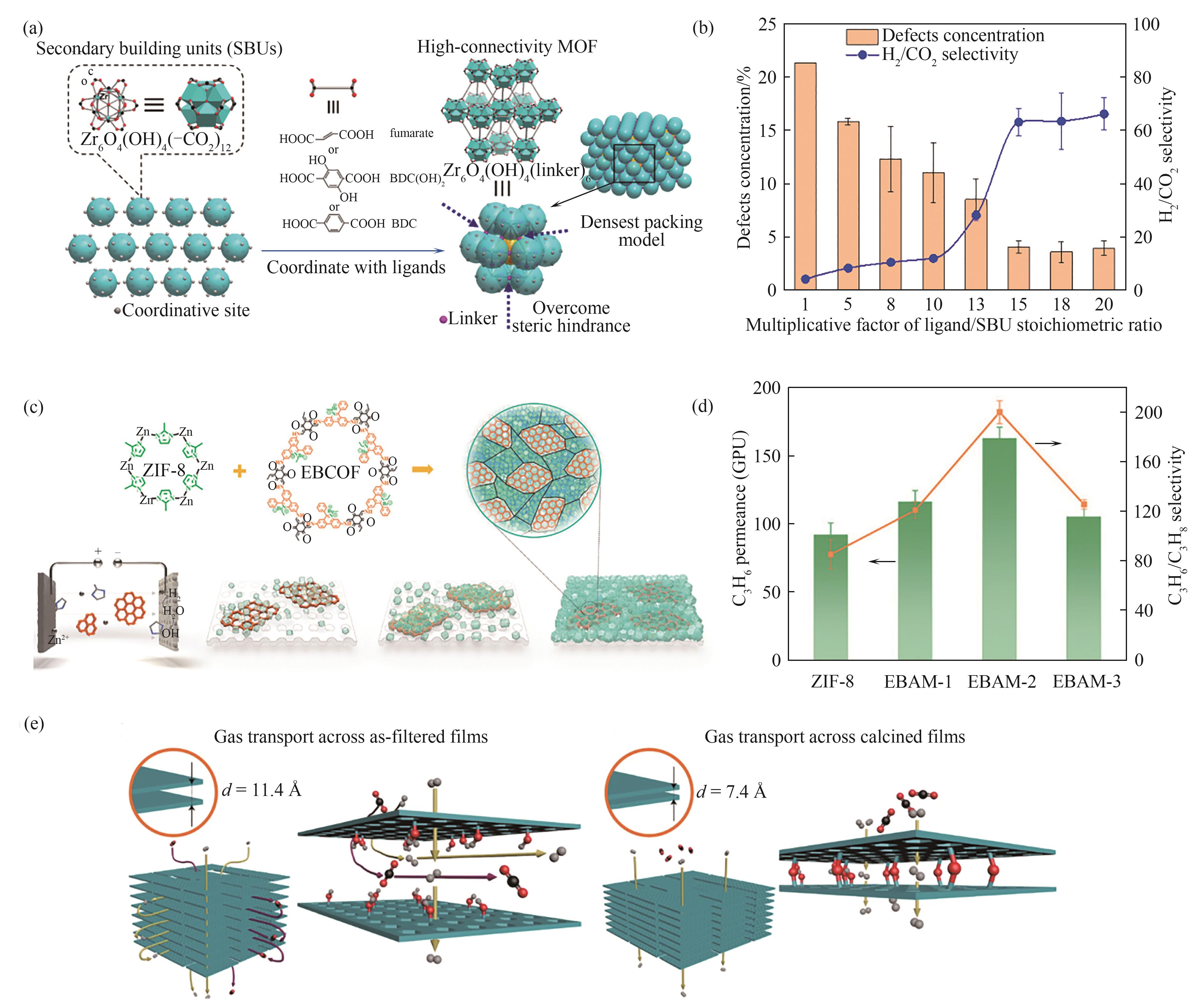
图7 (a)无晶格缺陷的MOF晶体膜制备过程示意图[71];(b)不同有机配体/金属团簇化学计量比倍增因子下Zr-MOF(富马酸)膜的缺陷浓度及H2/CO2选择性[71];(c)MOF-COF合金膜制备过程示意图[72];(d)MOF-COF合金膜的C3H6/C3H8混合气分离性能[72];(e)热处理前后RUB沸石膜内气体传递通道结构对比[36]
Fig.7 (a) Schematic diagram of the formation of MOF membranes with perfect lattices[71]; (b) The defect concentration and H2/CO2 selectivity of Zr-MOF (fumarate) membranes prepared using various multiplicative factors of the ligand/SBU stoichiometric ratio[71]; (c) Schematic diagram of the construction of MOF-COF alloy membranes[72]; (d) Mixed C3H6/C3H8 separation performance of the MOF-COF alloy membrane[72]; (e) Comparison of the gas transport channel strctures in RUB zeolite membranes before and after calcination[36]
| 1 | Sholl D S, Lively R P. Seven chemical separations to change the world[J]. Nature, 2016, 532(7600): 435-437. |
| 2 | 金万勤, 徐南平. 限域传质分离膜[J]. 化工学报, 2018, 69(1): 50-56. |
| Jin W Q, Xu N P. Membrane separation based on mechanism of confined mass transfer[J]. CIESC Journal, 2018, 69(1): 50-56. | |
| 3 | 朱育丹, 陆小华, 谢文龙, 等. 基于限域传质机制的膜过程定量描述的研究进展[J]. 科学通报, 2017, 62(S1): 223-232. |
| Zhu Y D, Lu X H, Xie W L, et al. The progress of quantitatively description of membrane process based on the mechanism of nanoconfined mass transfer[J]. Chinese Science Bulletin, 2017, 62(S1): 223-232. | |
| 4 | 刘壮, 汪伟, 巨晓洁, 等. 具有限域传质效应的碳基分离膜: 从碳纳米管膜到石墨烯膜[J]. 化工学报, 2018, 69(1): 166-174. |
| Liu Z, Wang W, Ju X J, et al. Carbon-based membranes with confinement effect for mass transport: from carbon nano-tube membranes to graphene membranes[J]. CIESC Journal, 2018, 69(1): 166-174. | |
| 5 | Robeson L M. Correlation of separation factor versus permeability for polymeric membranes[J]. Journal of Membrane Science, 1991, 62(2): 165-185. |
| 6 | Freeman B D. Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes[J]. Macromolecules, 1999, 32(2): 375-380. |
| 7 | Shen J, Liu G P, Han Y, et al. Artificial channels for confined mass transport at the sub-nanometre scale[J]. Nature Reviews Materials, 2021, 6: 294-312. |
| 8 | Shen Y X, Song W, Barden D R, et al. Achieving high permeability and enhanced selectivity for angstrom-scale separations using artificial water channel membranes[J]. Nature Communications, 2018, 9(1): 2294. |
| 9 | Song W, Joshi H, Chowdhury R, et al. Artificial water channels enable fast and selective water permeation through water-wire networks[J]. Nature Nanotechnology, 2020, 15(1): 73-79. |
| 10 | Kocsis I, Sorci M, Vanselous H, et al. Oriented chiral water wires in artificial transmembrane channels[J]. Science Advances, 2018, 4(3): eaao5603. |
