化工学报 ›› 2019, Vol. 70 ›› Issue (3): 801-816.DOI: 10.11949/j.issn.0438-1157.20180965
骆枫1,2( ),林力1,李振臣1,李文钰1,陈先林1,沙沙1,罗涛2(
),林力1,李振臣1,李文钰1,陈先林1,沙沙1,罗涛2( )
)
收稿日期:2018-08-27
修回日期:2018-11-02
出版日期:2019-03-05
发布日期:2019-03-05
通讯作者:
罗涛
作者简介:<named-content content-type="corresp-name">骆枫</named-content>(1989—),男,博士,助理研究员,<email>luofenghxf@foxmail.com</email>|罗涛(1987—),男,博士,助理研究员,<email>tao.luo@scu.edu.cn</email>
Feng LUO1,2( ),Li LIN1,Zhenchen LI1,Wenyu LI1,Xianlin CHEN1,Sha SHA1,Tao LUO2(
),Li LIN1,Zhenchen LI1,Wenyu LI1,Xianlin CHEN1,Sha SHA1,Tao LUO2( )
)
Received:2018-08-27
Revised:2018-11-02
Online:2019-03-05
Published:2019-03-05
Contact:
Tao LUO
摘要:
生物质通过电化学转化合成燃料和高附加值化学品是未来化学工业发展的一个重要方向,也是现代社会实现可持续发展的重要保障。在可再生能源产能不断提升,而现阶段暂无成熟的大规模能源存储技术的背景下,如何有效地利用可再生能源所产电能进行生物质的电化学转化是目前学术界和工业界关注的一个热点。本文介绍了近年来该领域的研究进展,着重阐释了关键的电化学反应和相关反应器的设计。从生物质衍生的平台分子的电化学转化取得了一定的进展,然而从生物质到平台分子的电化学转化还面临较大的挑战。提高平台分子和生物质电化学反应的选择性有赖于合适的电极材料和催化剂,而将原位分离与电极反应耦合的设计能够提高产物的收率,特别是在生物质直接电化学转化的过程中。
中图分类号:
骆枫, 林力, 李振臣, 李文钰, 陈先林, 沙沙, 罗涛. 生物质的电化学转化反应及反应器[J]. 化工学报, 2019, 70(3): 801-816.
Feng LUO, Li LIN, Zhenchen LI, Wenyu LI, Xianlin CHEN, Sha SHA, Tao LUO. Electrochemical reactions and reactors for biomass valorisation[J]. CIESC Journal, 2019, 70(3): 801-816.
| Item | Cellulose | Hemicellulose | Lignin |
|---|---|---|---|
| contents/%(mass) | 40—45 | 25—35 | 15—30 |
| monomer | D-glucose | C5 sugars (xylose) | 3 phenols |
| polymer(chain) | linear,β-1,4 glucosidic | brached | cross-linked, 3D network |
| Mw | 50—2500 | 50—400 | huge |
| crystallinity | crystalline | amorphous | amorphous |
| solubility | water[-],organics[-] | water[-] | water[-] |
| solvents | dilute H2SO4, Cu(NH3)4(OH)2 | dilute acid,base | strong base |
| hydrolysis | H2SO4 solutions | dilute acid,base | — |
表1 木质纤维素的组成及特点
Table 1 Characteristics of lignocellulose components
