• •
收稿日期:2024-06-24
修回日期:2024-09-17
出版日期:2024-09-18
通讯作者:
苗青
作者简介:苗青(1988—),女,博士,副教授,miaoqing@sust.edu.cn
基金资助:Received:2024-06-24
Revised:2024-09-17
Online:2024-09-18
Contact:
Qing MIAO
摘要:
蛋白质是细胞内含量丰富的生物分子之一,其参与几乎所有的生化过程。总结了蛋白质中氨基酸残基和游离氨基酸的反应类型,包括半胱氨酸的巯基通过烷基化、不饱和键加成、氟取代等反应类型与探针或试剂反应;赖氨酸的氨基与醛或酯发生缩合反应,还与烯键或炔键发生加成反应,少量可与双官能团底物发生环化反应;精氨酸的胍基更多的是通过与探针形成非共价氢键实现修饰;含羧基侧链的谷氨酸和天冬氨酸与不同类型的底物进行酯化和自由基等反应;酪氨酸的酚羟基和邻位C上的氢可发生自由基及亲核取代反应;含氮杂环的吲哚基(色氨酸)及咪唑基(组氨酸)有不同反应位点,可发生特定类型的反应。同时,对于蛋白质残基修饰在医药与工程领域的相关应用进行了概括。通过以上总结对不同氨基酸的修饰及应用提出不足和对未来的展望。
中图分类号:
苗青, 石睿思. 蛋白质氨基酸残基及游离氨基酸的化学修饰与应用[J]. 化工学报, DOI: 10.11949/0438-1157.20240707.
Qing MIAO, Ruisi SHI. Chemical modification and applications of protein amino acid residues and free amino acids[J]. CIESC Journal, DOI: 10.11949/0438-1157.20240707.

图1 与巯基发生卤代烷基化反应的机理及相应探针或试剂
Fig.1 Mechanisms of haloalkylation reactions with sulfhydryl groups and corresponding probes or reagents(X=F,Cl,Br,螺旋结构为蛋白质)
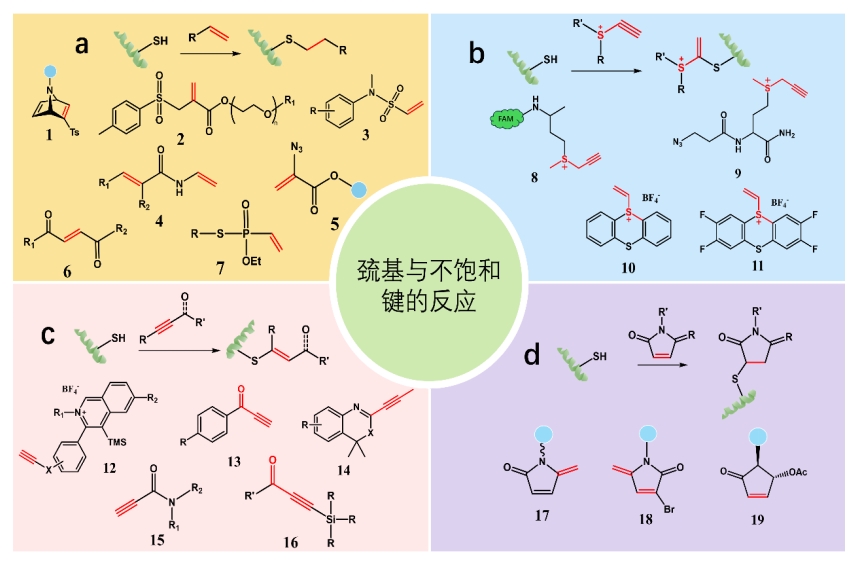
图2 不饱和键与巯基的反应类型:(a)烯键与半胱氨酸巯基的加成反应;(b)锍中心引发的硫醇-炔型反应;(c)巯基与炔键的反应;(d)巯基与类马来酰亚胺的反应
Fig.2 Reaction types of unsaturated bonds with sulfhydryl groups:(a) the addition of alkene bond to cysteine sulfhydryl group; (b) thiol-alkyne type reactions initiated by sulfonium centres; (c) reaction of sulfhydryl groups with alkyne bonds; (d) reaction of sulfhydryl groups with maleimide analogues
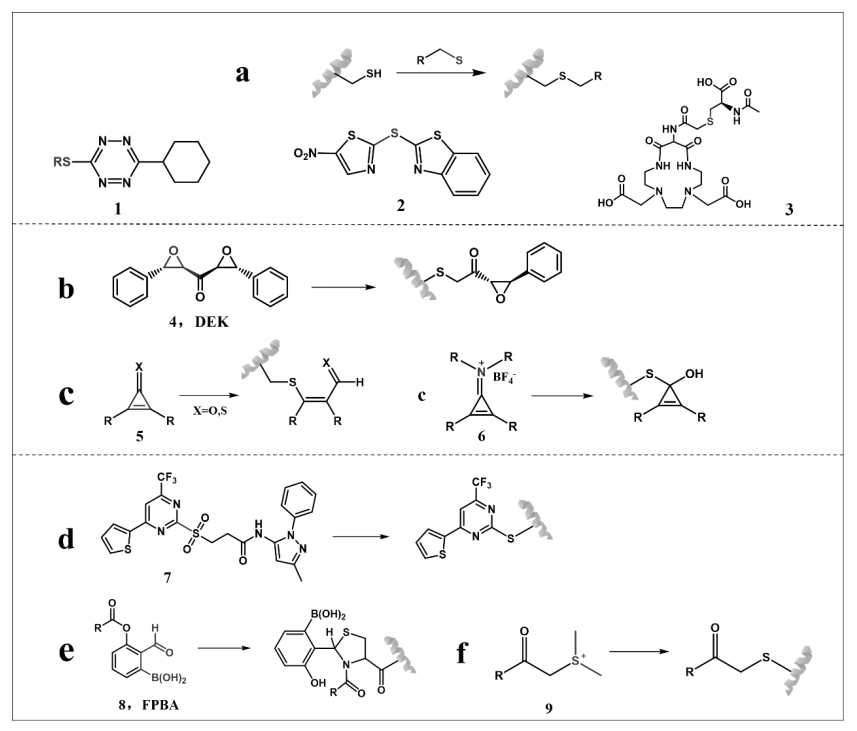
图4 与半胱氨酸残基的各种类型反应:(a)类似硫交换反应;(b)和(c)三元环类型;(d)、(e)和(f)其它类型反应
Fig.4 Various types of reactions with cysteine residues: (a) sulfur-like exchange reaction; (b) and (c) ternary ring types; (d), (e) and (f) other types of reactions
图5 游离半胱氨酸的修饰反应:(a)和(b)卤代烷基化反应;(c)和(d)硫交换反应;(e)和(f)形成五元环反应;(g)取代反应
Fig.5 Modification reactions of free cysteine: (a) and (b) haloalkylation reactions; (c) and (d) sulphur exchange reactions; (e) and (f) form a five-membered ring reaction; (g) substitution reactions
图6 丙烯酸酯的特殊不饱和键与巯基反应示意图和相应的探针结构
Fig.6 Schematic of the reaction of special unsaturated bond with sulfhydryl group of acrylate and corresponding probe structure(圆圈代表不同探针的丙烯酸酯外的其他部分)
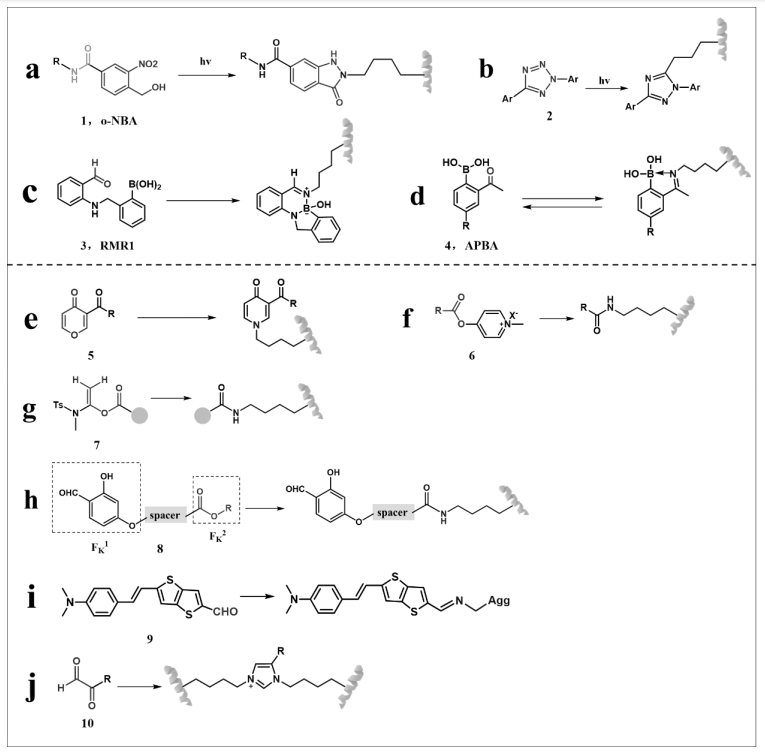
图7 与赖氨酸残基的环化反应及与醛或酯的反应:(a)、(b)、(c)和(d)环化反应;(e)、(f)、(g)、(h)、(i)和(j)醛或酯的反应
Fig.7 Cyclisation reactions with lysine residues and reactions with aldehydes or esters: (a), (b), (c) and (d) cyclisation reactions; (e), (f), (g), (h), (i) and (j) aldehyde or ester reactions
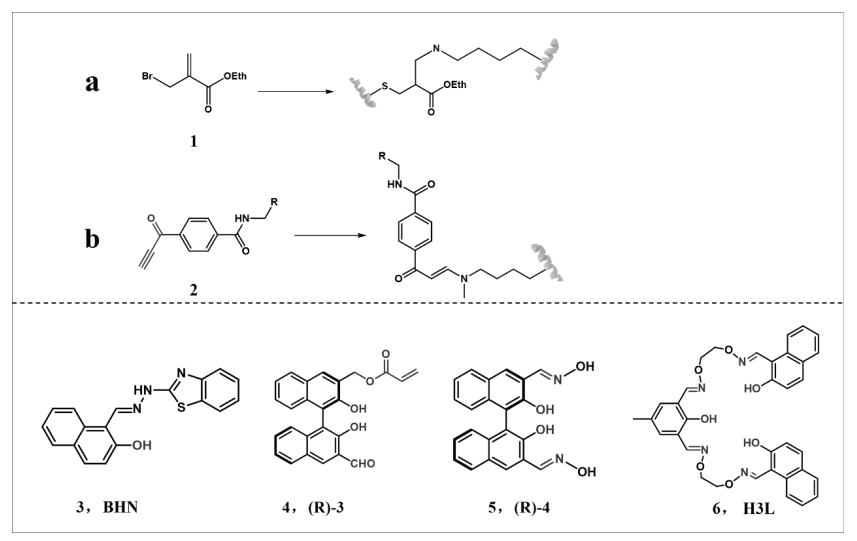
图8 赖氨酸与烯(a)、炔(b)键的反应以及既可修饰游离赖氨酸又可修饰游离精氨酸的探针
Fig.8 Reactions of lysine with alkene (a) and alkynyl (b) bonds and probes that modify both free lysine and free arginine
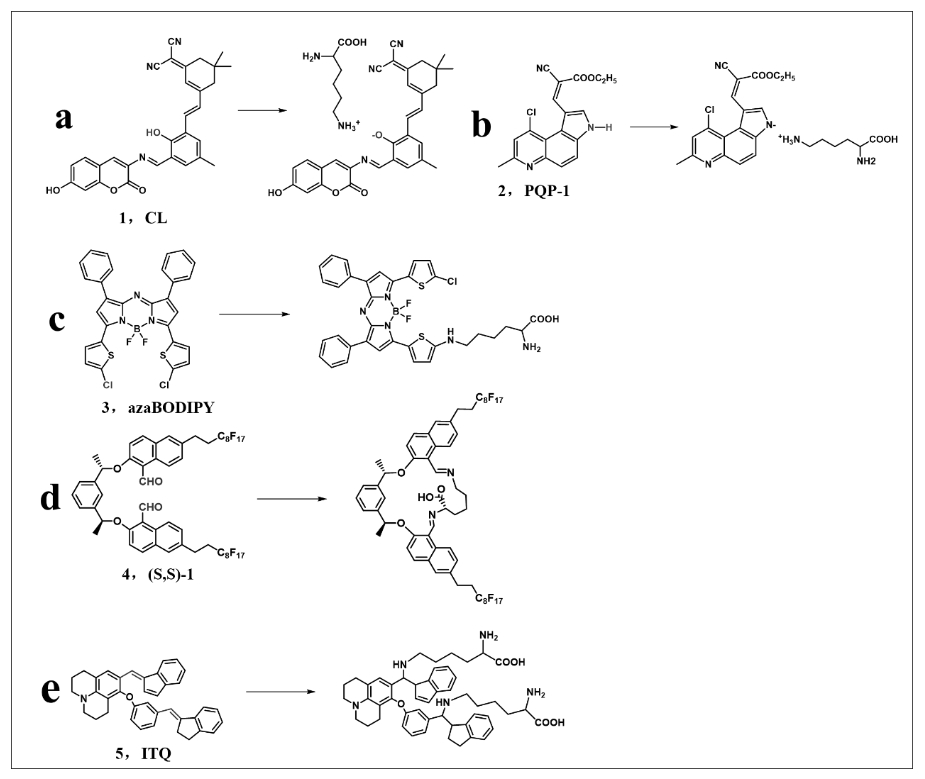
图9 探针或试剂与游离赖氨酸的修饰:(a)和(b)质子化反应;(c)亲核取代反应;(d)环化反应;(e)加成反应
Fig.9 Modification of probes or reagents with free lysine: (a) and (b) protonation reactions; (c) nucleophilic substitution reactions; (d) cyclisation reactions; (e) addition reactions
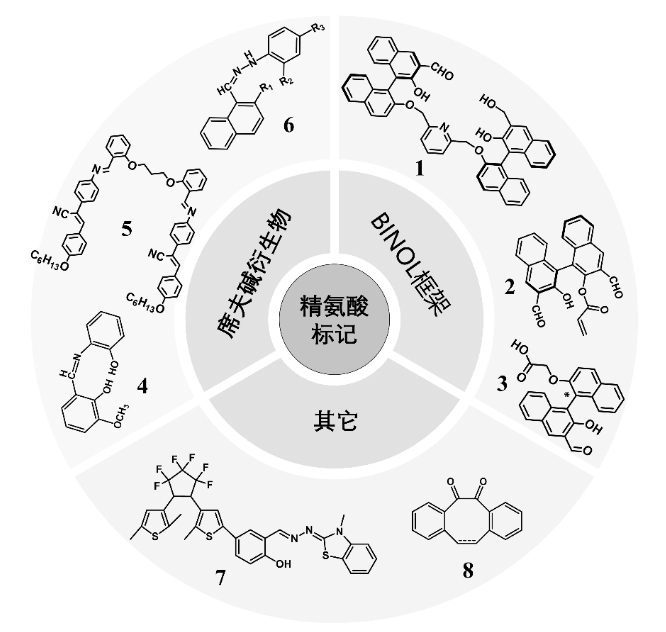
图10 精氨酸的修饰:1-7为游离精氨酸的标记探针;8为蛋白质中精氨酸残基的标记探针
Fig.10 Arginine modification: 1-7 are labelled probes for free arginine; 8 is a labelling probe for arginine residues in proteins
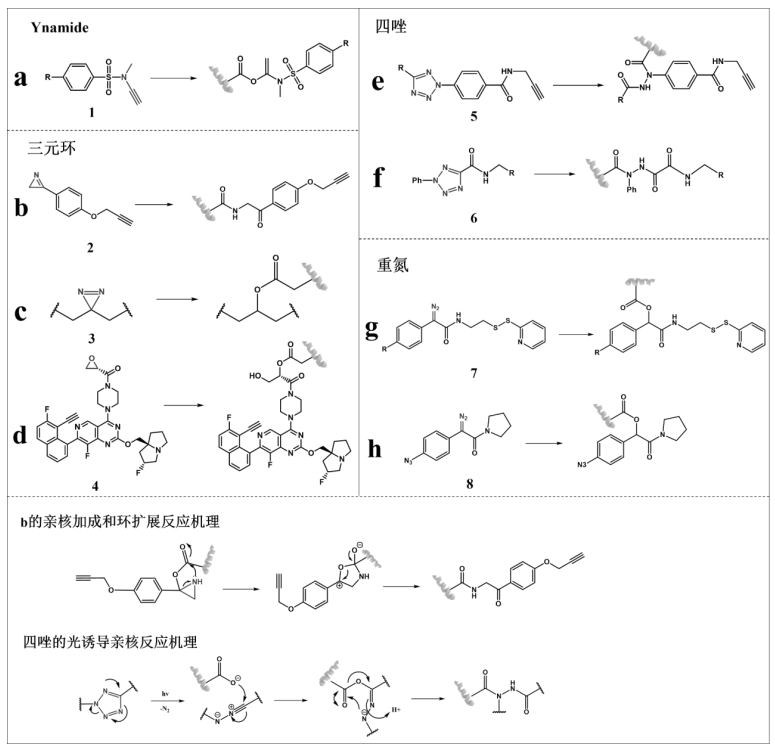
图11 谷氨酸或天冬氨酸的羧基修饰分为四类:(a)通过Ynamide试剂反应;(b)、(c)、(d)与三元环反应;(e)、(f)与四唑的反应;(g)、(h)与重氮基团反应
Fig.11 Carboxyl modifications of glutamic acid or aspartic acid are divided into four categories: (a) reaction by Ynamide reagent; (b), (c), (d) reactions with the ternary ring; (e), (f) reactions with tetrazoles; (g), (h) reactions with diazo groups
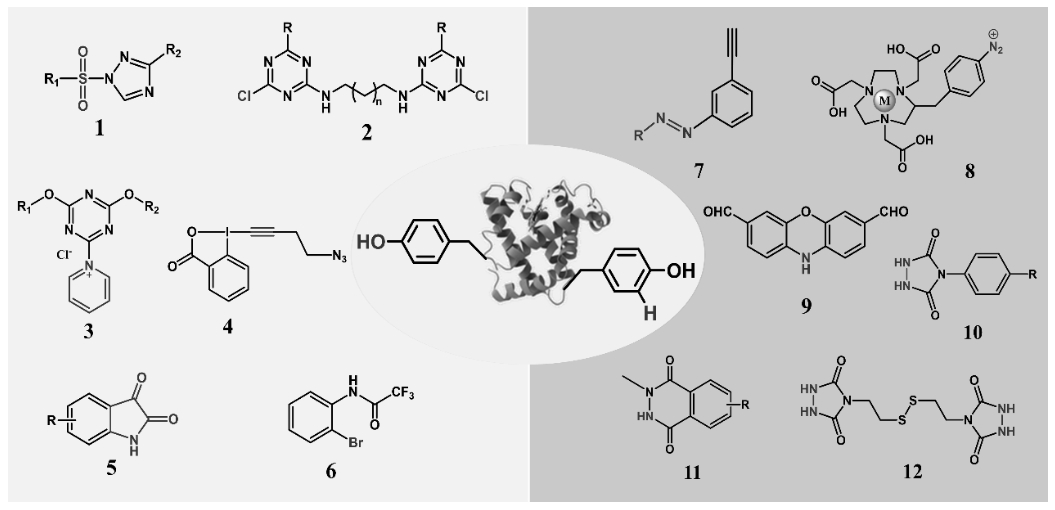
图12 酪氨酸残基两种类型的修饰:1-6为修饰酪氨酸侧链酚羟基的探针;7-12为修饰与酪氨酸侧链酚羟基邻位C上H的探针
Fig.12 Two types of modifications of tyrosine residues: 1-6 are probes for modification of the phenolic hydroxyl group of the tyrosine side chain; 7-12 is the probe that modifies H on the ortho C of the phenol hydroxyl group of the side chain of tyrosine
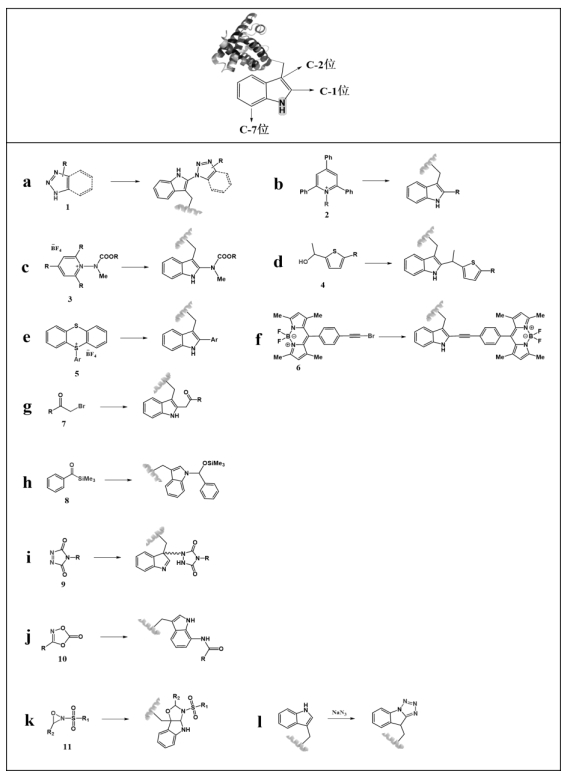
图13 色氨酸通过吲哚环上的C-1、N-H、C-2、C-7位点及不同位点环的修饰
Fig.13 Tryptophan is modified by the C-1, N-H, C-2, and C-7 sites on the indole ring and different site-specific rings
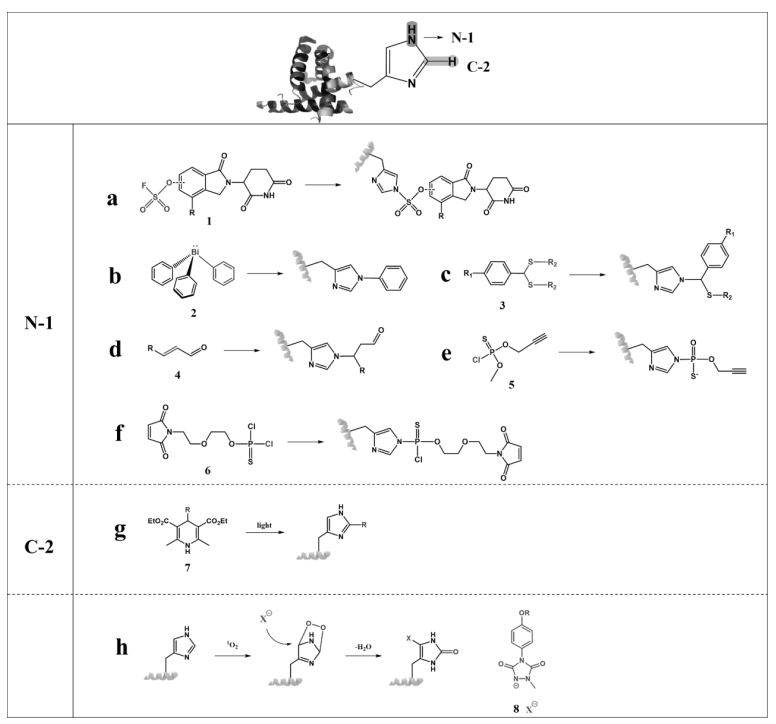
图14 组氨酸不同位点的修饰(分别为通过N-1、C-2位点进行反应,以及氧化环化反应)
Fig.14 Modification of different sites of histidine (reactions through the N-1 and C-2 sites, and oxidative cyclisation, respectively)
| 143 | Jia S, He D, Chang C J. Bioinspired thiophosphorodichloridate reagents for chemoselective histidine bioconjugation[J]. Journal of the American Chemical Society, 2019, 141(18): 7294-7301. |
| 144 | Lei H, Zhang J S, Li Y, et al. Histidine-specific bioconjugation for single-molecule force spectroscopy[J]. ACS Nano, 2022, 16(9): 15440-15449. |
| 145 | Chen X P, Ye F R, Luo X S, et al. Histidine-specific peptide modification via visible-light-promoted C-H alkylation[J]. Journal of the American Chemical Society, 2019, 141(45): 18230-18237. |