| 11 | Hinds B J, Chopra N, Rantell T, et al. Aligned multiwalled carbon nanotube membranes[J]. Science, 2004, 303(5654): 62-65. |
| 12 | Secchi E, Marbach S, Niguès A, et al. Massive radius-dependent flow slippage in carbon nanotubes[J]. Nature, 2016, 537(7619): 210-213. |
| 13 | Vatanpour V, Ali Naziri Mehrabani S, Keskin B, et al. A comprehensive review on the applications of boron nitride nanomaterials in membrane fabrication and modification[J]. Industrial & Engineering Chemistry Research, 2021, 60(37): 13391-13424. |
| 14 | Kim W G, Nair S. Membranes from nanoporous 1D and 2D materials: a review of opportunities, developments, and challenges[J]. Chemical Engineering Science, 2013, 104: 908-924. |
| 15 | Hummer G, Rasaiah J C, Noworyta J P. Water conduction through the hydrophobic channel of a carbon nanotube[J]. Nature, 2001, 414: 188-190. |
| 16 | Huang L B, Hardiagon A, Kocsis I, et al. Hydroxy channels-adaptive pathways for selective water cluster permeation[J]. Journal of the American Chemical Society, 2021, 143(11): 4224-4233. |
| 17 | Xu T, Zhao N N, Ren F, et al. Subnanometer porous thin films by the co-assembly of nanotube subunits and block copolymers[J]. ACS Nano, 2011, 5(2): 1376-1384. |
| 18 | Shen J, Liu G P, Huang K, et al. Subnanometer two-dimensional graphene oxide channels for ultrafast gas sieving[J]. ACS Nano, 2016, 10(3): 3398-3409. |
| 19 | Wang S F, Wu Y Z, Zhang N, et al. A highly permeable graphene oxide membrane with fast and selective transport nanochannels for efficient carbon capture[J]. Energy & Environmental Science, 2016, 9(10): 3107-3112. |
| 20 | Shen J, Liu G Z, Ji Y F, et al. 2D MXene nanofilms with tunable gas transport channels[J]. Advanced Functional Materials, 2018, 28(31): 1801511. |
| 21 | Lu Z, Wu Y, Ding L, et al. A lamellar MXene (Ti3C2T x )/PSS composite membrane for fast and selective lithium-ion separation[J]. Angewandte Chemie International Edition, 2021, 60(41): 22265-22269. |
| 22 | Mei L, Cao Z L, Ying T, et al. Simultaneous electrochemical exfoliation and covalent functionalization of MoS2 membrane for ion sieving[J]. Advanced Materials, 2022, 34(26): 2201416. |
| 23 | Fan Z W, Zhang J, Zuo B Y, et al. Biomimetic guttation feature in 2D hydrotalcite membranes for self-sustaining water purification[J]. Advanced Functional Materials, 2024, 34(26): 2316247. |
| 24 | Liu G P, Jin W Q, Xu N P. Two-dimensional-material membranes: a new family of high-performance separation membranes[J]. Angewandte Chemie International Edition, 2016, 55(43): 13384-13397. |