| Item | Cellulose | Hemicellulose | Lignin |
|---|---|---|---|
| contents/%(mass) | 40—45 | 25—35 | 15—30 |
| monomer | D-glucose | C5 sugars (xylose) | 3 phenols |
| polymer(chain) | linear,β-1,4 glucosidic | brached | cross-linked, 3D network |
| Mw | 50—2500 | 50—400 | huge |
| crystallinity | crystalline | amorphous | amorphous |
| solubility | water[-],organics[-] | water[-] | water[-] |
| solvents | dilute H2SO4, Cu(NH3)4(OH)2 | dilute acid,base | strong base |
| hydrolysis | H2SO4 solutions | dilute acid,base | — |
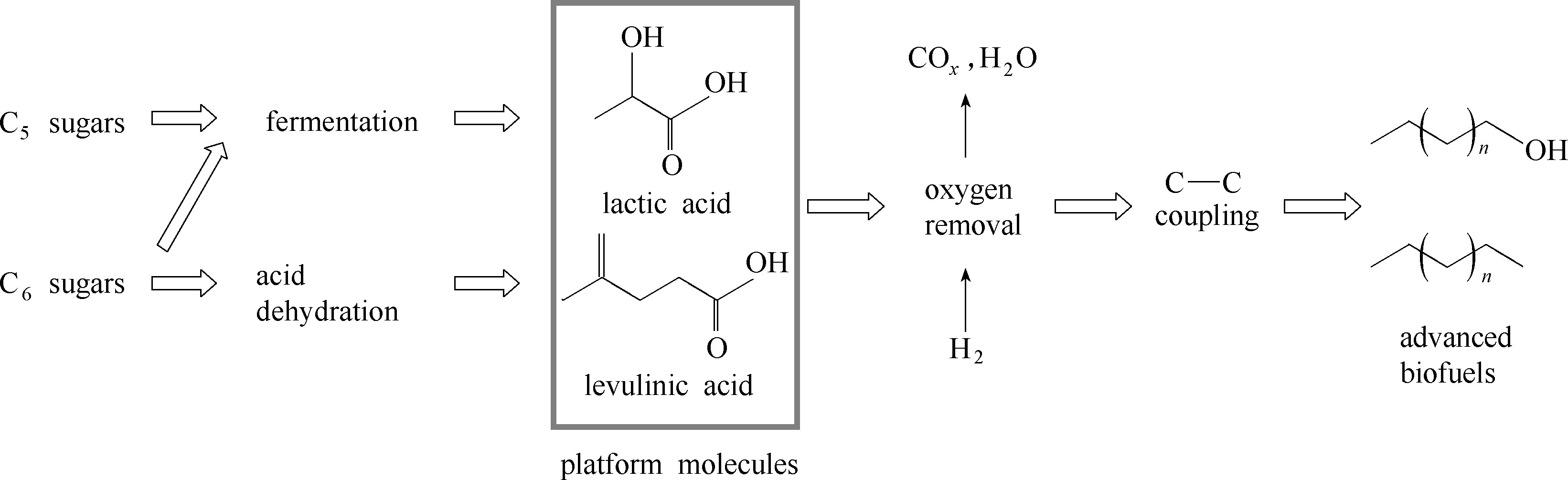
图2 作为平台分子的有机酸是从木质纤维素到生物燃料转化路径上的关键节点[34]
Fig.2 Organic acids, platform molecules derived from lignocellulose, stand at the crossroad of lignocellulose conversion route to advanced biofuels[34]
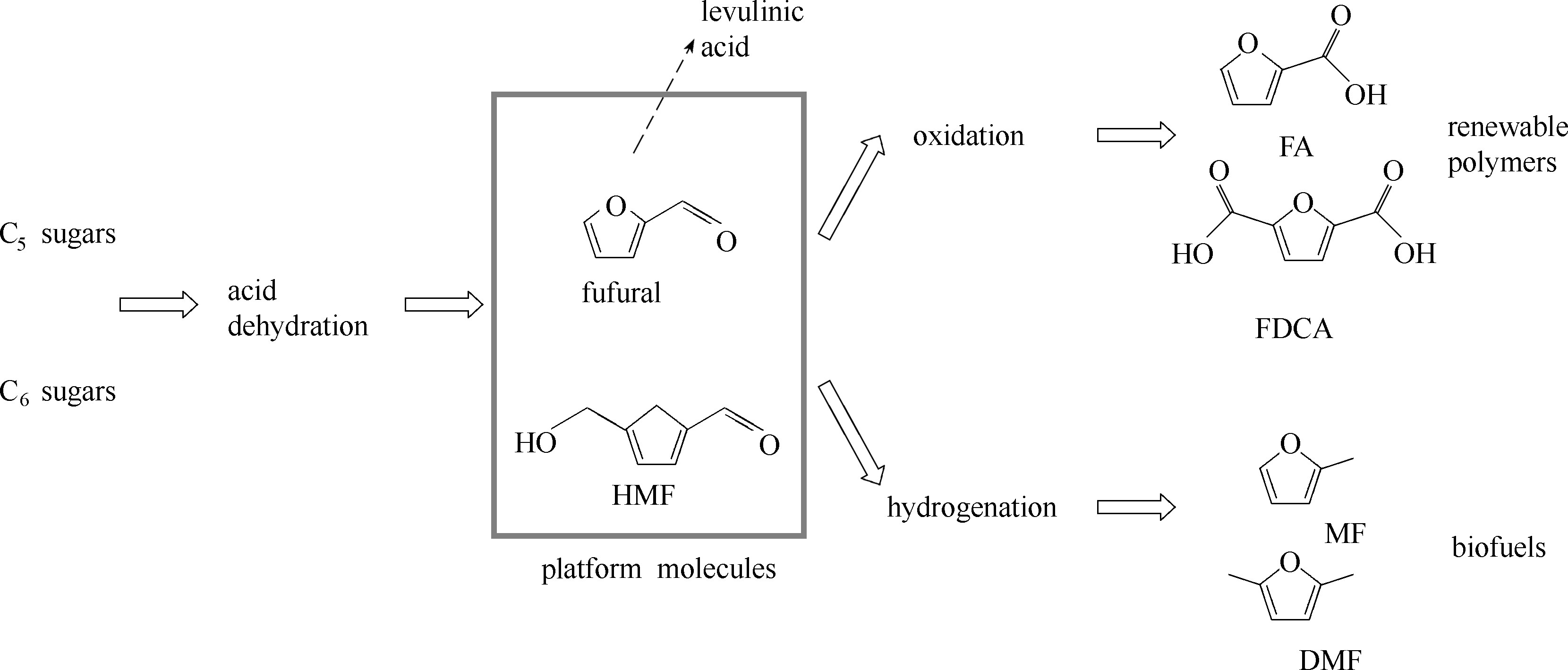
图3 糠醛 (fufural)和5-羟甲基糠醛 (5-hydroxylmethylfufural, HMF),作为糖类衍生的代表性平台分子,可以被转化为合成可再生聚合物所需的单体;还原后也可以直接作为生物燃料[23]。糠醛也可以被转化为乙酰丙酸 (图中虚线箭头),从而进入图2所示的转化路径
Fig.3 Fufural and 5-hydroxylmethylfufural (HMF) are representative platform molecules that can be (electrochemically) converted to monomers for renewable polymers (FA, fufuranic acid; FDCA, 2,5-furandicarboxylic acid) and biofuels (MF, 2-methylfuran; DMF, 2,5-dimethylfuran)[23]. Fufural can also be converted to levulinic acid (the dashed arrow), then undergoes another route of transformation as shown in Fig.2
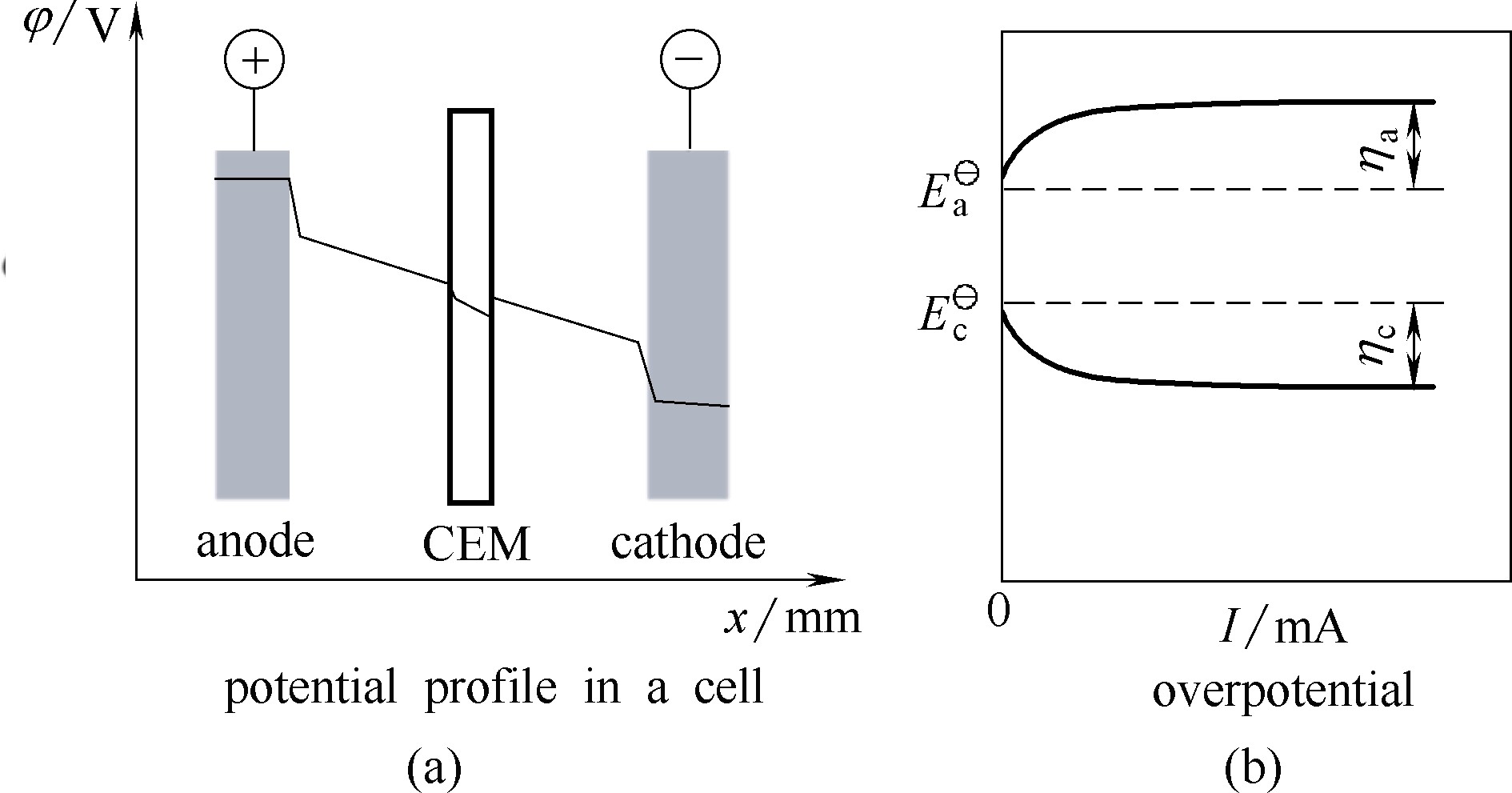
图4 电解池内的电势曲线 (a), 其中CEM指阳离子交换膜,是一种用于分隔阳极和阴极电解液的典型隔膜[49];阳极过电势 (ηa) 和阴极过电势 (ηc) 随电流的变化 (b)[50]
Fig.4 Potential profile in an electrolyzer for electrochemical conversion of biomass (a). CEM denotes cation exchange membrane, which is a representative separator between anolyte and catholyte[49]. Anodic overpotential (ηa) and cathodic overpential (ηc) as a function of cell current (b)[50]
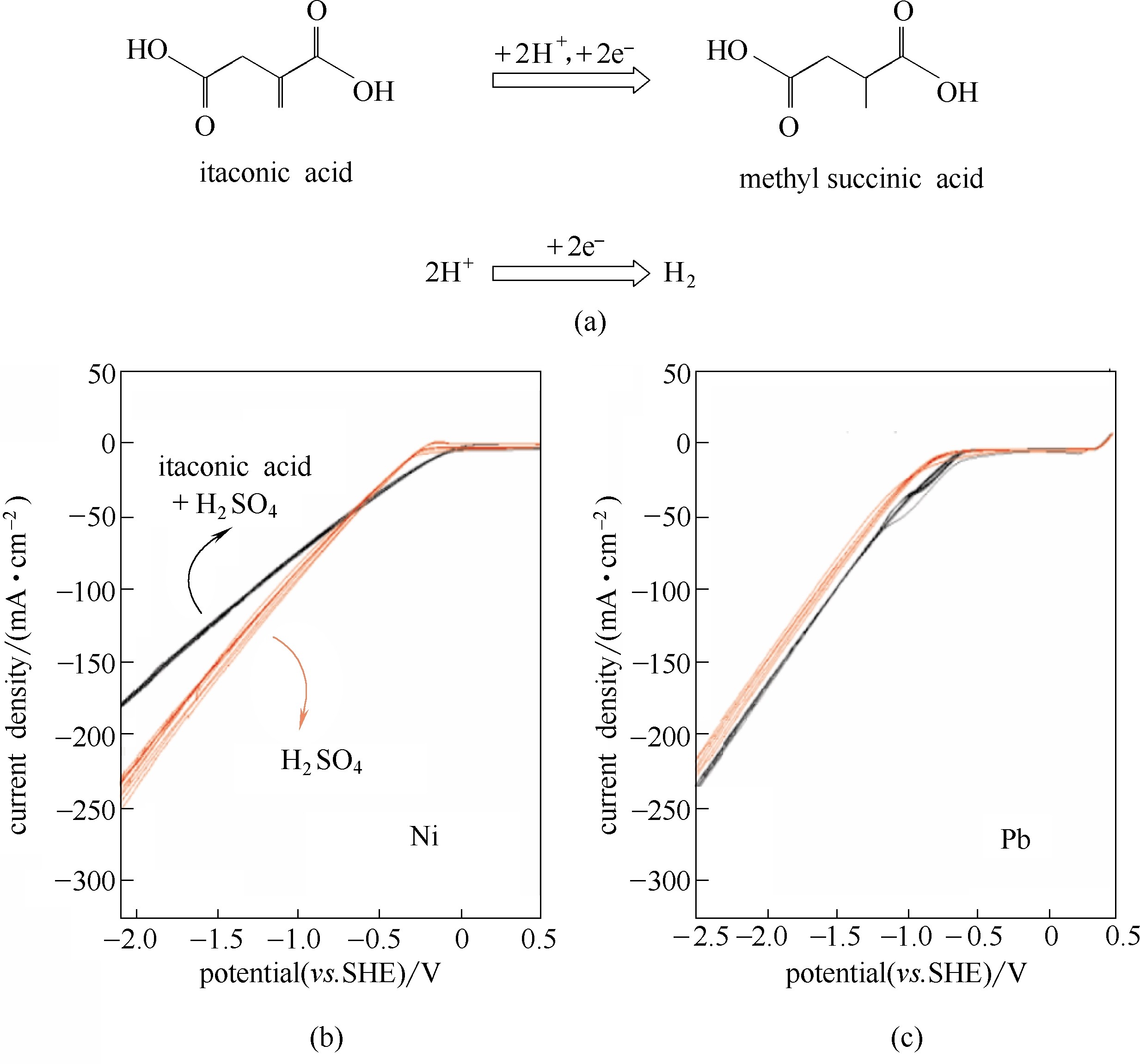