| 146 | Nakane K, Sato S, Niwa T, et al. Proximity histidine labeling by umpolung strategy using singlet oxygen[J]. Journal of the American Chemical Society, 2021, 143(20): 7726-7731. |
| 147 | López-Laguna H, Rueda A, Martínez-Torró C, et al. Biofabrication of self-assembling covalent protein nanoparticles through histidine-templated cysteine coupling[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(10): 4133-4144. |
| 148 | Pinto A M, Pereira R, Martins A J, et al. Designing an antimicrobial film for wound applications incorporating bacteriophages and ε-poly-L-lysine[J]. International Journal of Biological Macromolecules, 2024, 268. |
| 149 | Hu S N, Yue F X, Peng F, et al. Lysine-mediated surface modification of cellulose nanocrystal films for multi-channel anti-counterfeiting[J]. Carbohydrate Polymers, 2024, 340: 122315. |
| 150 | Zhang T T, Zhang D Q, Liu D D, et al. Polyaspartic acid modified by fluorescent carbon quantum dots as an environmentally friendly scale inhibitor for calcium sulphate[J]. Desalination, 2024, 584: 117740. |
| 151 | Anas M, Mandal T K. Side-chain functionality driven thermoresponsive and semicrystalline poly(L-glutamate)s and self-assembly[J]. European Polymer Journal, 2023, 199: 112469. |
| 152 | Zhao A, Cai L Z, Li R, et al. Environmental risk mitigation of lambda-cyhalothrin using tryptophan-modified magnetic nanoparticles: A sustainable emergency response strategy[J]. Chemical Engineering Journal, 2024, 490: 151808. |
| 153 | Moyosore A, Ahmad H, Latif M A M, et al. Potential of Phenylalanine‐, Tryptophan‐, and Tyrosine‐MOF‐5 Composites for Selective Carbon Dioxide and Methane Adsorption[J]. Macromolecular Theory and Simulations, 2024: 2400051. |
| 154 | Prasad S, Nayak P S, D'Silva P. A histidine-functionalized ROS scavenging hybrid nanozyme for therapeutic application in Parkinson's disease pathogenesis[J]. Materials Advances, 2024, 5(6): 2388-2399. |
| 1 | Li W Z, Li Y G, Kang J Q, et al. 4-octyl itaconate as a metabolite derivative inhibits inflammation via alkylation of STING[J]. Cell Reports, 2023, 42(3): 112145. |
| 2 | Kim S, Kim S, Kim S, et al. Affinity-directed site-specific protein labeling and its application to antibody-drug conjugates[J]. Advanced Science, 2024, 11(4): e2306401. |
| 3 | Wang H T, Jing X Y, Feng K J, et al. In situ tuning the structure of Geobacter biofilm for bioelectricity enhancement[J]. Environmental Science & Technology Letters, 2024, 11(2): 106-113. |
| 4 | Naik S S, Torris A, Choudhury N R, et al. Biodegradable and 3D printable lysine functionalized polycaprolactone scaffolds for tissue engineering applications[J]. Biomaterials Advances, 2024, 159: 213816. |
| 5 | Yu J R, Fan J, Song Y X, et al. Near-infrared fluorescent probe with large Stokes shift for specific detection of lysine[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2024, 308: 123734. |
| 6 | Reja R M, Wang W J, Lyu Y H, et al. Lysine-targeting reversible covalent inhibitors with long residence time[J]. Journal of the American Chemical Society, 2022, 144(3): 1152-1157. |
| 7 | Zhao Y Y, Duan K, Fan Y L, et al. Catalyst-free late-stage functionalization to assemble α-acyloxyenamide electrophiles for selectively profiling conserved lysine residues[J]. Communications Chemistry, 2024, 7(1): 31. |
| 8 | Kavitha V, Snega V, Viswanathamurthi P, et al. A simple selective probe for lysine detection in tablets, food samples and cells[J]. Journal of Fluorescence, 2023. |
| 9 | Xu J W, Cao F L, Lu C X, et al. Synthesis of novel fluorescence probes and their application in the enantioselective recognition of arginine[J]. RSC Advances, 2024, 14(3): 1970-1976. |
| 10 | Zhou H, Huang X Y, Zheng S N, et al. An effect fluorescence sensor for arginine based on bis-cyanodistyrene Schiff-base[J]. Journal of Molecular Structure, 2024, 1305: 137798. |
| 11 | Jun J V, Petri Y D, Erickson L W, et al. Modular diazo compound for the bioreversible late-stage modification of proteins[J]. Journal of the American Chemical Society, 2023, 145(12): 6615-6621. |
| 12 | Thomas R P, Grant E K, Dickinson E R, et al. Reactive fragments targeting carboxylate residues employing direct to biology, high-throughput chemistry[J]. RSC Medicinal Chemistry, 2023, 14(4): 671-679. |
| 13 | Li S R, Zhang P W, Xu F, et al. Ynamide electrophile for the profiling of ligandable carboxyl residues in live cells and the development of new covalent inhibitors[J]. Journal of Medicinal Chemistry, 2022, 65(15): 10408-10418. |
| 14 | Ma N, Hu J, Zhang Z M, et al. 2 H-azirine-based reagents for chemoselective bioconjugation at carboxyl residues inside live cells[J]. Journal of the American Chemical Society, 2020, 142(13): 6051-6059. |