| 25 | Liu G P, Jin W Q, Xu N P. Graphene-based membranes[J]. Chemical Society Reviews, 2015, 44(15): 5016-5030. |
| 26 | Deng J J, Lu Z, Ding L, et al. Fast electrophoretic preparation of large-area two-dimensional titanium carbide membranes for ion sieving[J]. Chemical Engineering Journal, 2021, 408: 127806. |
| 27 | Nair R R, Wu H A, Jayaram P N, et al. Unimpeded permeation of water through helium-leak-tight graphene-based membranes[J]. Science, 2012, 335(6067): 442-444. |
| 28 | Zheng S X, Tu Q S, Urban J J, et al. Swelling of graphene oxide membranes in aqueous solution: characterization of interlayer spacing and insight into water transport mechanisms[J]. ACS Nano, 2017, 11(6): 6440-6450. |
| 29 | Chen L, Shi G S, Shen J, et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing[J]. Nature, 2017, 550(7676): 380-383. |
| 30 | Zhang M C, Mao Y Y, Liu G Z, et al. Molecular bridges stabilize graphene oxide membranes in water[J]. Angewandte Chemie International Edition, 2020, 59(4): 1689-1695. |
| 31 | Zhang M C, Zhao P X, Li P S, et al. Designing biomimic two-dimensional ionic transport channels for efficient ion sieving[J]. ACS Nano, 2021, 15(3): 5209-5220. |
| 32 | Cheng L, Guo Y N, Liu Q, et al. Metal confined in 2D membranes for molecular recognition and sieving towards ethylene/ethane separation[J]. Advanced Materials, 2022, 34(44): 2206349. |
| 33 | Akbari A, Sheath P, Martin S T, et al. Large-area graphene-based nanofiltration membranes by shear alignment of discotic nematic liquid crystals of graphene oxide[J]. Nature Communications, 2016, 7: 10891. |
| 34 | Liu S, Liu G Z, Chen G N, et al. Scale-up fabrication of two-dimensional material membranes: challenges and opportunities[J]. Current Opinion in Chemical Engineering, 2023, 39: 100892. |
| 35 | Liu Z Y, Ma Z, Qian B T, et al. A facile and scalable method of fabrication of large-area ultrathin graphene oxide nanofiltration membrane[J]. ACS Nano, 2021, 15(9): 15294-15305. |
| 36 | Dakhchoune M, Villalobos L F, Semino R, et al. Gas-sieving zeolitic membranes fabricated by condensation of precursor nanosheets[J]. Nature Materials, 2021, 20(3): 362-369. |
| 37 | Du P, Zhang Y T, Wang X R, et al. Control of zeolite framework flexibility for ultra-selective carbon dioxide separation[J]. Nature Communications, 2022, 13(1): 1427. |
| 38 | Li L, Xu R S, Song C W, et al. A review on the progress in nanoparticle/C hybrid CMS membranes for gas separation[J]. Membranes, 2018, 8(4): 134. |