图7 衣康酸电催化还原成甲基琥珀酸的反应,以及竞争性的析氢反应(a); 以Ni为阴极时纯硫酸溶液(红色曲线)及衣康酸溶液的循环伏安曲线(黑色曲线)(b); 以Pb为阴极时的循环伏安曲线(c)[62]
Fig.7 Electrocatalytic reduction of itaconic acid to methyl succinic acid, and competitive hydrogen evolution reaction(a); Cyclic voltagram of pure supporting electrolye (H2SO4, red curves) and itaconic acid solution (black curves) with Ni cathode(b), and with Pb cathode(c)[62]
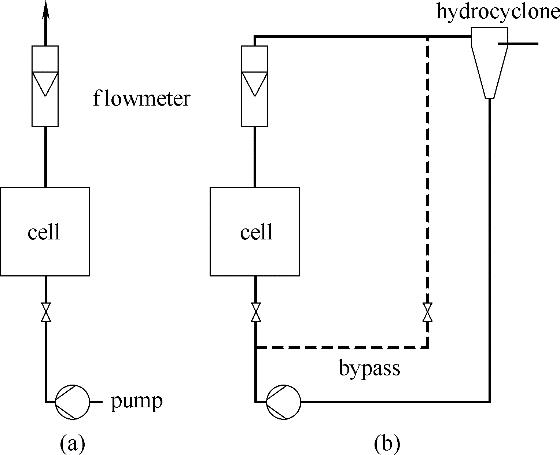
图9 电解液单次通过电化学反应器(a); 电解液循环通过电化学反应器(b)(流程图中的分离装置以水力旋流器示意[58])
Fig.9 Single pass of electrolyte in electrochemical cell(a); multiple passes of electrolyte in cell, with a hydrocyclone as a representative separation unit(b)[58]
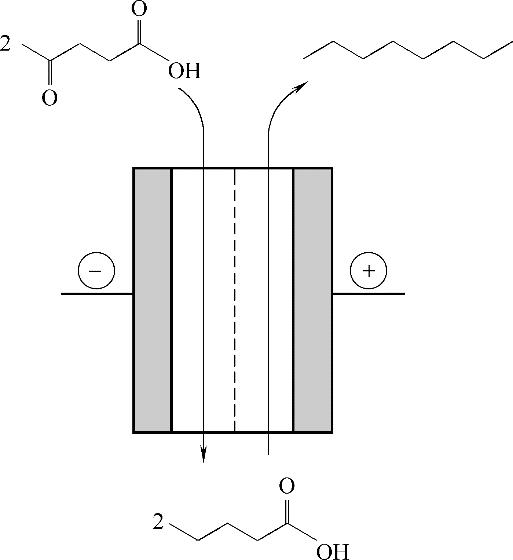
图10 乙酰丙酸在同一个电化学反应器里的还原及后续的氧化反应,最终产物是辛烷 (经授权修改自文献[40]; Copyright ? 2017, 英国皇家化学学会)
Fig.10 Consecutive reduction and oxidation of levulinic acid stream in a single electrochemical cell for the synthesis of octane
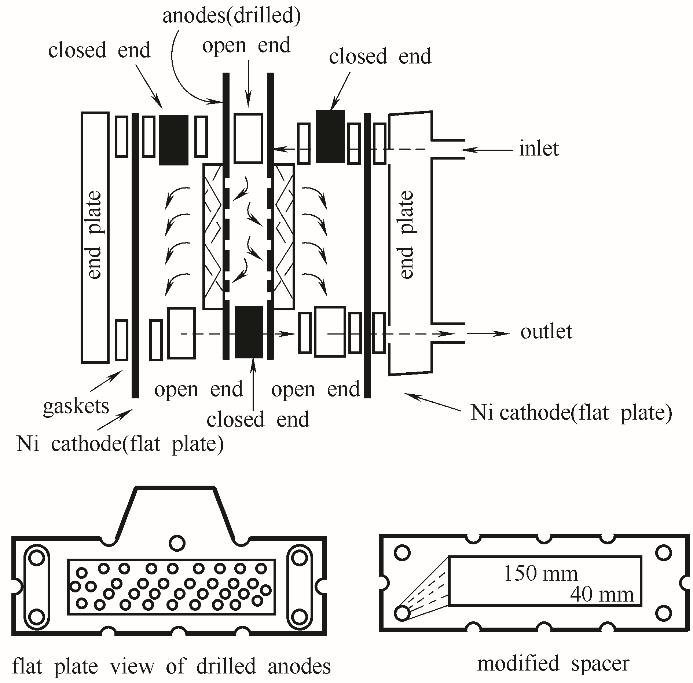
图11 用于木质素降解的板框式电化学反应器示意图(该反应器的部件都是商业化产品,其中的多孔阳极被凿出直径3 mm的孔(左下图)以便电解液能穿过阳极流动。经过流道改造的垫片(右下图)可以将电解液导入反应器中间的入口,使电解液能穿过阳极[84])
Fig.11 Schematic of a press-filter type electrochemical reactor for lignin depolymerization(The components of the reactor are all of commerical sources. The planar porous anode is drilled with holes of 3 mm diameter (lower left), allowing the electrolyte to flow through the anodes. Spacers with modified flow channels (lower right) could feed the electrolyte to the anodes in the interior of the reactor[84])
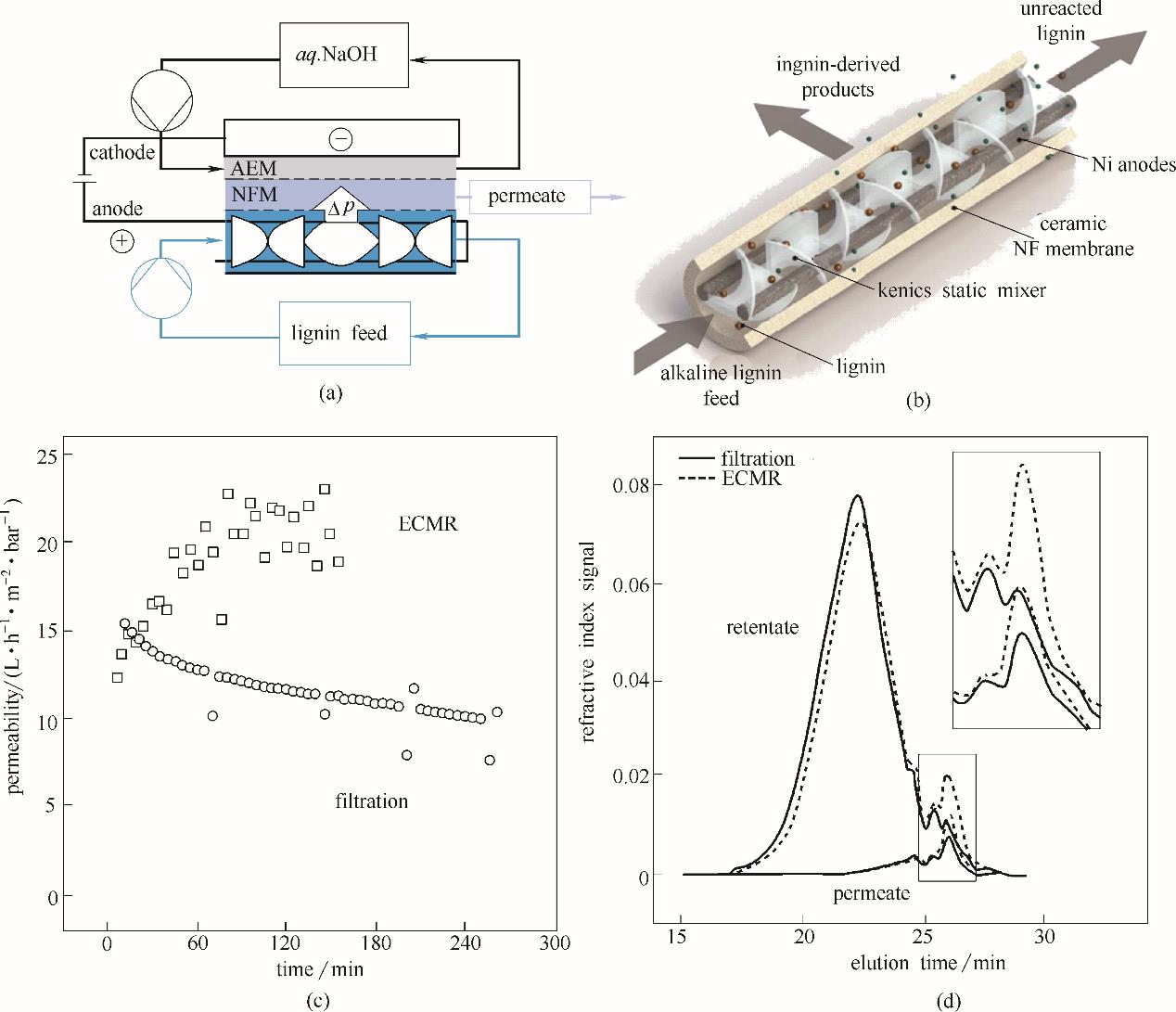
图12 用于木质素降解反应的电化学膜反应器(ECMR)示意图(a)(AEM指阴离子交换膜,NFM指纳滤膜);反应器阳极室3D示意图(b)(其中镍阳极附近的静态混合器(static mixers)可以促进阳极电解液的混合,并促进阳极附近小分子产物通过陶瓷纳滤膜的原位分离); ECMR里的纳滤膜原位过滤比反应之后的纳滤分离过程有更好的渗透性能(c); ECMR过程比普通的电化学降解及后续膜过滤过程有更高的小分子产物收率(d)[77] (1bar=105 Pa)