| 15 | Yu Z T, He X Q, Wang R L, et al. Simultaneous covalent modification of K-ras(G12D) and K-ras(G12C) with tunable oxirane electrophiles[J]. Journal of the American Chemical Society, 2023, 145(37): 20403-20411. |
| 16 | Nakane K, Niwa T, Tsushima M, et al. BODIPY catalyzes proximity-dependent histidine labelling[J]. ChemCatChem, 2022, 14(9): e202200077. |
| 17 | Shindo N, Fuchida H, Sato M, et al. Selective and reversible modification of kinase cysteines with chlorofluoroacetamides[J]. Nature Chemical Biology, 2019, 15(3): 250-258. |
| 18 | Byun D P, Ritchie J, Jung Y, et al. Covalent inhibition by a natural product-inspired latent electrophile[J]. Journal of the American Chemical Society, 2023, 145(20): 11097-11109. |
| 19 | Abegg D, Tomanik M, Qiu N, et al. Chemoproteomic profiling by cysteine fluoroalkylation reveals myrocin G as an inhibitor of the nonhomologous end joining DNA repair pathway[J]. Journal of the American Chemical Society, 2021, 143(48): 20332-20342. |
| 20 | Pieters B J G E, Hintzen J C J, Grobben Y, et al. Installation of trimethyllysine analogs on intact histones via cysteine alkylation[J]. Bioconjugate Chemistry, 2019, 30(3): 952-958. |
| 21 | Liu Z, Wu Y P, Mao X, et al. Development of multifunctional synthetic nucleosomes to interrogate chromatin-mediated protein interactions[J]. Science Advances, 2023, 9(18): eade5186. |
| 22 | Wang D Y, Yu M Y, Liu N, et al. A sulfonium tethered peptide ligand rapidly and selectively modifies protein cysteine in vicinity[J]. Chemical Science, 2019, 10(19): 4966-4972. |
| 23 | Grison C M, Burslem G M, Miles J A, et al. Double quick, double click reversible peptide "stapling"[J]. Chemical Science, 2017, 8(7): 5166-5171. |
| 24 | Wan C, Zhang Y C, Wang J P, et al. Traceless peptide and protein modification via rational tuning of pyridiniums[J]. Journal of the American Chemical Society, 2024, 146(4): 2624-2633. |
| 25 | Gil de Montes E, Jiménez-Moreno E, Oliveira B L, et al. Azabicyclic vinyl sulfones for residue-specific dual protein labelling[J]. Chemical Science, 2019, 10(16): 4515-4522. |
| 26 | Wang T, Riegger A, Lamla M, et al. Water-soluble allyl sulfones for dual site-specific labelling of proteins and cyclic peptides[J]. Chemical Science, 2016, 7(5): 3234-3239. |
| 27 | Huang R, Li Z H, Sheng Y, et al. N-methyl-N-phenylvinylsulfonamides for cysteine-selective conjugation[J]. Organic Letters, 2018, 20(20): 6526-6529. |
| 28 | Ahangarpour M, Kavianinia I, Hume P A, et al. N-vinyl acrylamides: versatile heterobifunctional electrophiles for thiol-thiol bioconjugations[J]. Journal of the American Chemical Society, 2022, 144(30): 13652-13662. |
| 29 | Ariyasu S, Hayashi H, Xing B G, et al. Site-specific dual functionalization of cysteine residue in peptides and proteins with 2-azidoacrylates[J]. Bioconjugate Chemistry, 2017, 28(4): 897-902. |
| 30 | Bernardim B, Cal P M S D, Matos M J, et al. Stoichiometric and irreversible cysteine-selective protein modification using carbonylacrylic reagents[J]. Nature Communications, 2016, 7(1): 13128. |
| 31 | Baumann A L, Schwagerus S, Broi K, et al. Chemically induced vinylphosphonothiolate electrophiles for thiol-thiol bioconjugations[J]. Journal of the American Chemical Society, 2020, 142(20): 9544-9552. |
| 32 | Hou Z F, Wang D Y, Li Y, et al. A sulfonium triggered thiol-yne reaction for cysteine modification[J]. The Journal of Organic Chemistry, 2020, 85(3): 1698-1705. |
| 33 | Wang R, Yang D Y, Tian T, et al. Low-toxicity sulfonium-based probes for cysteine-specific profiling in live cells[J]. Analytical Chemistry, 2022, 94(10): 4366-4372. |
| 34 | Hartmann P, Bohdan K, Hommrich M, et al. Chemoselective umpolung of thiols to episulfoniums for cysteine bioconjugation[J]. Nature Chemistry, 2024, 16(3): 380-388. |
| 35 | Kung K K Y, Xu C F, O W Y, et al. Functionalized quinolizinium-based fluorescent reagents for modification of cysteine-containing peptides and proteins[J]. RSC Advances, 2022, 12(10): 6248-6254. |
| 36 | Maes D, Nicque M, Iftikhar M, et al. Phenylpropynones as selective disulfide rebridging bioconjugation reagents[J]. Organic Letters, 2024, 26(4): 895-899. |
| 37 | McAulay K, Hoyt E A, Thomas M, et al. Alkynyl benzoxazines and dihydroquinazolines as cysteine targeting covalent warheads and their application in identification of selective irreversible kinase inhibitors[J]. Journal of the American Chemical Society, 2020, 142(23): 10358-10372. |
| 38 | Petit E, Bosch L, Costa A M, et al. (Z)-oxopropene-1,3-diyl, a linker for the conjugation of the thiol group of cysteine with amino-derivatized drugs[J]. The Journal of Organic Chemistry, 2019, 84(17): 11170-11176. |
| 39 | Teng S H, Zhang Z G, Li B H, et al. Thiol-specific silicon-containing conjugating reagent: β-silyl alkynyl carbonyl compounds[J]. Angewandte Chemie (International Ed. In English), 2023, 62(45): e202311906. |