| 39 | Koh D Y, McCool B A, Deckman H W, et al. Reverse osmosis molecular differentiation of organic liquids using carbon molecular sieve membranes[J]. Science, 2016, 353(6301): 804-807. |
| 40 | Wang H J, Zhai Y M, Li Y, et al. Covalent organic framework membranes for efficient separation of monovalent cations[J]. Nature Communications, 2022, 13(1): 7123. |
| 41 | Hasell T, Cooper A I. Porous organic cages: soluble, modular and molecular pores[J]. Nature Reviews Materials, 2016, 1(9): 16053. |
| 42 | Zhao S, Zhao Z Y, Zha Z Y, et al. Amine-rich molecular nodule-assembled membrane having 5 angstrom channels for CO2/N2 separation[J]. Advanced Functional Materials, 2024, 34(27): 2314469. |
| 43 | Yu G L, Zou X Q, Sun L, et al. Constructing connected paths between UiO-66 and PIM-1 to improve membrane CO2 separation with crystal-like gas selectivity[J]. Advanced Materials, 2019, 31(15): 1806853. |
| 44 | Cooper A I. Conjugated microporous polymers[J]. Advanced Materials, 2009, 21(12): 1291-1295. |
| 45 | Ma Y, Cui F C, Rong H Z, et al. Continuous porous aromatic framework membranes with modifiable sites for optimized gas separation[J]. Angewandte Chemie International Edition, 2022, 61(1): e202113682. |
| 46 | Du W G, Liu L, Yin L Y, et al. Ultrathin free-standing porous aromatic framework membranes for efficient anion transport[J]. Angewandte Chemie International Edition, 2024, 63(22): e202402943. |
| 47 | Wang H J, Wang M D, Liang X, et al. Organic molecular sieve membranes for chemical separations[J]. Chemical Society Reviews, 2021, 50(9): 5468-5516. |
| 48 | Yuan D Q, Lu W G, Zhao D, et al. Highly stable porous polymer networks with exceptionally high gas-uptake capacities[J]. Advanced Materials, 2011, 23(32): 3723-3725. |
| 49 | Chen G N, Liu G Z, Pan Y, et al. Zeolites and metal-organic frameworks for gas separation: the possibility of translating adsorbents into membranes[J]. Chemical Society Reviews, 2023, 52(14): 4586-4602. |
| 50 | Peng Y, Li Y S, Ban Y J, et al. Metal-organic framework nanosheets as building blocks for molecular sieving membranes[J]. Science, 2014, 346(6215): 1356-1359. |
| 51 | Li J R, Zhou H C. Bridging-ligand-substitution strategy for the preparation of metal-organic polyhedra[J]. Nature Chemistry, 2010, 2(10): 893-898. |
| 52 | Qiao Z H, Zhao S, Sheng M L, et al. Metal-induced ordered microporous polymers for fabricating large-area gas separation membranes[J]. Nature Materials, 2019, 18(2): 163-168. |
| 53 | Chen G N, Chen C L, Guo Y N, et al. Solid-solvent processing of ultrathin, highly loaded mixed-matrix membrane for gas separation[J]. Science, 2023, 381(6664): 1350-1356. |