Fig.12 Flow scheme showing the electrochemical membrane reactor (ECMR) for lignin depolymerization(a)(AEM is anion exchange membrane, NFM is nanofiltration membrane); 3 D scheme of the anode compartment with static mixers right next to the Ni anodes to promote the liquid flow and in-situ product removal through the ceramic nanofiltration (NF) membrane(b); ECMR has better permeability through the NF membrane compared with post-reaction filtration of the reaction medium(c); Gel permeation chromotography shows that ECMR process has improvent in yield of small molecular components(d)[77]

图13 羟甲基糠醛转化为2,5-糠醛二酸的连续氧化电化学反应实验室装置图(2,5-糠醛二酸是一种合成生物塑料的重要单体[88])
Fig.13 Picture of the bench scale electrochemical cells for the continuous oxidation of HMF to FDCA, an important monomer for bioplastics[88]
| 1 | ShafieeS, TopalE. When will fossil fuel reserves be diminished?[J]. Energy Policy, 2009, 37(1): 181-189. |
| 2 | RagauskasA J, WilliamsC K, DavisonB H, et al. The path forward for biofuels and biomaterials[J]. Science, 2006, 311(5760): 484-489. |
| 3 | CherubiniF. The biorefinery concept: using biomass instead of oil for producing energy and chemicals[J]. Energy Conversion and Management, 2010, 51(7): 1412-1421. |
| 4 | MckendryP. Energy production from biomass (Part 1): Overview of biomass[J]. Bioresource Technology, 2002, 83(1): 37-46. |
| 5 | RuizH A, Rodriguez-JassoR M, FernandesB D, et al. Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: a review[J]. Renewable & Sustainable Energy Reviews, 2013, 21: 35-51. |
| 6 | KanT, StrezovV, EvansT J. Lignocellulosic biomass pyrolysis: a review of product properties and effects of pyrolysis parameters[J]. Renewable & Sustainable Energy Reviews, 2016, 57: 1126-1140. |
| 7 | SansaniwalS K, PalK, RosenM A, et al. Recent advances in the development of biomass gasification technology: a comprehensive review[J]. Renewable & Sustainable Energy Reviews, 2017, 72: 363-384. |