| 40 | Zhang Y Q, Zhou X P, Xie Y H, et al. Thiol specific and tracelessly removable bioconjugation via Michael addition to 5-methylene pyrrolones[J]. Journal of the American Chemical Society, 2017, 139(17): 6146-6151. |
| 41 | Zhang Y Q, Zang C L, An G C, et al. Cysteine-specific protein multi-functionalization and disulfide bridging using 3-bromo-5-methylene pyrrolones[J]. Nature Communications, 2020, 11(1): 1015. |
| 42 | Yu J, Yang X Y, Sun Y, et al. Highly reactive and tracelessly cleavable cysteine-specific modification of proteins via 4-substituted cyclopentenone[J]. Angewandte Chemie (International Ed. In English), 2018, 57(36): 11598-11602. |
| 43 | Ryu K A, Reyes-Robles T, Wyche T P, et al. Near-infrared photoredox catalyzed fluoroalkylation strategy for protein labeling in complex tissue environments[J]. ACS Catalysis, 2024, 14(5): 3482-3491. |
| 44 | Embaby A M, Schoffelen S, Kofoed C, et al. Rational tuning of fluorobenzene probes for cysteine-selective protein modification[J]. Angewandte Chemie (International Ed.in English), 2018, 57(27): 8022-8026. |
| 45 | Lee L C C, Tsang A W Y, Liu H W, et al. Photofunctional cyclometalated iridium(III) polypyridine complexes bearing a perfluorobiphenyl moiety for bioconjugation, bioimaging, and phototherapeutic applications[J]. Inorganic Chemistry, 2020, 59(20): 14796-14806. |
| 46 | Zhuang H L, Guo Z D, Zhuang R Q, et al. Synthesis of 18 F-radiolabeled organophosphine fluorides for thiol-chemoselective peptide conjugation[J]. Journal of Labelled Compounds & Radiopharmaceuticals, 2020, 63(14): 597-607. |
| 47 | Lipka B M, Betti V M, Honeycutt D S, et al. Rapid electrophilic cysteine arylation with pyridinium salts[J]. Bioconjugate Chemistry, 2022, 33(11): 2189-2196. |
| 48 | White A M, Dellsén A, Larsson N, et al. Late-stage functionalization with cysteine staples generates potent and selective melanocortin receptor-1 agonists[J]. Journal of Medicinal Chemistry, 2022, 65(19): 12956-12969. |
| 49 | Su X C, Zhang L Y, Zhao L N, et al. Efficient protein-protein couplings mediated by small molecules under mild conditions[J]. Angewandte Chemie (International Ed.in English), 2022, 61(35): e202205597. |
| 50 | Tallon A M, Xu Y R, West G M, et al. Thiomethyltetrazines are reversible covalent cysteine warheads whose dynamic behavior can be "switched off" via bioorthogonal chemistry inside live cells[J]. Journal of the American Chemical Society, 2023, 145(29): 16069-16080. |
| 51 | Li P Z, Tian Y C, Shang Q H, et al. Discovery of a highly potent NPAS3 heterodimer inhibitor by covalently modifying ARNT[J]. Bioorganic Chemistry, 2023, 139: 106676. |
| 52 | Prakash S, Hazari P P, Meena V K, et al. Radiolabeling and preclinical evaluation of a new S-alkylated cysteine derivative conjugated to C-substituted macrocycle for positron emission tomography[J]. ACS Omega, 2018, 3(6): 6497-6505. |
| 53 | de Munnik M, Lithgow J, Brewitz L, et al. αβ, α'β'-Diepoxyketones are mechanism-based inhibitors of nucleophilic cysteine enzymes[J]. Chemical Communications, 2023, 59(86): 12859-12862. |
| 54 | Lv S M, Xu F, Fan Y L, et al. Cyclopropenone, cyclopropeniminium ion, and cyclopropenethione as novel electrophilic warheads for potential target discovery of triple-negative breast cancer[J]. Journal of Medicinal Chemistry, 2023, 66(4): 2851-2864. |
| 55 | Förster T, Shang E C, Shimizu K, et al. 2-sulfonylpyrimidines target the kinesin HSET via cysteine alkylation[J]. European Journal of Organic Chemistry, 2019, 2019(31-32): 5486-5496. |
| 56 | Li K C, Wang W J, Gao J M. Fast and stable N-terminal cysteine modification through thiazolidino boronate mediated acyl transfer[J]. Angewandte Chemie International Edition, 2020, 59(34): 14246-14250. |
| 57 | Wan C, Yang D Y, Guo X C, et al. β-Carbonyl sulfonium enables cysteine-specific bioconjugation for activity-based protein profiling in live cells[J]. Chemical Communications, 2024, 60(27): 3725-3728. |
| 58 | An S X, Lin Y F, Ye T Q, et al. An extra-large Stokes shift near-infrared fluorescent probe for specific detection and imaging of cysteine[J]. Talanta, 2024, 267: 125247. |
| 59 | An S X, Lin Y F, Wang J B, et al. Near-infrared mitochondria-targeted fluorescent probe with a large Stokes shift for rapid and sensitive detection of cysteine/homocysteine and its bioimaging application[J]. Sensors and Actuators B: Chemical, 2023, 374: 132799. |
| 60 | Qi S L, Zhang H Y, Wang X Y, et al. Development of a NIR fluorescent probe for highly selective and sensitive detection of cysteine in living cells and in vivo[J]. Talanta, 2021, 234: 122685. |
| 61 | Gu Q S, Yang Z C, Chao J J, et al. Tumor-targeting probe for dual-modal imaging of cysteine in vivo[J]. Analytical Chemistry, 2023, 95(33): 12478-12486. |