| 54 | Tan X Y, Robijns S, Thür R, et al. Truly combining the advantages of polymeric and zeolite membranes for gas separations[J]. Science, 2022, 378(6625): 1189-1194. |
| 55 | Keerthi A, Goutham S, You Y, et al. Water friction in nanofluidic channels made from two-dimensional crystals[J]. Nature Communications, 2021, 12(1): 3092. |
| 56 | Xie Q, Alibakhshi M A, Jiao S P, et al. Fast water transport in graphene nanofluidic channels[J]. Nature Nanotechnology, 2018, 13(3): 238-245. |
| 57 | Tunuguntla R H, Henley R Y, Yao Y C, et al. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins[J]. Science, 2017, 357(6353): 792-796. |
| 58 | Keerthi A, Geim A K, Janardanan A, et al. Ballistic molecular transport through two-dimensional channels[J]. Nature, 2018, 558(7710): 420-424. |
| 59 | 覃瑶, 张禹萌, 潘雪玲, 等. 限域传递机制初探:以限域状态为切入点描述传递阻力[J]. 化工学报, 2023, 74(1): 74-85. |
| Qin Y, Zhang Y M, Pan X L, et al. Preliminary study on mechanism of transfer in confined space: description of confined transfer resistance based on confined fluid state[J]. CIESC Journal, 2023, 74(1): 74-85. | |
| 60 | Chen X F, Qin Y, Zhu Y D, et al. Accurate prediction of solvent flux in sub-1-nm slit-pore nanosheet membranes[J]. Science Advances, 2024, 10(17): eadl1455. |
| 61 | 高庆伟, 覃瑶, 张禹萌, 等. 限域传质分离机制初探:界面吸附层的“二次限域”效应[J]. 化工学报, 2020, 71(10): 4688-4695. |
| Gao Q W, Qin Y, Zhang Y M, et al. Preliminary study on mechanism of confined mass transfer and separation: “secondary confinement” effect of interfacial adsorption layer[J]. CIESC Journal, 2020, 71(10): 4688-4695. | |
| 62 | Pan X L, Ma Z H, Qin Y, et al. Modeling of alcohol/water separation in graphene-based membranes: the roles of interfacial adsorption and the effective transfer path[J]. Industrial & Engineering Chemistry Research, 2024, 63(14): 6399-6410. |
| 63 | Lu J, Zhang H C, Hou J, et al. Efficient metal ion sieving in rectifying subnanochannels enabled by metal-organic frameworks[J]. Nature Materials, 2020, 19(7): 767-774. |
| 64 | Zhao C, Feng F, Hou J, et al. Unlocking direct lithium extraction in harsh conditions through thiol-functionalized metal-organic framework subnanofluidic membranes[J]. Journal of the American Chemical Society, 2024, 146(20): 14058-14066. |
| 65 | Zuo P P, Ye C C, Jiao Z R, et al. Near-frictionless ion transport within triazine framework membranes[J]. Nature, 2023, 617(7960): 299-305. |
| 66 | Zhao S, Jiang C H, Fan J C, et al. Hydrophilicity gradient in covalent organic frameworks for membrane distillation[J]. Nature Materials, 2021, 20(11): 1551-1558. |