| 8 | YangH, YanR, ChenH, et al. Characteristics of hemicellulose, cellulose and lignin pyrolysis[J]. Fuel, 2007, 86(12/13): 1781-1788. |
| 9 | DorrestijnE, LaarhovenL J J, ArendsI, et al. The occurrence and reactivity of phenoxyl linkages in lignin and low rank coal[J]. Journal of Analytical and Applied Pyrolysis, 2000, 54(1/2): 153-192. |
| 10 | BalatM. Biomass energy and biochemical conversion processing for fuels and chemicals[J]. Energy Sources Part A - Recovery Utilization and Environmental Effects, 2006, 28(6): 517-525. |
| 11 | ParsellT, YoheS, DegensteinJ, et al. A synergistic biorefinery based on catalytic conversion of lignin prior to cellulose starting from lignocellulosic biomass[J]. Green Chemistry, 2015, 17(3): 1492-1499. |
| 12 | ChhedaJ N, HuberG W, DumesicJ A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals[J]. Angewandte Chemie-International Edition, 2007, 46(38): 7164-7183. |
| 13 | 江泽民. 对中国能源问题的思考[J]. 上海交通大学学报, 2008, (3): 345 - 359. |
| JiangZ M. Reflections on energy issues in China[J]. Journal of Shanghai Jiao Tong University, 2008, (3): 345 - 359. | |
| 14 | ITER. 60 years of progress[EB/OL]. [2018-10-31]. https: //. |
| 15 | YangZ G, ZhangJ L, Kintner-MeyerM C W, et al. Electrochemical energy storage for green grid[J]. Chemical Reviews, 2011, 111(5): 3577-3613. |
| 16 | CarrascoJ M, FranqueloL G, BialasiewiczJ T, et al. Power-electronic systems for the grid integration of renewable energy sources: a survey[J]. IEEE Transactions on Industrial Electronics, 2006, 53(4): 1002-1016. |
| 17 | DunnB, KamathH, TarasconJ M. Electrical energy storage for the grid: a battery of choices[J]. Science, 2011, 334(6058): 928-935. |
| 18 |
AustB, MorscherC. Negative strompreise in Deutschland[J]. Wirtschaftdienst, 2017, 97(4): 304-306. DOI: 10.1007/s10273-017-2135-0.