| 62 | An J M, Jeong M, Jung J, et al. Next-generation femtech: urine-based cervical cancer diagnosis using a fluorescent biothiol probe with controlled smiles rearrangement[J]. ACS Applied Materials & Interfaces, 2024, 16(4): 4493-4504. |
| 63 | Zhang Y D, Wang X, Bai X Y, et al. Highly specific cys fluorescence probe for living mouse brain imaging via evading reaction with other biothiols[J]. Analytical Chemistry, 2019, 91(13): 8591-8594. |
| 64 | Wu T H, Huang S Q, Feng X, et al. Visualizing drug release from a stimuli-responsive soft material based on amine-thiol displacement[J]. ACS Applied Materials & Interfaces, 2023, 15(19): 22967-22976. |
| 65 | Li S J, Wang P P, Ye M T, et al. Cysteine-activatable near-infrared fluorescent probe for dual-channel tracking lipid droplets and mitochondria in epilepsy[J]. Analytical Chemistry, 2023, 95(11): 5133-5141. |
| 66 | Wang B J, Liu R J, Fang J G, et al. A water-soluble dual-site fluorescent probe for the rapid detection of cysteine with high sensitivity and specificity[J]. Chemical Communications, 2019, 55(78): 11762-11765. |
| 67 | Wang Z L, Zhang Y, Liang Y Y, et al. Novel bis-camphor-derived colorimetric and fluorescent probe for rapid and visual detection of cysteine and its versatile applications in food analysis and biological imaging[J]. Journal of Agricultural and Food Chemistry, 2022, 70(2): 669-679. |
| 68 | Zhang X Y, Liu H, Ma Y Y, et al. Development of a novel near-infrared fluorescence light-up probe with a large Stokes shift for sensing of cysteine in aqueous solution, living cells and zebrafish[J]. Dyes and Pigments, 2019, 171: 107722. |
| 69 | Wang H, Zhang Y X, Yang Y Y, et al. In situ photoacoustic imaging of cysteine to reveal the mechanism of limited GSH synthesis in pulmonary fibrosis[J]. Chemical Communications, 2019, 55(65): 9685-9688. |
| 70 | Qiao L Q, Yang Y X, Cai J H, et al. Long wavelength emission fluorescent probe for highly selective detection of cysteine in living cells[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2022, 264: 120247. |
| 71 | Liu D J, Lv Y, Chen M, et al. A long wavelength emission two-photon fluorescent probe for highly selective detection of cysteine in living cells and an inflamed mouse model[J]. Journal of Materials Chemistry B, 2019, 7(25): 3970-3975. |
| 72 | Zhao L H, He X, Huang Y B, et al. A novel near-infrared fluorescent probe for intracellular detection of cysteine [J]. Analytical and Bioanalytical Chemistry, 2020, 412(26): 7211-7217. |
| 73 | Qin J C, Wang J, Bian Y, et al. D-A-D type based NIR fluorescence probe for monitoring the cysteine levels in pancreatic cancer cell during ferroptosis[J]. Bioorganic Chemistry, 2024, 146: 107260. |
| 74 | Zhou L P, Yang T A, Zhang T R, et al. A novel dual-function fluorescent probe for the detection of cysteine and its applications in vitro[J]. Talanta, 2024, 272: 125769. |
| 75 | Li X, Liu M, Yi Q Y, et al. A hepatocyte-targeting fluorescent probe of dicyanomethylene-4H-pyran to detect cysteine in living cells and zebrafish: Design, synthesis and evaluation[J]. Dyes and Pigments, 2024, 224: 112031. |
| 76 | Kavitha V, Viswanathamurthi P, Haribabu J, et al. An aqueous mediated ultrasensitive facile probe incorporated with acrylate moiety to monitor cysteine in food samples and live cells[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2023, 293: 122447. |
| 77 | Ding X D, Yang B, Liu Z L, et al. A novel intramolecular charge transfer-based near-infrared fluorescent probe with large Stokes shift for highly sensitive detection of cysteine in vivo[J]. Analytica Chimica Acta, 2023, 1280: 341873. |
| 78 | Luo L, Guo R R, Wang L J, et al. A novel fluorescent probe with a large Stokes shift for colorimetric and selective detection of cysteine in water, milk, cucumber, pear and tomato[J]. Analytical Methods: Advancing Methods and Applications, 2024, 16(15): 2322-2329. |
| 79 | Liu H B, Xu H, Guo X, et al. A novel near-infrared fluorescent probe based on isophorone for the bioassay of endogenous cysteine[J]. Organic & Biomolecular Chemistry, 2021, 19(4): 873-877. |
| 80 | Huang H J, Ji X R, Jiang Y Q, et al. NBD-based fluorescent probes for separate detection of cysteine and biothiols via different reactivities[J]. Organic & Biomolecular Chemistry, 2020, 18(21): 4004-4008. |
| 81 | Guo A D, Wei D, Nie H J, et al. Light-induced primary amines and o-nitrobenzyl alcohols cyclization as a versatile photoclick reaction for modular conjugation[J]. Nature Communications, 2020, 11(1): 5472. |
| 82 | Zhang J, Liu J L, Li X F, et al. Unexpected cyclization product discovery from the photoinduced bioconjugation chemistry between tetrazole and amine[J]. Journal of the American Chemical Society, 2024, 146(3): 2122-2131. |