| 67 | Wang M D, Zhang P H, Liang X, et al. Ultrafast seawater desalination with covalent organic framework membranes[J]. Nature Sustainability, 2022, 5: 518-526. |
| 68 | Zhang M C, Guan K C, Shen J, et al. Nanoparticles@rGO membrane enabling highly enhanced water permeability and structural stability with preserved selectivity[J]. AIChE Journal, 2017, 63(11): 5054-5063. |
| 69 | Liang F, Liu Q, Zhao J, et al. Ultrafast water-selective permeation through graphene oxide membrane with water transport promoters[J]. AIChE Journal, 2020, 66(2): e16812. |
| 70 | Yang G, Xie Z L, Cran M, et al. Functionalizing graphene oxide framework membranes with sulfonic acid groups for superior aqueous mixture separation[J]. Journal of Materials Chemistry A, 2019, 7(34): 19682-19690. |
| 71 | Liu G Z, Guo Y N, Chen C L, et al. Eliminating lattice defects in metal-organic framework molecular-sieving membranes[J]. Nature Materials, 2023, 22(6): 769-776. |
| 72 | Liu Y T, Wu H, Li R L, et al. MOF-COF “alloy” membranes for efficient propylene/propane separation[J]. Advanced Materials, 2022, 34(24): 2201423. |
| [1] | 陈森洋, 靳蒲航, 谭志明, 谢公南. 质子交换膜燃料电池中蛇形流道液滴运动数值仿真研究[J]. 化工学报, 2024, 75(S1): 183-194. |
| [2] | 李匡奚, 于佩潜, 王江云, 魏浩然, 郑志刚, 冯留海. 微气泡旋流气浮装置内流动分析与结构优化[J]. 化工学报, 2024, 75(S1): 223-234. |
| [3] | 谢慧慧, 姜佳鑫, 王鑫, 李正, 郭鑫, 吕欣然, 王凌云, 刘杨. 深共晶溶剂聚合物包覆膜传输分离铂、钯的研究[J]. 化工学报, 2024, 75(S1): 235-243. |
| [4] | 邱知, 谭明. 聚离子液体膜的制备及其在低钠高钾健康酱油中的应用[J]. 化工学报, 2024, 75(S1): 244-250. |
| [5] | 徐英宇, 杨国强, 彭璟, 孙海宁, 张志炳. 微界面高级氧化处理煤化工废水的研究[J]. 化工学报, 2024, 75(S1): 283-291. |
| [6] | 刘律, 刘洁茹, 范亮亮, 赵亮. 基于层流效应的被动式颗粒分离微流控方法研究[J]. 化工学报, 2024, 75(S1): 67-75. |
| [7] | 张丽萍, 孟晓荣, 宋锦峰, 杜金晶. VO2@KH550/570@PS复合薄膜的制备及其热致相变性能[J]. 化工学报, 2024, 75(9): 3348-3359. |
| [8] | 唐昊, 胡定华, 李强, 张轩畅, 韩俊杰. 抗加速度双切线弧流道内气泡动力学行为数值与可视化研究[J]. 化工学报, 2024, 75(9): 3074-3082. |
| [9] | 陈引, 赵霄, 杜王芳, 杨竹强, 李凯, 赵建福. 喷雾冷却液膜流动特性测试方案优化及传热规律分析[J]. 化工学报, 2024, 75(8): 2734-2743. |
| [10] | 王皓宇, 杨杨, 荆文婕, 杨斌, 唐雨, 刘毅. 不同旋流器作用下气液螺旋环状流动特性研究[J]. 化工学报, 2024, 75(8): 2744-2755. |
| [11] | 罗正航, 李敬宇, 陈伟雄, 种道彤, 严俊杰. 摇摆运动下低流率蒸汽冷凝换热特性和气泡受力数值模拟[J]. 化工学报, 2024, 75(8): 2800-2811. |
| [12] | 王倩倩, 李冰, 郑伟波, 崔国民, 赵兵涛, 明平文. 氢燃料电池局部动态特征三维模型[J]. 化工学报, 2024, 75(8): 2812-2820. |
| [13] | 曾港, 陈林, 杨董, 袁海专, 黄彦平. 矩形通道内超临界CO2局部热流场可视化实验[J]. 化工学报, 2024, 75(8): 2831-2839. |
| [14] | 李彦熹, 王晔春, 谢向东, 王进芝, 王江, 周煜, 潘盈秀, 丁文涛, 郭烈锦. 蜗壳式多通道气液旋流分离器结构优化及分离特性研究[J]. 化工学报, 2024, 75(8): 2875-2885. |
| [15] | 白炳林, 杜燊, 李明佳, 张传琪. 基于水相剥离的单壁碳纳米管薄膜透光和导电特性[J]. 化工学报, 2024, 75(7): 2680-2687. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号