DOI URL |
| 19 | de GraaffM. Power-2-Chemicals[EB/OL]. [2018-10-31] https: //. |
| 20 | DuL, ShaoY Y, SunJ M, et al. Electrocatalytic valorisation of biomass derived chemicals[J]. Catalysis Science & Technology, 2018, 8(13): 3216-3232. |
| 21 | MitsosA, AsprionN, FloudasC A, et al. Challenges in process optimization for new feedstocks and energy sources[J]. Computers & Chemical Engineering, 2018, 113: 209-221. |
| 22 | BebelisS, BouzekK, CornellA, et al. Highlights during the development of electrochemical engineering[J]. Chemical Engineering Research & Design, 2013, 91(10): 1998-2020. |
| 23 | KwonY, SchoutenK J P, van der WaalJ C, et al. Electrocatalytic conversion of furanic compounds[J]. ACS Catalysis, 2016, 6(10): 6704-6717. |
| 24 | SimoesM, BarantonS, CoutanceauC. Electrochemical valorisation of glycerol[J]. ChemSusChem, 2012, 5(11): 2106-2124. |
| 25 | MoehleS, ZirbesM, RodrigoE, et al. Modern electrochemical aspects for the synthesis of value-added organic products[J]. Angewandte Chemie-International Edition, 2018, 57(21): 6018-6041. |
| 26 | ZirbesM, WaldvogelS R. Electro-conversion as sustainable method for the fine chemical production from the biopolymer lignin[J]. Current Opinion in Green and Sustainable Chemistry, 2018, 14: 19-25. |
| 27 | Maki-ArvelaP, SalmiT, HolmbomB, et al. Synthesis of sugars by hydrolysis of hemicelluloses—a review[J]. Chemical Reviews, 2011, 111(9): 5638-5666. |
| 28 | RinaldiR, JastrzebskiR, CloughM T, et al. Paving the way for lignin valorisation: Recent advances in bioengineering, biorefining and catalysis[J]. Angewandte Chemie-International Edition, 2016, 55(29): 8164-8215. |
| 29 | ShuaiL, AmiriM T, Questell-SantiagoY M, et al. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization[J]. Science, 2016, 354(6310): 329-333. |
| 30 | YuJ C, BaizerM M, NobeK. Electrochemical generated acid catalysis of cellulose hydrolysis[J]. Journal of The Electrochemical Society, 1988, 135(1): 83-87. |
| 31 | MengD, LiG, LiuZ, et al. Study of depolymerization of cotton cellulose by Pb/PbO2 anode electrochemical catalysis in sulfuric acid solution[J]. Polymer Degradation and Stability, 2011, 96(7): 1173-1178. |
| 32 | WerpyT, PetersenG, AdenA, et al., Results of screening for potential candidates from sugars and synthesis gas[M]//Top Value-Added Chemicals from Biomass: Volume 1. Oak Ridge: U.S. Department of Energy, 2004. |
| 33 | HolladayJ E, WhiteJ F, BozellJ J, et al. Results of screening for potential candidates from biorefinery lignin[M]//Top Value-Added Chemicals from Biomass: Volume 2. Oak Ridge: U.S. Department of Energy, 2007. |
| 34 | Carlos Serrano-RuizJ, PinedaA, Mariana BaluA, et al. Catalytic transformations of biomass-derived acids into advanced biofuels[J]. Catalysis Today, 2012, 195(1): 162-168. |
| 35 | DengL, LiJ, LaiD M, et al. Catalytic conversion of biomass-derived carbohydrates into gamma-valerolactone without using an external H2 supply[J]. Angewandte Chemie-International Edition, 2009, 48(35): 6529-6532. |
| 36 | EppingerJ, HuangK W. Formic acid as a hydrogen energy carrier[J]. ACS Energy Letters, 2017, 2(1): 188-195. |
| 37 | VoltaChem. Formic acid, the new energy carrier[EB/OL]. [2018-10-31]. https: //. |
| 38 | BozellJ J, MoensL, ElliottD C, et al. Production of levulinic acid and use as a platform chemical for derived products[J]. Resources Conservation and Recycling, 2000, 28(3/4): 227-239. |
| 39 | HayesM H B. Biochar and biofuels for a brighter future[J]. Nature, 2006, 443(7108): 144-144. |
| 40 | NilgesP, Dos SantosT R, HarnischF, et al. Electrochemistry for biofuel generation: electrochemical conversion of levulinic acid to octane[J]. Energy & Environmental Science, 2012, 5(1): 5231-5235. |
| 41 | RosatellaA A, SimeonovS P, FradeR F M, et al. 5-Hydroxymethylfurfural (HMF) as a building block platform: biological properties, synthesis and synthetic applications[J]. Green Chemistry, 2011, 13(4): 754-793. |
| 42 | van PuttenR J, van der WaalJ C, de JongE, et al. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources[J]. Chemical Reviews, 2013, 113(3): 1499-1597. |
| 43 | PanM, ShuG, PanJ, et al. Performance comparison of 2-methylfuran and gasoline on a spark-ignition engine with cooled exhaust gas recirculation[J]. Fuel, 2014, 132: 36-43. |
| 44 | AlipourS, LiB, VaranasiS, et al. Methods for high yield production of furans from biomass sugars at mild operating conditions: US20160281120[P]. 2016-09-29. |
| 45 | RieserK P, de VriesA. BASF and Avantium intend to establish Joint Venture[EB/OL]. [2018-10-31].https: //. |
| 46 | ChengF, BrewerC E. Producing jet fuel from biomass lignin: potential pathways to alkyl benzenes and cycloalkanes[J]. Renewable & Sustainable Energy Reviews, 2017, 72: 673-722. |
| 47 | AtkinsP, PaulaJ D. Physical Chemistry[M]. 8th ed. Great Britain: Oxford University Press, 2006. |
| 48 | LapicqueF. Electrochemical reactors[M]//Chemical Engineering and Chemical Process Technology: Volume 3. United Nations Educational Scientific and Cultural Organization, and Encyclopedia of Life Support Systems (UNESCO - EOLSS), 2010: 160-191. |
| 49 | reactorsElectrochemical [EB/OL]. [2018-10-31]. https: //web.vscht.cz/~paidarm/ACHP/erasmus/ElectrochemReactors_eng.pdf. |
| 50 | 刘俊吉, 周亚平, 李松林. 物理化学[M]. 6版. 北京: 高等教育出版社, 2009. |
| LiuJ J, ZhouY P, LiS L. Physical Chemistry[M]. 6th ed. Beijing: Higher Education Press, 2009 | |