| 83 | Zheng M M, Chen F J, Li K C, et al. Lysine-targeted reversible covalent ligand discovery for proteins via phage display[J]. Journal of the American Chemical Society, 2022, 144(34): 15885-15893. |
| 84 | Yi S D, Wei S Y, Wu Q S, et al. Azaphilones as activation-free primary-amine-specific bioconjugation reagents for peptides, proteins and lipids[J]. Angewandte Chemie (International Ed. in English), 2022, 61(6): e202111783. |
| 85 | Nong K Y, Zhao Y L, Yi S D, et al. 3-acyl-4-pyranone as a lysine residue-selective bioconjugation reagent for peptide and protein modification[J]. Bioconjugate Chemistry, 2024, 35(3): 286-299. |
| 86 | Wan C, Yang D Y, Song C L, et al. A pyridinium-based strategy for lysine-selective protein modification and chemoproteomic profiling in live cells[J]. Chemical Science, 2024, 15(14): 5340-5348. |
| 87 | Adusumalli S R, Rawale D G, Thakur K, et al. Chemoselective and site-selective lysine-directed lysine modification enables single-site labeling of native proteins[J]. Angewandte Chemie (International Ed. in English), 2020, 59(26): 10332-10336. |
| 88 | Shen D, Jin W H, Zhao Q, et al. Covalent solvatochromic proteome stress sensor based on the schiff base reaction[J]. Analytical Chemistry, 2022, 94(41): 14143-14150. |
| 89 | Guo P, Chu X, Wu C J, et al. Peptide stapling by crosslinking two amines with α-ketoaldehydes through diverse modified glyoxal-lysine dimer linkers[J]. Angewandte Chemie (International Ed. in English), 2024, 63(16): e202318893. |
| 90 | Gabizon R, Tivon B, Reddi R N, et al. A simple method for developing lysine targeted covalent protein reagents[J]. Nature Communications, 2023, 14(1): 7933. |
| 91 | Gao F, Chang M Y, Meng X, et al. Site-selective modification of secondary amine moieties on native peptides, proteins, and natural products with ynones[J]. Bioconjugate Chemistry, 2023, 34(9): 1553-1562. |
| 92 | Wang T R, Pang Q D, Tong Z P, et al. A hydrazone-based spectroscopic off-on probe for sensing of basic arginine and lysine[J]. Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 2021, 258: 119824. |
| 93 | Liao P, Yu X Z, Fan C L, et al. Acrylate-guided chemoselective fluorescent detection of arginine and lysine in aqueous media[J]. Dyes and Pigments, 2023, 215: 111288. |
| 94 | Huang J R, Zhao H L, Yu X Z, et al. An oxime-based fluorescent-ON probe for the rapid detection of arginine and lysine in aqueous solution[J]. Dyes and Pigments, 2023, 217: 111414. |
| 95 | Pan Y Q, Xu X, Zhang Y, et al. A highly sensitive and selective bis(salamo)-type fluorescent chemosensor for identification of Cu2+ and the continuous recognition of S2-, Arginine and Lysine[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2020, 229: 117927. |
| 96 | Yang B, Zhou J H, Huang X, et al. A new pyrroloquinoline-derivative-based fluorescent probe for the selective detection and cell imaging of lysine[J]. Pharmaceuticals, 2022, 15(4): 474. |
| 97 | Jiang X D, Yue S, Jia L, et al. NIR fluorescent AzaBODIPY-based probe for the specific detection of L-lysine[J]. ChemistrySelect, 2018, 3(26): 7581-7585. |
| 98 | Yang J Q, Jiang L, Tian J, et al. Fluorous phase-enhanced fluorescent sensitivity for enantioselective recognition of lysine[J]. Organic Letters, 2022, 24(50): 9327-9331. |
| 99 | Yu X Z, Zhang B J, Liao P, et al. A chemoselective fluorescent probe for arginine in aqueous phase[J]. Dyes and Pigments, 2022, 203: 110339. |
| 100 | Yu X Z, Zhang B J, Fan C L, et al. Rapid, enantioselective and colorimetric detection of D-arginine[J]. iScience, 2022, 25(9): 104964. |
| 101 | Shang X F, Li J, Guo K R, et al. Development and cytotoxicity of Schiff base derivative as a fluorescence probe for the detection of L-Arginine[J]. Journal of Molecular Structure, 2017, 1134: 369-373. |
| 102 | Li J, Zhang Y, Chen Y M, et al. Synthesis, binding ability, and cell cytotoxicity of fluorescent probes for L-arginine detection based on naphthalene derivatives: Experiment and theory[J]. Journal of Molecular Recognition, 2018, 31(1): e2657. |
| 103 | Jia Y M, Lu M M, Cui S Q, et al. A new diarylethene based chemosensor for colorimetric recognition of arginine and fluorescent detection of Cu2+ [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2022, 423: 113592. |
| 104 | Shih C T, Kuo B H, Tsai C Y, et al. Dibenzocyclooctendiones (DBCDOs): arginine-selective chemical labeling reagents obtained through benzilic acid rearrangement[J]. Organic Letters, 2022, 24(25): 4694-4698. |
| 105 | Xuan W M, Ma J N. Pinpointing acidic residues in proteins[J]. ChemMedChem, 2024, 19(5): e202300623. |
| 106 | Li S R, Zhu C J, Zhao Q, et al. Ynamide coupling reagent for the chemical cross-linking of proteins in live cells[J]. ACS Chemical Biology, 2023, 18(6): 1405-1415. |