| 51 | AgarJ N, BowdenF P. The kinetics of electrode reactions (I and II)[J]. Proceedings of the Royal Society of London Series A-Mathematical and Physical Sciences, 1938, 169(A937): 0206-0234. |
| 52 | WalshF C. The kinetics of electrode-reactions (2): Mass-transfer and mixed control[J]. Transactions of the Institute of Metal Finishing, 1992, 70: 95-99. |
| 53 | RodunerE. Selected fundamentals of catalysis and electrocatalysis in energy conversion reactions—a tutorial[J]. Catalysis Today, 2018, 309: 263-268. |
| 54 | BagotskyV S. Fundamentals of Electrochemistry[M]. 2nd ed. John Wiley & Sons, Inc., 2005. |
| 55 | WalshF, ReadeG. Design and performance of electrochemical reactors for efficient synthesis and environmental treatment (1): Electrode gemoetry and figures of merit[J]. Analyst, 1994, 119(5): 791-796. |
| 56 | PletcherD, GreenR A, BrownR C D. Flow electrolysis cells for the synthetic organic chemistry laboratory[J]. Chemical Reviews, 2018, 118(9): 4573-4591. |
| 57 | ParkK, PintauroP N, BaizerM M, et al. Current efficiencies and regeneration of poisoned Raney-Nickel in the electrohydrogenation of glucose to sorbitol[J]. Journal of Applied Electrochemistry, 1986, 16(6): 941-946. |
| 58 | PintauroP N, JohnsonD K, ParkK, et al. The paired electrochemical synthesis of sorbitol and gluconic acid in undivided flow cels(1)[J]. Journal of Applied Electrochemistry, 1984, 14(2): 209-220. |
| 59 | Garcia-MateosF J, Cordero-LanzacT, BerenguerR, et al. Lignin-derived Pt supported carbon (submicron)fiber electrocatalysts for alcohol electro-oxidation[J]. Applied Catalysis B-Environmental, 2017, 211: 18-30. |
| 60 | DingY, ChenM W. Nanoporous metals for catalytic and optical applications[J]. MRS Bulletin, 2009, 34(8): 569-576. |
| 61 | KwonY. Biomass electrochemistry: from cellulose to sorbitol[D]. Leiden University, 2013. |
| 62 | HolzhaeuserF J, ArtzJ, PalkovitsS, et al. Electrocatalytic upgrading of itaconic acid to methylsuccinic acid using fermentation broth as a substrate solution[J]. Green Chemistry, 2017, 19(10): 2390-2397. |
| 63 | ZhangZ, SongJ, HanB. Catalytic transformation of lignocellulose into chemicals and fuel products in ionic liquids[J]. Chemical Reviews, 2017, 117(10): 6834-6880. |
| 64 | Di MarinoD, StoeckmannD, KriescherS, et al. Electrochemical depolymerisation of lignin in a deep eutectic solvent[J]. Green Chemistry, 2016, 18(22): 6021-6028. |
| 65 | DierT K F, RauberD, DurneataD, et al. Sustainable electrochemical depolymerization of lignin in reusable ionic liquids[J]. Scientific Reports, 2017, 7(1): 5041-5052. |
| 66 | ReichertE, WintringerR, VolmerD A, et al. Electro-catalytic oxidative cleavage of lignin in a protic ionic liquid[J]. Physical Chemistry Chemical Physics, 2012, 14(15): 5214-5221. |
| 67 | HossainM M, AldousL. Ionic liquids for lignin processing: dissolution, isolation, and conversion[J]. Australian Journal of Chemistry, 2012, 65(11): 1465-1477. |
| 68 | 瞿广飞, 赵茜, 李军燕, 等. 以离子液体和超临界CO2为介质的生物质电化学液化方法及装置: 105441107 A [P]. 2016-03-30. |
| QuG F, ZhaoQ, LiJ Y, et al. Methods and set-ups for the electrochemical liquification of biomass with ionic liquids and super-critical CO2 as reaction medium: 105441107 A [P]. 2016-03-30. | |
| 69 | TianM, WenJ, MacdonaldD, et al. A novel approach for lignin modification and degradation[J]. Electrochemistry Communications, 2010, 12(4): 527-530. |
| 70 | ShaoD, LiangJ, CuiX, et al. Electrochemical oxidation of lignin by two typical electrodes: Ti/Sb-SnO2 and Ti/PbO2[J]. Chemical Engineering Journal, 2014, 244: 288-295. |
| 71 | ZhuH, WangL, ChenY, et al. Electrochemical depolymerization of lignin into renewable aromatic compounds in a non-diaphragm electrolytic cell[J]. RSC Advances, 2014, 4(56): 29917-29924. |
| 72 | ZhuH, ChenY, QinT, et al. Lignin depolymerization via an integrated approach of anode oxidation and electro-generated H2O2 oxidation[J]. RSC Advances, 2014, 4(12): 6232-6238. |
| 73 | LiuM, WenY, QiJ, et al. Fine chemicals prepared by bamboo lignin degradation through electrocatalytic redox between Cu cathode and Pb/PbO2 anode in alkali solution[J]. Chemistry Select, 2017, 2(17): 4956-4962. |
| 74 | LuoT, AbduS, WesslingM. Selectivity of ion exchange membranes: a review[J]. Journal of Membrane Science, 2018, 555: 429-454. |
| 75 | StiefelS, SchmitzA, PetersJ, et al. An integrated electrochemical process to convert lignin to value-added products under mild conditions[J]. Green Chemistry, 2016, 18(18): 4999-5007. |
| 76 | StiefelS, MarksC, SchmidtT, et al. Overcoming lignin heterogeneity: reliably characterizing the cleavage of technical lignin[J]. Green Chemistry, 2016, 18(2): 531-540. |
| 77 | StiefelS, LoelsbergJ, KipshagenL, et al. Controlled depolymerization of lignin in an electrochemical membrane reactor[J]. Electrochemistry Communications, 2015, 61: 49-52. |
| 78 | Di MarinoD, AnikoV, StoccoA, et al. Emulsion electro-oxidation of kraft lignin[J]. Green Chemistry, 2017, 19(20): 4778-4784. |
| 79 | KimH J, LeeJ, GreenS K, et al. Selective glycerol oxidation by electrocatalytic dehydrogenation[J]. ChemSusChem, 2014, 7(4): 1051-1056. |
| 80 |
SekarN, RamasamyR P. Electrochemical impedance spectroscopy for microbial fuel cell characterization[J]. Journal of Microbial and Biochemical Technology, 2013, S6: 004. DOI: 10.4172/1948-5948.S6-004.