| 107 | West A V, Muncipinto G, Wu H Y, et al. Labeling preferences of diazirines with protein biomolecules[J]. Journal of the American Chemical Society, 2021, 143(17): 6691-6700. |
| 108 | Bach K, Beerkens B L H, Zanon P R A, et al. Light-activatable, 2,5-disubstituted tetrazoles for the proteome-wide profiling of aspartates and glutamates in living bacteria[J]. ACS Central Science, 2020, 6(4): 546-554. |
| 109 | Zhang X R, Huang H R, Liu Y, et al. Optical control of protein functions via genetically encoded photocaged aspartic acids[J]. Journal of the American Chemical Society, 2023, 145(35): 19218-19224. |
| 110 | Petri Y D, Gutierrez C S, Raines R T. Chemoselective caging of carboxyl groups for on-demand protein activation with small molecules[J]. Angewandte Chemie (International Ed. in English), 2023, 62(22): e202215614. |
| 111 | Brulet J W, Borne A L, Yuan K, et al. Liganding functional tyrosine sites on proteins using sulfur-triazole exchange chemistry[J]. Journal of the American Chemical Society, 2020, 142(18): 8270-8280. |
| 112 | Huang T, Hosseinibarkooie S, Borne A L, et al. Chemoproteomic profiling of kinases in live cells using electrophilic sulfonyl triazole probes[J]. Chemical Science, 2021, 12(9): 3295-3307. |
| 113 | Zhang Y, Yin R J, Jiang H, et al. Peptide stapling through site-directed conjugation of triazine moieties to the tyrosine residues of a peptide[J]. Organic Letters, 2023, 25(13): 2248-2252. |
| 114 | Jiang H F, Zhang Q, Zhang Y, et al. Triazine-pyridine chemistry for protein labelling on tyrosine[J]. Chemical Communications, 2022, 58(50): 7066-7069. |
| 115 | Declas N, Maynard J R J, Menin L, et al. Tyrosine bioconjugation with hypervalent iodine[J]. Chemical Science, 2022, 13(43): 12808-12817. |
| 116 | You S Q, Wang R T, Ma C, et al. Electrochemical chemoselective hydroxyl group transformation: anthranilic acyl modification of tyrosine bioconjugations[J]. Organic Chemistry Frontiers, 2023, 10(18): 4606-4615. |
| 117 | Chinn A J, Hwang J, Kim B, et al. Application of high-throughput competition experiments in the development of aspartate-directed site-selective modification of tyrosine residues in peptides[J]. The Journal of Organic Chemistry, 2020, 85(14): 9424-9433. |
| 118 | Sun F X, Suttapitugsakul S, Wu R H. An azo coupling-based chemoproteomic approach to systematically profile the tyrosine reactivity in the human proteome[J]. Analytical Chemistry, 2021, 93(29): 10334-10342. |
| 119 | Leier S, Richter S, Bergmann R, et al. Radiometal-containing aryl diazonium salts for chemoselective bioconjugation of tyrosine residues[J]. ACS Omega, 2019, 4(26): 22101-22107. |
| 120 | San Segundo M, Correa A. Site-selective aqueous C-H acylation of tyrosine-containing oligopeptides with aldehydes[J]. Chemical Science, 2020, 11(42): 11531-11538. |
| 121 | Li B X, Kim D K, Bloom S, et al. Site-selective tyrosine bioconjugation via photoredox catalysis for native-to-bioorthogonal protein transformation[J]. Nature Chemistry, 2021, 13(9): 902-908. |
| 122 | Depienne S, Alvarez-Dorta D, Croyal M, et al. Luminol anchors improve the electrochemical-tyrosine-click labelling of proteins[J]. Chemical Science, 2021, 12(46): 15374-15381. |
| 123 | Depienne S, Bouzelha M, Courtois E, et al. Click-electrochemistry for the rapid labeling of virus, bacteria and cell surfaces[J]. Nature Communications, 2023, 14(1): 5122. |
| 124 | Cui L L, Ma Y G, Li M, et al. Tyrosine-reactive cross-linker for probing protein three-dimensional structures[J]. Analytical Chemistry, 2021, 93(10): 4434-4440. |
| 125 | Keyes E D, Mifflin M C, Austin M J, et al. Chemoselective, oxidation-induced macrocyclization of tyrosine containing peptides[J]. Journal of the American Chemical Society, 2023, 145(18): 10071-10081. |
| 126 | Watanabe S, Wada Y, Kawano M, et al. Selective modification of tryptophan in polypeptides via C-N coupling with azoles using in situ-generated iodine-based oxidants in aqueous media[J]. Chemical Communications, 2023, 59(87): 13026-13029. |
| 127 | Laroche B, Tang X J, Archer G, et al. Photochemical chemoselective alkylation of tryptophan-containing peptides[J]. Organic Letters, 2021, 23(2): 285-289. |
| 128 | Tower S J, Hetcher W J, Myers T E, et al. Selective modification of tryptophan residues in peptides and proteins using a biomimetic electron transfer process[J]. Journal of the American Chemical Society, 2020, 142(20): 9112-9118. |
| 129 | Hoopes C R, Garcia F J, Sarkar A M, et al. Donor-acceptor pyridinium salts for photo-induced electron-transfer driven modification of tryptophan in peptides, proteins, and proteomes using visible light[J]. Journal of the American Chemical Society, 2022, 144(14): 6227-6236. |
| 130 | Nuruzzaman M, Colella B M, Uzoewulu C P, et al. Hexafluoroisopropanol as a bioconjugation medium of ultrafast, tryptophan-selective catalysis[J]. Journal of the American Chemical Society, 2024, 146(10): 6773-6783. |
| 131 | Kaplaneris N, Puet A, Kallert F, et al. Late-stage C-H functionalization of tryptophan-containing peptides with thianthrenium salts: conjugation and ligation[J]. Angewandte Chemie (International Ed. in English), 2023, 62(9): e202216661. |
| 132 | Kaplaneris N, Son J, Mendive-Tapia L, et al. Chemodivergent manganese-catalyzed C-H activation: modular synthesis of fluorogenic probes[J]. Nature Communications, 2021, 12(1): 3389. |
| 155 | Suganthi S, Vignesh S, Al-Ansari M M, et al. Development of PVA/sodium alginate incorporated with histidine capped silver nanoparticles for food packaging application[J]. Polymers for Advanced Technologies, 2024, 35(5). |
| 156 | Dadakhani S, Dehghan G, Khataee A, et al. Design and application of histidine-functionalized ZnCr-LDH nanozyme for promoting bacteria-infected wound healing[J]. RSC Advances, 2024, 14(2): 1195-1206. |
| 157 | Bhutani P, Joshi G, Raja N, et al. U.S. FDA approved drugs from 2015-June 2020: a perspective[J]. Journal of Medicinal Chemistry, 2021, 64(5): 2339-2381. |
| 158 | Jiang L L, Liu S X, Jia X L, et al. ABPP-CoDEL: activity-based proteome profiling-guided discovery of tyrosine targeting covalent inhibitors from DNA-encoded libraries[J]. Journal of the American Chemical Society, 2023, 145(46): 25283-25292. |
| 133 | Lima R N, Delgado J A C, Bernardi D I, et al. Post-synthetic functionalization of tryptophan protected peptide sequences through indole (C-2) photocatalytic alkylation[J]. Chemical Communications, 2021, 57(47): 5758-5761. |
| 134 | Reimler J, Studer A. Visible-light mediated tryptophan modification in oligopeptides employing acylsilanes[J]. Chemistry, 2021, 27(62): 15392-15395. |
| 135 | Decoene K W, Unal K, Staes A, et al. Triazolinedione protein modification: from an overlooked off-target effect to a tryptophan-based bioconjugation strategy[J]. Chemical Science, 2022, 13(18): 5390-5397. |
| 136 | Wang W, Wu J, Kuniyil R, et al. Peptide late-stage diversifications by rhodium-catalyzed tryptophan C7 amidation[J]. Chem, 2020, 6(12): 3428-3439. |
| 137 | Xie X, Moon P J, Crossley S W M, et al. Oxidative cyclization reagents reveal tryptophan cation–π interactions[J]. Nature, 2024, 627(8004): 680-687. |
| 138 | Weng Y Y, Xu X B, Chen H T, et al. Tandem electrochemical oxidative azidation/heterocyclization of tryptophan containing peptides under buffer conditions[J]. Angewandte Chemie (International Ed. in English), 2022, 61(41): e202206308. |
| 139 | Cruite J T, Nowak R P, Donovan K A, et al. Covalent stapling of the cereblon sensor loop histidine using sulfur heterocycle exchange[J]. ACS Medicinal Chemistry Letters, 2023, 14(11): 1576-1581. |
| 140 | Chan H C, Bueno B, Le Roch A, et al. Copper-promoted N-arylation of the imidazole side chain of protected histidine by using triarylbismuth reagents[J]. Chemistry – A European Journal, 2021, 27(53): 13330-13336. |
| 141 | Wan C, Wang Y N, Lian C S, et al. Histidine-specific bioconjugation via visible-light-promoted thioacetal activation[J]. Chemical Science, 2022, 13(28): 8289-8296. |
| 142 | Li J Y, Zhou J H, Xu H, et al. ACR-based probe for the quantitative profiling of histidine reactivity in the human proteome[J]. Journal of the American Chemical Society, 2023, 145(9): 5252-5260. |
| [1] | 李云璇, 刘新悦, 陈熙, 刘文, 周明月, 蓝兴英. 基于固液氧化还原靶向反应的能量存储技术:材料、器件及动力学[J]. 化工学报, 2024, 75(4): 1222-1240. |
| [2] | 常蕊, 邢蕊蕊, 闫学海. 基于非共价化学的绿色生物可循环肽材料[J]. 化工学报, 2024, 75(4): 1317-1332. |
| [3] | 李诗浩, 吴振华, 赵展烽, 吴洪, 杨冬, 石家福, 姜忠义. 化工过程中的电子传递、质子传递和分子传递[J]. 化工学报, 2024, 75(3): 1052-1064. |
| [4] | 咸国义, 陈立芳, 漆志文. 基于DFT的环己酮肟液相贝克曼重排机理研究[J]. 化工学报, 2024, 75(1): 302-311. |
| [5] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [6] | 何晓崐, 刘锐, 薛园, 左然. MOCVD生长AlN单晶薄膜的气相和表面化学反应综述[J]. 化工学报, 2023, 74(7): 2800-2813. |
| [7] | 陈雅鑫, 袁航, 刘冠章, 毛磊, 杨纯, 张瑞芳, 张光亚. 蛋白质纳米笼介导的酶自固定化研究进展[J]. 化工学报, 2023, 74(7): 2773-2782. |
| [8] | 张全碧, 羊依金, 郭旭晶. 芬顿氧化法对利福平制药废水中溶解性有机物的催化降解[J]. 化工学报, 2023, 74(5): 2217-2227. |
| [9] | 侯文起, 孙彦, 董晓燕. 碱化修饰甲状腺素运载蛋白显著增强对淀粉样β蛋白聚集的抑制作用[J]. 化工学报, 2023, 74(5): 2100-2110. |
| [10] | 禹进, 余彬彬, 蒋新生. 一种基于虚拟组分的燃烧调控化学作用量化及分析方法研究[J]. 化工学报, 2023, 74(3): 1303-1312. |
| [11] | 周光正, 王学重, 周浩宇. 溶酶菌蛋白质结晶的多目标优化与模拟[J]. 化工学报, 2023, 74(10): 4191-4200. |
| [12] | 安绍杰, 许洪峰, 李思, 许远航, 李佳锡. 利用分子机器的组装与分解构建pH敏感性谷胱甘肽过氧化物人工酶[J]. 化工学报, 2022, 73(8): 3669-3678. |
| [13] | 郑默, 李晓霞. ReaxFF MD模拟揭示的煤热解挥发分自由基反应的竞争与协调[J]. 化工学报, 2022, 73(6): 2732-2741. |
| [14] | 李岩, 田阿慧, 周毅. 反应性双射流中标量输运和化学反应特性[J]. 化工学报, 2022, 73(5): 1947-1963. |
| [15] | 余彬彬, 蒋新生, 禹进, 蔡运雄, 李玉玺, 何东海, 于佳佳. 全氟己酮抑制航空煤油燃烧实验及化学动力学研究[J]. 化工学报, 2022, 73(4): 1834-1844. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号