DOI URL |
| 81 | 赵博. 电化学方法在生物质催化转化中的应用研究[D]. 合肥: 中国科学技术大学, 2015. |
| ZhaoB. Studies on the catalytic conversion of biomass with electrochemical methods[D]. Hefei: University of Science and Technology of China, 2015. | |
| 82 | SchmittD, RegenbrechtC, HartmerM, et al. Highly selective generation of vanillin by anodic degradation of lignin: a combined approach of electrochemistry and product isolation by adsorption[J]. Beilstein Journal of Organic Chemistry, 2015, 11: 473-480. |
| 83 | HarrisonK W, MartinG D, RamsdenT G, et al. The wind-to-hydrogen project: operational experience, testingperformance, and systems integration[R/OL].Colorado: U.S. Department of Energy, 2009. https: //. |
| 84 | SmithC Z, UtleyJ H P, HammondJ K. Electro-organic reactions. Part 60[1]. The electro-oxidative conversion at laboratory scale of a lignosulfonate into vanillin in an FM01 filter press flow reactor: preparative and mechanistic aspects[J]. Journal of Applied Electrochemistry, 2011, 41(4): 363-375. |
| 85 | Ponce De LeonC, HusseyW, FrazaoF, et al. The 3D printing of a polymeric electrochemical cell body and its characterisation[J]. Chemical Engineering Transactions, 2014, 41(Special Issue): 1-6. |
| 86 | LölsbergJ, StarckO, StiefelS, et al. 3D-printed electrodes with improved mass transport properties[J]. ChemElectroChem, 2017, 4(12): 3309-3313. |
| 87 |
ZirbesM, SchmittD, BeiserN, et al. Anodic degradation of lignin at active transition metal-based alloys and performance-enhanced anodes[J]. ChemElectroChem, 2018. DOI: 10.1002/celc.201801218.
DOI URL |
| 88 | de GraaffM. VoltaChem demonstrates continuous electrochemical FDCA production[EB/OL]. [2018-10-31].https: //, 2017. |
| 89 | 李振环, 曹磊, 苏坤梅, 等. 一种次氯酸钠电催化5-HMF制备FDCA的方法: 108130554A [P]. 2018-06-08. |
| LiZ H, CaoL, SuK M, et al. A method for the electrochemical conversion of 5-HMF to FDCA with sodium hypochlorite as mediator: 108130554A [P]. 2018-06-08. |
| [1] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [2] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| [3] | 郑玉圆, 葛志伟, 韩翔宇, 王亮, 陈海生. 中高温钙基材料热化学储热的研究进展与展望[J]. 化工学报, 2023, 74(8): 3171-3192. |
| [4] | 吴文涛, 褚良永, 张玲洁, 谭伟民, 沈丽明, 暴宁钟. 腰果酚生物基自愈合微胶囊的高效制备工艺研究[J]. 化工学报, 2023, 74(7): 3103-3115. |
| [5] | 董茂林, 陈李栋, 黄六莲, 吴伟兵, 戴红旗, 卞辉洋. 酸性助水溶剂制备木质纳米纤维素及功能应用研究进展[J]. 化工学报, 2023, 74(6): 2281-2295. |
| [6] | 杨峥豪, 何臻, 常玉龙, 靳紫恒, 江霞. 生物质快速热解下行式流化床反应器研究进展[J]. 化工学报, 2023, 74(6): 2249-2263. |
| [7] | 周小文, 杜杰, 张战国, 许光文. 基于甲烷脉冲法的Fe2O3-Al2O3载氧体还原特性研究[J]. 化工学报, 2023, 74(6): 2611-2623. |
| [8] | 张建华, 陈萌萌, 孙雅雯, 彭永臻. 部分短程硝化同步除磷耦合Anammox实现生活污水高效脱氮除磷[J]. 化工学报, 2023, 74(5): 2147-2156. |
| [9] | 葛泽峰, 吴雨青, 曾名迅, 查振婷, 马宇娜, 侯增辉, 张会岩. 灰化学成分对生物质气化特性的影响规律[J]. 化工学报, 2023, 74(5): 2136-2146. |
| [10] | 张兰河, 赖青燚, 王铁铮, 关潇卓, 张明爽, 程欣, 徐小惠, 贾艳萍. H2O2对SBR脱氮效率和污泥性能的影响[J]. 化工学报, 2023, 74(5): 2186-2196. |
| [11] | 王泽栋, 石至平, 刘丽艳. 考虑气泡非均匀耗散的矩形反应器声流场数值模拟及结构优化[J]. 化工学报, 2023, 74(5): 1965-1973. |
| [12] | 刘海芹, 李博文, 凌喆, 刘亮, 俞娟, 范一民, 勇强. 羟基-炔点击化学改性半乳甘露聚糖薄膜的制备及性能研究[J]. 化工学报, 2023, 74(3): 1370-1378. |
| [13] | 祖凌鑫, 胡荣庭, 李鑫, 陈余道, 陈广林. 木质生物质化学组分的碳释放产物特征和反硝化利用程度[J]. 化工学报, 2023, 74(3): 1332-1342. |
| [14] | 郑杰元, 张先伟, 万金涛, 范宏. 丁香酚环氧有机硅树脂的制备及其固化动力学研究[J]. 化工学报, 2023, 74(2): 924-932. |
| [15] | 付家崴, 陈帅帅, 方凯伦, 蒋新. 微反应器共沉淀反应制备铜锰催化剂[J]. 化工学报, 2023, 74(2): 776-783. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号